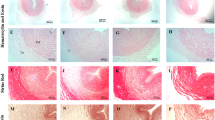
Abstract
Most tissues, including those to be decellularized for tissue engineering applications, are frozen for long term preservation. Such conventional cryopreservation has been shown to alter the structure and mechanical properties of tissues. Little is known, however, how freezing affects decellularization of tissues. The purpose of this study was two-fold: to examine the effects of freezing on decellularization of human umbilical arteries (HUAs), which represent a potential scaffolding material for small-diameter tissue-engineered vascular grafts, and to examine how decellularization affects the mechanical properties of frozen HUAs. Among many decellularization methods, hypotonic sodium dodecyl sulfate solution was selected as the decellularizing agent and tested on fresh HUAs to optimize decellularization conditions. The efficiency of decellularization was evaluated by DNA assay and histology every 12 up to 48 h. The optimized decellularization protocol was then performed on frozen HUAs. The stiffness, burst pressure, and suture retention strength of fresh HUAs and frozen HUAs before and after decellularization were also examined. It appeared that freezing decreased the efficiency of decellularization, which may be attributed to the condensed extracellular matrix caused by freezing. While the stiffness of fresh HUAs did not change significantly after decellularization, decellularization reduced the compliance of frozen HUAs. Interestingly, the stiffness of decellularized frozen HUAs was similar to that of decellularized fresh HUAs. Although little difference in stiffness was observed, we suggest avoiding freezing if more efficient and complete decellularization is desired.








Similar content being viewed by others
References
Abousleiman, R. I., Y. Reyes, P. McFetridge, et al. The human umbilical vein: a novel scaffold for musculoskeletal soft tissue regeneration. Artif. Organs 32:735–742, 2008.
Adham, M., J. P. Gournier, J. P. Favre, et al. Mechanical characteristics of fresh and frozen human descending thoracic aorta. J. Surg. Res. 64:32–34, 1996.
Bakhach, J. The cryopreservation of composite tissues: principles and recent advancement on cryopreservation of different type of tissues. Organogenesis 5:119–126, 2009.
Ballyk, P. D., C. Walsh, J. Butany, et al. Compliance mismatch may promote graft-artery intimal hyperplasia by altering suture-line stresses. J. Biomech. 31:229–237, 1998.
Bergmeister, H., M. Strobl, C. Grasl, et al. Tissue engineering of vascular grafts European Surgery. Acta Chirurgica Austriaca 45:187–193, 2013.
Bishopric, N. H., L. Dousman, and Y. M. M. Yao. Matrix Substrate for a Viable Body Tissue-Derived Prosthesis and Method for Making the Same. Saint Paul: St. Jude Medical Inc, 1999.
Blondel, W. C. P. M., B. Lehalle, G. Maurice, et al. Rheological properties of fresh and cryopreserved human arteries tested in vitro. Rheol. Acta 39:461–468, 2000.
Couet, F., S. Meghezi, and D. Mantovani. Fetal development, mechanobiology and optimal control processes can improve vascular tissue regeneration in bioreactors: an integrative review. Med. Eng. Phys. 34:269–278, 2012.
Cox, B., and A. Emili. Tissue subcellular fractionation and protein extraction for use in mass-spectrometry-based proteomics. Nat. Protoc. 1:1872–1878, 2006.
Crapo, P. M., T. W. Gilbert, and S. F. Badylak. An overview of tissue and whole organ decellularization processes. Biomaterials 32:3233–3243, 2011.
Crouzier, T., T. McClendon, Z. Tosun, et al. Inverted human umbilical arteries with tunable wall thicknesses for nerve regeneration. J. Biomed. Mater. Res. A 89:818–828, 2009.
Dahl, S. L. M., J. Koh, V. Prabhakar, et al. Decellularized native and engineered arterial scaffolds for transplantation. Cell Transplant. 12:659–666, 2003.
Ferruzzi, J., M. R. Bersi, and J. D. Humphrey. Biomechanical phenotyping of central arteries in health and disease: advantages of and methods for murine models. Ann. Biomed. Eng. 41:1311–1330, 2013.
Fitzpatrick, J. C., P. M. Clark, and F. M. Capaldi. Effect of decellularization protocol on the mechanical behavior of porcine descending aorta. Int. J. Biomater., 2010. doi:10.1155/2010/620503.
Gilbert, T. W., T. L. Sellaro, and S. F. Badylak. Decellularization of tissues and organs. Biomaterials 27:3675–3683, 2006.
Greenwald, S. E., and C. L. Berry. Improving vascular grafts: the importance of mechanical and haemodynamic properties. J. Pathol. 190:292–299, 2000.
Gui, L. Q., A. Muto, S. A. Chan, et al. Development of decellularized human umbilical arteries as small-diameter vascular grafts. Tissue Eng. Part A 15:2665–2676, 2009.
Hoenicka, M., K. Lehle, V. R. Jacobs, et al. Properties of the human umbilical vein as a living scaffold for a tissue-engineered vessel graft. Tissue Eng. 13:219–229, 2007.
Hu, J. J., A. Ambrus, T. W. Fossum, et al. Time courses of growth and remodeling of porcine aortic media during hypertension: a quantitative immunohistochemical examination. J. Histochem. Cytochem. 56:359–370, 2008.
Hu, J. J., W. C. Chao, P. Y. Lee, et al. Construction and characterization of an electrospun tubular scaffold for small-diameter tissue-engineered vascular grafts: a scaffold membrane approach. J. Mech. Behav. Biomed. Mater. 13:140–155, 2012.
Hu, J. J., T. W. Fossum, M. W. Miller, et al. Biomechanics of the porcine basilar artery in hypertension. Ann. Biomed. Eng. 35:19–29, 2007.
Humphrey, J. D. Cardiovascular Solid Mechanics: Cells, Tissues, and Organs. New York: Springer, 2002.
Isenberg, B. C., C. Williams, and R. T. Tranquillo. Small-diameter artificial arteries engineered in vitro. Circ. Res. 98:25–35, 2006.
Kannan, R. Y., H. J. Salacinski, P. E. Butler, et al. Current status of prosthetic bypass grafts: a review. J. Biomed. Mater. Res. Part B 74B:570–581, 2005.
Ketchedjian, A., A. L. Jones, P. Krueger, et al. Recellularization of decellularized allograft scaffolds in ovine great vessel reconstructions. Ann. Thorac. Surg. 79:888–896, 2005.
Masson, I., A. Fialaire-Legendre, C. Godin, et al. Mechanical properties of arteries cryopreserved at −80 degrees C and −150 degrees C. Med. Eng. Phys. 31:825–832, 2009.
Muller-Schweinitzer, E. Cryopreservation of vascular tissues. Organogenesis 5:97–104, 2009.
Nseir, N., O. Regev, T. Kaully, et al. Biodegradable scaffold fabricated of electrospun albumin fibers: mechanical and biological characterization. Tissue Eng. Part C 19:257–264, 2013.
Pellegata, A. F., M. A. Asnaghi, I. Stefani, et al. Detergent-enzymatic decellularization of swine blood vessels: insight on mechanical properties for vascular tissue engineering. Biomed. Res. Int. 20:918753, 2013.
Pukacki, F., T. Jankowski, M. Gabriel, et al. The mechanical properties of fresh and cryopreserved arterial homografts. Eur. J. Vasc. Endovasc. Surg. 20:21–24, 2000.
Rosario, D. J., G. C. Reilly, E. A. Salah, et al. Decellularization and sterilization of porcine urinary bladder matrix for tissue engineering in the lower urinary tract. Regen. Med. 3:145–156, 2008.
Rosset, E., A. Friggi, G. Novakovitch, et al. Effects of cryopreservation on the viscoelastic properties of human arteries. Ann. Vasc. Surg. 10:262–272, 1996.
Roy, S., P. Silacci, and N. Stergiopulos. Biomechanical properties of decellularized porcine common carotid arteries. Am. J. Physiol. Heart Circ. Physiol. 289:H1567–H1576, 2005.
Schaner, P. J., N. D. Martin, T. N. Tulenko, et al. Decellularized vein as a potential scaffold for vascular tissue engineering. J. Vasc. Surg. 40:146–153, 2004.
Schutte, S. C., Z. Z. Chen, K. G. M. Brockbank, et al. Cyclic strain improves strength and function of a collagen-based tissue-engineered vascular media. Tissue Eng. Part A 16:3149–3157, 2010.
Sheridan, W. S., G. P. Duffy, and B. P. Murphy. Mechanical characterization of a customized decellularized scaffold for vascular tissue engineering. J. Mech. Behav. Biomed. Mater. 8:58–70, 2012.
Song, Y. C., B. S. Khirabadi, F. Lightfoot, et al. Vitreous cryopreservation maintains the function of vascular grafts. Nat. Biotechnol. 18:296–299, 2000.
Stegemann, J. P., S. N. Kaszuba, and S. L. Rowe. Review: advances in vascular tissue engineering using protein-based Biomaterials. Tissue Eng. 13:2601–2613, 2007.
Stemper, B. D., N. Yoganandan, M. R. Stineman, et al. Mechanics of fresh, refrigerated, and frozen arterial tissue. J. Surg. Res. 139:236–242, 2007.
Thakrar, R. R., V. P. Patel, G. Hamilton, et al. Vitreous cryopreservation maintains the viscoelastic property of human vascular grafts. FASEB J. 20:874–881, 2006.
Vaz, C. M., S. van Tuijl, C. V. C. Bouten, et al. Design of scaffolds for blood vessel tissue engineering using a multi-layering electrospinning technique. Acta Biomater. 1:575–582, 2005.
Veith, F. J., S. K. Gupta, E. Ascer, et al. 6-Year prospective multicenter randomized comparison of autologous saphenous-vein and expanded polytetrafluoroethylene grafts in infrainguinal arterial reconstructions. J. Vasc. Surg. 3:104–114, 1986.
Veith, F. J., C. M. Moss, S. Sprayregen, et al. Preoperative saphenous venography in arterial reconstructive surgery of the lower-extremity. Surgery 85:253–256, 1979.
Venkatasubramanian, R. T., E. D. Grassl, V. H. Barocas, et al. Effects of freezing and cryopreservation on the mechanical properties of arteries. Ann. Biomed. Eng. 34:823–832, 2006.
Williams, C., J. Liao, E. M. Joyce, et al. Altered structural and mechanical properties in decellularized rabbit carotid arteries. Acta Biomater. 5:993–1005, 2009.
Xiong, Y., W. Y. Chan, A. W. C. Chua, et al. Decellularized porcine saphenous artery for small-diameter tissue-engineered conduit graft. Artif. Organs 37:E74–E87, 2013.
Xu, C. C., R. W. Chan, and N. Tirunagari. A biodegradable, acellular xenogeneic scaffold for regeneration of the vocal fold lamina propria. Tissue Eng. 13:551–566, 2007.
Zou, Y., and Y. H. Zhang. Mechanical evaluation of decellularized porcine thoracic aorta. J. Surg. Res. 175:359–368, 2012.
Acknowledgment
This research was supported by grants from the National Science Council (NSC-100-2221-E-006-097) and the National Health Research Institute (NHRI-EX102-10217EC) in Taiwan.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Dan Elson oversaw the review of this article.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tuan-Mu, HY., Yu, CH. & Hu, JJ. On the Decellularization of Fresh or Frozen Human Umbilical Arteries: Implications for Small-Diameter Tissue Engineered Vascular Grafts. Ann Biomed Eng 42, 1305–1318 (2014). https://doi.org/10.1007/s10439-014-1000-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-014-1000-1