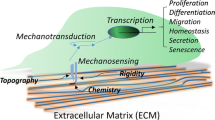
Abstract
The use of magnetic nanoparticles (MNPs) is a promising technique for future advances in biomedical applications. This idea is supported by the availability of MNPs that can target specific cell components, the variety of shapes of MNPs and the possibility of finely controlling the applied magnetic forces. To examine this opportunity, here we review the current developments in the use of MNPs to mechanically stimulate cells and, specifically, the cell mechanotransduction systems. We analyze the cell components that may act as mechanosensors and their effect on cell fate and we focus on the promising possibilities of controlling stem-cell differentiation, inducing cancer-cell death and treating nervous-system diseases.


Similar content being viewed by others
References
Ahamed, M., M. S. AlSalhi, and M. K. J. Siddiqui. Silver nanoparticle applications and human health. Clin. Chim. Acta 411:1841–1848, 2010.
Amano, M., M. Nakayama, and K. Kaibuchi. Rho-kinase/ROCK: a key regulator of the cytoskeleton and cell polarity. Cytoskeleton 67:545–554, 2010.
Arulmoli, J., M. M. Pathak, L. P. McDonnell, J. L. Nourse, F. Tombola, J. C. Earthman, and L. A. Flanagan. Static stretch affects neural stem cell differentiation in an extracellular matrix-dependent manner. Sci. Rep. 5:8499, 2015.
Asin, L., M. R. Ibarra, A. Tres, and G. F. Goya. Controlled cell death by magnetic hyperthermia: effects of exposure time, field amplitude, and nanoparticle concentration. Pharm. Res. 29:1319–1327, 2012.
Bernal, A., L. M. Perez, B. De Lucas, N. S. Martin, A. Kadow-Romacker, G. Plaza, K. Raum, and B. G. Galvez. Low-intensity pulsed ultrasound improves the functional properties of cardiac mesoangioblasts. Stem Cell Rev. 11:852–865, 2015.
Blumenthal, N. R., O. Hermanson, B. Heimrich, and V. P. Shastri. Stochastic nanoroughness modulates neuron-astrocyte interactions and function via mechanosensing cation channels. Proc. Natl. Acad. Sci. USA 111:16124–16129, 2014.
Bray, D. Axonal growth in response to experimentally applied nechanical tension. Dev. Biol. 102:379–389, 1984.
Chakraborty, M., S. Jain, and V. Rani. Nanotechnology: emerging tool for diagnostics and therapeutics. Appl. Biochem. Biotechnol. 165:1178–1187, 2011.
Chatelin, S., A. Constantinesco, and R. Willinger. Fifty years of brain tissue mechanical testing: from in vitro to in vivo investigations. Biorheology 47:255–276, 2010.
Cheng, D., X. Li, G. Zhang, and H. Shi. Morphological effect of oscillating magnetic nanoparticles in killing tumor cells. Nanoscale Res. Lett. 9:195, 2014.
Cho, M. H., E. J. Lee, M. Son, J. Lee, D. Yoo, J. Kim, S. W. Park, J. Shin, and J. Cheon. A magnetic switch for the control of cell death signalling in in vitro and in vivo systems. Nat. Mater. 11:1038–1043, 2012.
Choi, W. I., J. Kim, S. U. Heo, Y. Y. Jeong, Y. H. Kim, and G. Tae. The effect of mechanical properties of iron oxide nanoparticle-loaded functional nano-carrier on tumor targeting and imaging. J. Controlled Release 162:267–275, 2012.
Coste, B., J. Mathur, M. Schmidt, T. J. Earley, S. Ranade, M. J. Petrus, A. E. Dubin, and A. Patapoutian. Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science 330:55–60, 2010.
del Rio, A., R. Perez-Jimenez, R. Liu, P. Roca-Cusachs, J. M. Fernandez, and M. P. Sheetz. Stretching single talin rod molecules activates vinculin binding. Science 323:638–641, 2009.
Di Carlo, D. A mechanical biomarker of cell state in medicine. J. Lab. Autom. 17:32–42, 2012.
Di Corato, R., A. Espinosa, L. Lartigue, M. Tharaud, S. Chat, T. Pellegrino, C. Menager, F. Gazeau, and C. Wilhelm. Magnetic hyperthermia efficiency in the cellular environment for different nanoparticle designs. Biomaterials 35:6400–6411, 2014.
Discher, D. E., P. Janmey, and Y. L. Wang. Tissue cells feel and respond to the stiffness of their substrate. Science 310:1139–1143, 2005.
Engler, A. J., S. Sen, H. L. Sweeney, and D. E. Discher. Matrix elasticity directs stem cell lineage specification. Cell 126:677–689, 2006.
Evangelisti, E., D. Wright, M. Zampagni, R. Cascella, C. Fiorillo, S. Bagnoli, A. Relini, D. Nichino, T. Scartabelli, and B. Nacmias. Lipid rafts mediate amyloid-induced calcium dyshomeostasis and oxidative stress in Alzheimer’s disease. Curr. Alzheimer Res. 10:143–153, 2013.
Fettiplace, R., and C. M. Hackney. The sensory and motor roles of auditory hair cells. Nat. Rev. Neurosci. 7:19–29, 2006.
Grashoff, C., B. D. Hoffman, M. D. Brenner, R. Zhou, M. Parsons, M. T. Yang, M. A. McLean, S. G. Sligar, C. S. Chen, T. Ha, and M. A. Schwartz. Measuring mechanical tension across vinculin reveals regulation of focal adhesion dynamics. Nature 466:263–266, 2010.
Hayakawa, K., H. Tatsumi, and M. Sokabe. Actin filaments function as a tension sensor by tension-dependent binding of cofilin to the filament. J. Cell Biol. 195:721–727, 2011.
Hemphill, M. A., S. Dauth, C. J. Yu, B. E. Dabiri, and K. K. Parker. Traumatic brain injury and the neuronal microenvironment: a potential role for neuropathological mechanotransduction. Neuron 85:1177–1192, 2015.
Henstock, J. R., M. Rotherham, H. Rashidi, K. M. Shakesheff, and A. J. El Haj. Remotely activated mechanotransduction via magnetic nanoparticles promotes mineralization synergistically with bone morphogenetic protein 2: applications for injectable cell therapy. Stem Cells Transl. Med. 3:1363–1374, 2014.
Huiskes, R., R. Ruimerman, G. H. van Lenthe, and J. D. Janssen. Effects of mechanical forces on maintenance and adaptation of form in trabecular bone. Nature 405:704–706, 2000.
Ingber, D. E. Cellular basis of mechanotransduction. Biol. Bull. 194:323–325, 1998.
Ito, Y., T. Kimura, K. Nam, A. Katoh, T. Masuzawa, and A. Kishida. Effects of vibration on differentiation of cultured PC12 Cells. Biotechnol. Bioeng. 108:592–599, 2011.
Iwasaki, H., S. Eguchi, H. Ueno, F. Marumo, and Y. Hirata. Mechanical stretch stimulates growth of vascular smooth muscle cells via epidermal growth factor receptor. Am. J. Physiol. Heart Circ. Physiol. 278:H521–H529, 2000.
Jun, Y., J. Lee, and J. Cheon. Chemical design of nanoparticle probes for high-performance magnetic resonance imaging. Angew. Chem. Int. Ed. 47:5122–5135, 2008.
Jutila, A. A., D. L. Zignego, B. K. Hwang, J. K. Hilmer, T. Hamerly, C. A. Minor, S. T. Walk, and R. K. June. Candidate mediators of chondrocyte mechanotransduction via targeted and untargeted metabolomic measurements. Arch. Biochem. Biophys. 545:116–123, 2014.
Kalil, K., and E. Dent. Touch and go: guidance cues signal to the growth cone cytoskelleton. Curr. Opin. Neurobiol. 15:521–526, 2005.
Kanczler, J. M., H. S. Sura, J. Magnay, D. Green, R. O. C. Oreffo, J. P. Dobson, and A. J. El Haj. Controlled differentiation of human bone marrow stromal cells using magnetic nanoparticle technology. Tissue Eng. Part A 16:3241–3250, 2010.
Kelley, S. K., and A. Ashkenazi. Targeting death receptors in cancer with Apo2/TRAIL. Curr. Opin. Pharmacol. 4:333–339, 2004.
Keung, A. J., E. M. de Juan-Pardo, D. V. Schaffer, and S. Kumar. Rho GTPases mediate the mechanosensitive lineage commitment of neural stem cells. Stem Cells 29:1886–1897, 2011.
Kholodenko, B. N. Cell-signalling dynamics in time and space. Nat. Rev. Mol. Cell Biol. 7:165–176, 2006.
Kilinc, D., A. Blasiak, J. J. O’Mahony, and G. U. Lee. Low piconewton towing of CNS axons against diffusing and surface-bound repellents requires the inhibition of motor protein-associated pathways. Sci. Rep. 4:7128, 2014.
Kim, D., E. A. Rozhkova, I. V. Ulasov, S. D. Bader, T. Rajh, M. S. Lesniak, and V. Novosad. Biofunctionalized magnetic-vortex microdiscs for targeted cancer-cell destruction. Nat. Mater. 9:165–171, 2010.
Kolhatkar, A. G., A. C. Jamison, D. Litvinov, R. C. Willson, and T. R. Lee. Tuning the magnetic properties of nanoparticles. Int. J. Mol. Sci. 14:15977–16009, 2013.
Labeit, S., B. Kolmerer, and W. Linke. The giant protein titin: emerging roles in physiology and pathophysiology. Circ. Res. 80:290–294, 1997.
Lange, S., F. Xiang, A. Yakovenko, A. Vihola, P. Hackman, E. Rostkova, J. Kristensen, B. Brandmeier, G. Franzen, B. Hedberg, L. Gunnarsson, S. Hughes, S. Marchand, T. Sejersen, I. Richard, L. Edstrom, E. Ehler, B. Udd, and M. Gautel. The kinase domain of titin controls muscle gene expression and protein turnover. Science 308:1599–1603, 2005.
Lo, C. M., H. B. Wang, M. Dembo, and Y. L. Wang. Cell movement is guided by the rigidity of the substrate. Biophys. J . 79:144–152, 2000.
Lu, D., C. Chen, C. Lai, S. Soni, T. Lam, C. Le, E. Y. Chen, T. Nguyen, and W. Chin. Microgrooved surface modulates neuron differentiation in human embryonic stem cells. Hum. Embryonic Stem Cell Protoc., 2016. doi:10.1007/7651_2014_81
Markin, V. S., and B. Martinac. Mechanosensitive ion channels as reporters of bilayer expansion: a theoretical-model. Biophys. J . 60:1120–1127, 1991.
Matsumoto, Y., R. Chen, P. Anikeeva, and A. Jasanoff. Engineering intracellular biomineralization and biosensing by a magnetic protein. Nat. Commun. 6:8721, 2015.
Mitra, S. K., and D. D. Schlaepfer. Integrin-regulated FAK-Src signaling in normal and cancer cells. Curr. Opin. Cell Biol. 18:516–523, 2006.
Nikukar, H., S. Reid, P. M. Tsimbouri, M. O. Riehle, A. S. G. Curtis, and M. J. Dalby. Osteogenesis of mesenchymal stem cells by nanoscale mechanotransduction. ACS Nano 7:2758–2767, 2013.
Nunnally, M. H., J. M. Dangelo, and S. W. Craig. Filamin concentration in cleavage furrow and midbody region: frequency of occurrence compared with that of alpha-actinin and myosin. J. Cell Biol. 87:219–226, 1980.
Paluch, E. K., C. M. Nelson, N. Biais, B. Fabry, J. Moeller, B. L. Pruitt, C. Wollnik, G. Kudryasheva, F. Rehfeldt, and W. Federle. Mechanotransduction: use the force(s). BMC Biol. 13:47, 2015.
Paszek, M. J., N. Zahir, K. R. Johnson, J. N. Lakins, G. I. Rozenberg, A. Gefen, C. A. Reinhart-King, S. S. Margulies, M. Dembo, D. Boettiger, D. A. Hammer, and V. M. Weaver. Tensional homeostasis and the malignant phenotype. Cancer Cell 8:241–254, 2005.
Pathak, M. M., J. L. Nourse, T. Tran, J. Hwe, J. Arulmoli, T. L. Dai Trang, E. Bernardis, L. A. Flanagan, and F. Tombola. Stretch-activated ion channel Piezo1 directs lineage choice in human neural stem cells. Proc. Natl. Acad. Sci. USA 111:16148–16153, 2014.
Pelham, R., and Y. Wang. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc. Natl. Acad. Sci. USA 94:13661–13665, 1997.
Philip, J. T., and K. N. Dahl. Nuclear mechanotransduction: response of the lamina to extracellular stress with implications in aging. J. Biomech. 41:3164–3170, 2008.
Plaza, G. R., and T. Q. P. Uyeda. Contraction speed of the actomyosin cytoskeleton in the absence of the cell membrane. Soft Matter 9:4390–4400, 2013.
Plaza, G. R., T. Q. P. Uyeda, Z. Mirzaei, and C. A. Simmons. Study of the influence of actin-binding proteins using linear analyses of cell deformability. Soft Matter 11:5435–5446, 2015.
Pounder, N. M., and A. J. Harrison. Low intensity pulsed ultrasound for fracture healing: a review of the clinical evidence and the associated biological mechanism of action. Ultrasonics 48:330–338, 2008.
Puchner, E. M., A. Alexandrovich, A. L. Kho, U. Hensen, L. V. Schaefer, B. Brandmeier, F. Graeter, H. Grubmueller, H. E. Gaub, and M. Gautel. Mechanoenzymatics of titin kinase. Proc. Natl. Acad. Sci. USA 105:13385–13390, 2008.
Qiao, R., Q. Jia, S. Huewel, R. Xia, T. Liu, F. Gao, H. Galla, and M. Gao. Receptor-mediated delivery of magnetic nanoparticles across the blood-brain barrier. ACS Nano 6:3304–3310, 2012.
Ren, Y., J. C. Effler, M. Norstrom, T. Luo, R. A. Firtel, P. A. Iglesias, R. S. Rock, and D. N. Robinson. Mechanosensing through cooperative interactions between myosin II and the actin crosslinker cortexillin I. Curr. Biol. 19:1421–1428, 2009.
Ren, X. D., W. B. Kiosses, D. J. Sieg, C. A. Otey, D. D. Schlaepfer, and M. A. Schwartz. Focal adhesion kinase suppresses Rho activity to promote focal adhesion turnover. J. Cell Sci. 113(Pt 20):3673–3678, 2000.
Sachs, F. Stretch-activated ion channels: what are they? Physiology 25:50–56, 2010.
Sadhukha, T., T. S. Wiedmann, and J. Panyam. Inhalable magnetic nanoparticles for targeted hyperthermia in lung cancer therapy. Biomaterials 34:5163–5171, 2013.
Samuel, M. S., J. I. Lopez, E. J. McGhee, D. R. Croft, D. Strachan, P. Timpson, J. Munro, E. Schroeder, J. Zhou, V. G. Brunton, N. Barker, H. Clevers, O. J. Sansom, K. I. Anderson, V. M. Weaver, and M. F. Olson. Actomyosin-mediated cellular tension drives increased tissue stiffness and beta-catenin activation to induce epidermal hyperplasia and tumor growth. Cancer Cell 19:776–791, 2011.
Sawada, Y., M. Tamada, B. J. Dubin-Thaler, O. Cherniavskaya, R. Sakai, S. Tanaka, and M. P. Sheetz. Force sensing by mechanical extension of the Src family kinase substrate p130Cas. Cell 127:1015–1026, 2006.
Schrenk-Siemens, K., H. Wende, V. Prato, K. Song, C. Rostock, A. Loewer, J. Utikal, G. R. Lewin, S. G. Lechner, and J. Siemens. PIEZO2 is required for mechanotransduction in human stem cell-derived touch receptors. Nat. Neurosci. 18:10–16, 2015.
Seong, J., N. Wang, and Y. Wang. Mechanotransduction at focal adhesions: from physiology to cancer development. J. Cell Mol. Med. 17:597–604, 2013.
Seppala, J., H. Tossavainen, N. Rodic, P. Permi, U. Pentikainen, and J. Ylanne. Flexible structure of peptide-bound filamin a mechanosensor domain pair 20-21. PLoS ONE 10:e0136969, 2015.
Shao, M., F. Ning, J. Zhao, M. Wei, D. G. Evans, and X. Duan. Preparation of Fe3O4@SiO2@layered double hydroxide core-shell microspheres for magnetic separation of proteins. J. Am. Chem. Soc. 134:1071–1077, 2012.
Shen, J., F. W. Luscinskas, A. Connolly, C. F. Dewey, and M. A. Gimbrone. Fluid shear-stress modulates cytosolic free calcium in vascular endothelial-cells. Am. J. Physiol. 262:C384–C390, 1992.
Shen, Y., C. Wu, T. Q. Uyeda, G. R. Plaza, B. Liu, Y. Han, M. S. Lesniak, and Y. Cheng. Elongated nanoparticle aggregates in cancer cells for mechanical destruction with low frequency rotating magnetic field. Theranostics 7:1735–1748, 2017.
Simi, A. K., A. S. Piotrowski, and C. M. Nelson. Mechanotransduction, metastasis and genomic instability: genomic instability and cancer metastasis. In: Mechanisms, emerging themes, and novel therapeutic strategies 20, edited by C. Maxwell, and C. Roskelley. Switzerland: Springer, 2015, pp. 139–158.
Smith, M. L., D. Gourdon, W. C. Little, K. E. Kubow, R. A. Eguiluz, S. Luna-Morris, and V. Vogel. Force-induced unfolding of fibronectin in the extracellular matrix of living cells. PLoS Biol. 5:e268, 2007.
Son, B., H. D. Kim, M. Kim, J. A. Kim, J. Lee, H. Shin, N. S. Hwang, and T. H. Park. Physical stimuli-induced chondrogenic differentiation of mesenchymal stem cells using magnetic nanoparticles. Adv. Healthc. Mater. 4:1339–1347, 2015.
Souza, G. R., J. R. Molina, R. M. Raphael, M. G. Ozawa, D. J. Stark, C. S. Levin, L. F. Bronk, J. S. Ananta, J. Mandelin, M. Georgescu, J. A. Bankson, J. G. Gelovani, T. C. Killian, W. Arap, and R. Pasqualini. Three-dimensional tissue culture based on magnetic cell levitation. Nat. Nanotechnol. 5:291–296, 2010.
Sun, Y., K. M. A. Yong, L. G. Villa-Diaz, X. Zhang, W. Chen, R. Philson, S. Weng, H. Xu, P. H. Krebsbach, and J. Fu. Hippo/YAP-mediated rigidity-dependent motor neuron differentiation of human pluripotent stem cells. Nat. Mater. 13:599–604, 2014.
Suresh, S. Biomechanics and biophysics of cancer cells. Acta Biomater. 3:413–438, 2007.
Swaminathan, V., K. Mythreye, E. T. O’Brien, A. Berchuck, G. C. Blobe, and R. Superfine. Mechanical stiffness grades metastatic potential in patient tumor cells and in cancer cell lines. Cancer Res. 71:5075–5080, 2011.
Swift, J., I. L. Ivanovska, A. Buxboim, T. Harada, P. C. D. P. Dingal, J. Pinter, J. D. Pajerowski, K. R. Spinler, J. Shin, M. Tewari, F. Rehfeldt, D. W. Speicher, and D. E. Discher. Nuclear lamin-A scales with tissue stiffness and enhances matrix-directed differentiation. Science 341:1240104, 2013.
Thomas, C. H., J. H. Collier, C. S. Sfeir, and K. E. Healy. Engineering gene expression and protein synthesis by modulation of nuclear shape. Proc. Natl. Acad. Sci. USA 99:1972–1977, 2002.
Tsimbouri, P. M. Adult stem cell responses to nanostimuli. J. Funct. Biomater. 6:598–622, 2015.
Uddin, S. M. Z., and Y. Qin. Enhancement of osteogenic differentiation and proliferation in human mesenchymal stem cells by a modified low intensity ultrasound stimulation under simulated microgravity. PLoS ONE 8:e73914, 2013.
Uyeda, T. Q. P., Y. Iwadate, N. Umeki, A. Nagasaki, and S. Yumura. Stretching actin filaments within cells enhances their affinity for the myosin II motor domain. PLoS ONE 6:e26200, 2011.
Vicente-Manzanares, M., X. Ma, R. S. Adelstein, and A. R. Horwitz. Non-muscle myosin II takes centre stage in cell adhesion and migration. Nat. Rev. Mol. Cell Biol. 10:778–790, 2009.
Vogel, V. Mechanotransduction involving multimodular proteins: converting force into biochemical signals. Annu. Rev. Biophys. Biomol. Struct. 35:459–488, 2006.
Weaver, V., O. Petersen, F. Wang, C. Larabell, P. Briand, C. Damsky, and M. Bissell. Reversion of the malignant phenotype of human breast cells in three-dimensional culture and in vivo by integrin blocking antibodies. J. Cell Biol. 137:231–245, 1997.
Weinbaum, S., Y. Duan, M. M. Thi, and L. You. An integrative review of mechanotransduction in endothelial, epithelial (renal) and dendritic cells (osteocytes). Cell. Mol. Bioeng. 4:510–537, 2011.
Wu, M., J. Fannin, K. M. Rice, B. Wang, and E. R. Blough. Effect of aging on cellular mechanotransduction. Ageing Res. Rev. 10:1–15, 2011.
Yonemura, S., Y. Wada, T. Watanabe, A. Nagafuchi, and M. Shibata. alpha-Catenin as a tension transducer that induces adherens junction development. Nat. Cell Biol. 12:533–542, 2010.
Yoshikawa, H. Y., T. Kawano, T. Matsuda, S. Kidoaki, and M. Tanaka. Morphology and adhesion strength of myoblast cells on photocurable gelatin under native and non-native micromechanical environments. J. Phys. Chem. B 117:4081–4088, 2013.
Zangwill, A. Modern electrodynamics. Cambridge: Cambridge Unicersity Press, 2013.
Zemel, A., F. Rehfeldt, A. E. X. Brown, D. E. Discher, and S. A. Safran. Optimal matrix rigidity for stress-fibre polarization in stem cells. Nat. Phys. 6:468–473, 2010.
Zhang, E., M. F. Kircher, M. Koch, L. Eliasson, S. N. Goldberg, and E. Renstrom. Dynamic magnetic fields remote-control apoptosis via nanoparticle rotation. ACS Nano 8:3192–3201, 2014.
Acknowledgments
YC thanks the National Science Foundation of China (No. 81571803) the Thousand Talents Plan and Shanghai Pujiang Program (No. 15PJ1407800) for support. GRP and TQPU received a team grant from The Program of High-end Foreign Experts of the State Administration of Foreign Experts Affairs, China. GRP received support from the Ministerio de Economía y Competitividad, Spain, througth the project MAT2016-76847-R.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Associate Editor James J Moon oversaw the review of this article.
Rights and permissions
About this article
Cite this article
Shen, Y., Cheng, Y., Uyeda, T.Q.P. et al. Cell Mechanosensors and the Possibilities of Using Magnetic Nanoparticles to Study Them and to Modify Cell Fate. Ann Biomed Eng 45, 2475–2486 (2017). https://doi.org/10.1007/s10439-017-1884-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-017-1884-7