Abstract
We investigated the effect of two commonly studied surfactants, sodium dodecyl sulfate (SDS) and dodecyl trimethylammonium bromide (C12TAB), on skin barrier properties. Using skin conductivity, FT-IR of stratum corneum samples, and penetration of radiolabelled SDS, we determined that addition of C12TAB lowers the ability of SDS to perturb skin’s barrier properties. Ultrafiltration experiments revealed that addition of C12TAB serves to decrease the concentration of monomers and sub-micellar aggregates. None of the measured skin properties including enhancement of skin conductivity, perturbation of lipid structure and skin concentration of SDS correlated with the total SDS concentration in the donor compartment (i.e., the total SDS concentration). However, all these parameters correlated well against the concentration of monomers and sub-micellar aggregates. These findings provide the evidence of the importance of monomer and sub-micellar components in altering skin barrier properties.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Delivery of actives into and across the skin plays an important role in several topical, transdermal, dermatological, and personal care applications.43 While small, lipophilic molecules permeate the skin relatively easily, permeation of larger and hydrophilic molecules is hindered by the low permeability of stratum corneum (SC), the outermost layer of skin. This is particularly challenging since a significant fraction of currently marketed therapeutic drugs are hydrophilic in nature.16 Transdermal drug delivery is also appealing for the delivery of macromolecules such as insulin and vaccines, which otherwise have to be injected using needles and syringes.29,43 Needle-based methods are severely limited by pain and needle-phobia and lead to severe patient non-compliance.24 In addition to avoiding needles, transdermal delivery offers sustained release of drugs and control over termination. Delivery of macromolecules across the skin, however, is severely limited by the low permeability of skin. To date, there are about 20 drugs that are delivered using FDA-approved transdermal delivery methods, not including drugs delivered as creams or ointments.38 This means that there is vast room for improvement in delivery through transdermal routes, which has resulted in the development of novel methodologies to transiently enhance skin permeability using physical or chemical means.2,23,33,39,47 Some physical means of penetration enhancement include the use of jet-injectors,17,44 sonophoresis (ultrasound),3,30 iontophoresis,7,20 electroporation,8 and microneedles.13,37 Penetration enhancement can also be achieved by using chemicals,23,39,45 such as surfactants, fatty acids, esters, and amines to penetrate into the skin and disrupt the structure and packing of lipids and proteins within the SC. Chemical penetration enhancers (CPEs) are capable of enhancing skin permeability to molecules; however, this enhancement is often accompanied by irritation,21,42 thereby limiting their applications. Use of CPE mixtures has been proposed as a potential solution to decouple the enhancing and irritating effects of CPEs.10,23
Mixtures of CPEs, in particular surfactants, have already been shown to yield potent formulations without necessarily elevating the irritation.23 Surfactants possess the ability to self-assemble to form micellar structures and therefore can exhibit more complex interactions with skin compared to single CPEs. This property allows for the manipulation of surfactant mixtures to control their interactions with the skin.10,11,31,46 Here, we report on the effect of mixing two extensively studied surfactants, sodium dodecyl sulfate (SDS) and dodecyl trimethylammonium bromide (C12TAB),19,34,35 on the perturbation of the skin barrier. We particularly focus on assessing how the presence of C12TAB influences the effect of SDS on skin barrier.
Experimental Section
Materials
Ultrapure SDS and C12TAB were purchased from Sigma-Aldrich (St Louis, MO) and used without further purification. All solutions were prepared in phosphate buffered saline (PBS) with the exception of samples that were prepared for ultrafiltration studies. Those samples were prepared in Millipure water and not PBS because the salts in PBS interfere with dye complexation. 14C-radiolabelled SDS was purchased from Sigma-Aldrich (St Louis, MO). Mixed micellar solutions were prepared with SDS alone or with SDS + C12TAB at various concentrations and SDS:C12TAB molar ratios.
Skin Preparation
Porcine skin was utilized as the skin model in all experiments. Skin was obtained from Lampire Biologicals (Pipersville, PA) and stored at −80 °C for use as needed. Storage at −80 °C has minimal effect on the barrier properties of skin. 23 Skin was removed from the freezer prior to the experiment and was allowed to thaw at room temperature. Skin was never exposed to repeated freeze and thaw cycle.
Skin Electrical Impedance (Conductivity)
Skin barrier properties can be analyzed by determining the ability of ions to flow across the skin (i.e., measuring the skin’s conductivity).22 The ability of the surfactant solutions to reduce skin barrier properties was measured by quantifying their effect on skin electrical impedance. Skin conductivity is a good indicator of skin permeation.22 It has been shown by Karande et al. 22 that significant agreement exists between the skin electrical resistance analysis and standard permeation experiments using a Franz diffusion cell (FDC). Full thickness porcine skin was cut into 1 cm2 pieces and secured in a FDC (Permegear Inc., Riegelsville, PA) with a 0.2 cm2 contact area and the SC side facing the donor compartment. The conductivity of each skin sample was initially measured in the presence of PBS to determine the integrity of the skin. Skin’s electrical conductivity was measured using procedures described by Karande et al. 22
Ultrafiltration
Ultrafiltration experiments were carried out following the experimental procedure used by James-Smith et al. 18 Ten thousand molecular weight cutoff (MWCO) Centricon YM-10 ultracentrifugation filter tubes were purchased from Fisher Scientific. Two milliliters of SDS:C12TAB mixed micellar solutions of various SDS:C12TAB ratios (from 100:0 to 90:10) with various SDS concentrations were placed into the top portion of the ultracentrifugation tubes and subsequently centrifuged at ~2,900g for approximately 10 min so that less than 10% (i.e., <200 μL) volume was collected as filtrate. Centrifugation was performed under these conditions so as to minimize the effect on micellar structure. All samples were centrifuged in an Allegra X-12R Centrifuge (Beckman Coulter, Fullerton, CA). The filtrate was collected in the bottom attachment and diluted for analysis by a slightly modified dye complexation method.40
Dye Complexation Method
Methylene blue reagent was purchased from Fisher Scientific. Two milliliters of methylene blue reagent was added to 2 mL of the diluted filtrate from the ultrafiltration experiments. Two milliliters of chloroform was added and the solution was shaken on a Vortex mixer for approximately 30 s. Any SDS that was present in the filtrate complexed with the positively charged methylene blue through electrostatic interactions, thus forming an oil-soluble complex that partitioned into the chloroform organic phase. The solution was allowed to phase separate and the organic phase was removed and placed into a separate test tube. This extraction process was repeated two more times (i.e., 2 mL of chloroform was added, shaken, and removed two more times for a total chloroform volume of 6 mL) and the organic phase was then analyzed by UV–Visible spectrometry at 652 nm. SDS concentration was determined using a calibration curve. Known concentrations of SDS from 5 to 30 μM were mixed with methylene blue reagent and extracted with chloroform to obtain the calibration curve. UV–Vis analysis was done in a Shimadzu UV-1601 UV–vis spectrometer (Shimadzu Scientific Instruments, Columbia, MD). It has been shown C12TAB molecules in C12TAB–SDS mixture in the concentration range studied here are incorporated in the micelles19 and should not interfere with binding of free SDS that passes into the filtrate with methylene blue.
Heat Stripping of Skin
Intact sheets of SC were obtained by heat stripping porcine skin to separate the epidermis from the dermis.25 The skin was first removed from the −80 °C freezer and allowed to thaw to room temperature. The skin was then placed in a 60 °C PBS solution for 2 min. The skin was taken out of the PBS and the epidermis was removed by scraping it away from the dermis with flat-edged tweezers. The isolated epidermis was then floated over 0.25% (w/v) trypsin (Sigma-Aldrich, St Louis, MO) solution overnight at room temperature (25 °C) to digest the epidermal matrix.26 The SC sample was removed from the trypsin after 24 h and washed with PBS until all traces of epidermal debris was removed. It was allowed to dry for 24 h and then cut into 1 sq. cm pieces.
FT-IR
Fourier Transform-Infrared Spectroscopy (FT-IR) of the dry SC samples was carried out on a Nicolet Magna 850 spectrometer (Thermo Electron Corp., Waltham, MA) setup at a resolution of 2 cm−1. FT-IR has often been used to determine the effect of penetration enhancers on the structural properties of the SC.1,23,27 The spectra were averaged over 100 scans and subsequently smoothed, baseline corrected, and saved in the comma separated value (csv) format for further analysis by ORIGIN software (OriginLabs, Northhampton, MA). The average initial peak area at 2850 for all samples was approximately 20.5 ± 4.0 (AU). After the initial spectra were taken, the SC samples were placed into vials containing 1.5 mL of surfactant solutions of various compositions and concentrations. The samples were allowed to soak for 24 h and then washed by soaking and gentle swirling in 10 mL of fresh PBS three times. After the third wash, the samples were dried and then final FT-IR spectra were taken and analyzed. Each formulation was studied in quadruplicate to determine its effect on the SC.
The structure of the lipids within the SC has been correlated with skin’s barrier properties.12 There are two main ways in which surfactants tend to interact with the lipids of the SC. They can partition into the lipid bilayer, thereby disrupting the packing and leading to the fluidization of the bilayer. They could also lead to the removal (extraction) of lipids from the SC. Therefore, the effect of the formulations on the lipid bilayers in the SC was determined by analyzing changes in the lipid content in the SC. The integrated peak area of the methylene symmetric stretching mode, CH2sym, at 2850 cm−1 was used to quantify the effect of formulations on SC. If the peak area at 2850 cm−1 increased after exposure to the surfactant formulations, then it was concluded that surfactant molecules had partitioned into the SC. In the case where the peak area decreased, it was determined that the lipid molecules had been extracted from the SC.23
SDS Partitioning into Skin
14C-radiolabelled SDS was used to quantify the amount of SDS that partitions into porcine skin following a slightly modified version of the procedure utilized by Moore et al.31 Full thickness porcine skin was cut into 1 cm2 pieces, weighed, and secured in a FDC with the SC side facing the donor compartment. The skin was allowed to hydrate with PBS for 1 h and then the PBS was removed from the donor well and replaced with 200 μL of surfactant solution. The surfactant solutions were composed of mixtures of SDS and C12TAB at different ratios and each solution contained approximately 0.5 μCi/mL of 14C-SDS. The surfactant solutions were allowed to remain in contact with the skin for 2.5 h at room temperature. This exposure time was chosen because the FDCs that were used were smaller than the ones that were utilized by Moore et al.31 The skin was then blotted with a paper towel to remove excess liquid, allowed to dry in a fume hood for about 4 days and subsequently weighed. The skin was solubilized by submerging it in 5 mL of Solvable solution (PerkinElmer Life and Analytical Sciences, Inc, Boston, MA) and heating overnight in an oven at 60 °C. After the skin was completely dissolved, a 2 mL aliquot of the solubilized solution was diluted with 10 mL of Ultima Gold scintillation cocktail (PerkinElmer Life and Analytical Sciences, Inc, Boston, MA). The concentration of radiolabelled SDS in the skin was measured in a scintillation counter (Packard Tri-Carb 2100 TR). The concentration of SDS in the skin ([SDS]skin) was subsequently calculated using the measured radioactivity in skin and total SDS concentration in the donor compartment in the following equation:
where [14C-SDS]skin is the measured radioactivity in skin, [SDS] is the total SDS concentration in the donor compartment, [14C-SDS]donor is the concentration of radiolabelled SDS in the donor compartment, and m is the dry weight of the skin.
Results
Application of SDS:C12TAB mixtures to skin led to a concentration- and composition-dependent enhancement of transepidermal current (Fig. 1a). The enhancement increased with increasing surfactant concentration regardless of the specific composition of SLS:C12TAB. This is expected since increasing concentration avails more surfactant for skin penetration. The dependence on composition, however, was peculiar. Specifically, addition of small amounts of C12TAB led to a significant decrease in transepidermal current (Fig. 1a). Note that SDS constituted the majority of surfactant at all compositions and hence was expected to be the primary determinant of barrier perturbation. The enhancement in transepidermal current, however, did not correlate with the portion of the total surfactant concentration represented by SDS (i.e., the SDS concentration in the donor) (Fig. 1b). Within each specific composition of SLS:C12TAB, perturbation in barrier properties correlated well with the total concentration, as is reported in the previous literature.48 However, the correlation did not hold true over the entire composition range. This suggests that donor SDS concentration, on its own, cannot explain perturbation in barrier properties.
(a) Enhancement in transepidermal current for mixed micellar solutions of SDS:C12TAB at different molar ratios and total surfactant concentrations: 35 mM (black bars), 50 mM (upward striped bars), 70 mM (empty bars) or 100 mM (downward striped bars). Each bar represents the average of 3–5 experiments. (b) Enhancement in transepidermal current as a function of SDS concentration in the donor compartment. Each point represents the average of 3–5 skin samples from 3 to 5 different sections of skin
FT-IR analysis of SC samples confirmed that the dependence of transepidermal current on SDS:C12TAB mirrored that of SC lipids (Fig. 2a). In particular, higher concentrations of surfactants led to higher increase in the area at 2850 cm−1. In addition, in most cases, the change in area decreased with the addition of C12TAB to SDS. For the 35 mM micelles, the change in area actually increased when the C12TAB molar ratio was increased from 5 to 10% (i.e., from 95:5 to 90:10). This could possibly be explained by the fact that these values fall within the noise range of the instrument (i.e., ±10% for 100 scans). This could also explain the negative change in peak area for 35 mM at 95:5 SDS:C12TAB. The point to note is that addition of C12TAB leads to significant decrease in change in peak area. Also, for 70 mM, there appears to be a slight increase in change in peak area when the C12TAB molar ratio was increased from 2 to 5% (i.e., 98:2 to 95:5). This change is not statistically significant; as such, emphasis must be placed on the overall change in peak area as the C12TAB molar ratio is increased from 0 to 10%, which is a statistically significant change (p = 0.002). Note that the FT-IR analysis was not performed for solutions at 100 mM because the SC was very fragile at this concentration of SDS. Change in area for all compositions and concentrations correlated with the enhancement in transepidermal current (Fig. 2b, r 2 = 0.88).
(a) Change in absorbance peak area at 2850 cm−1 for stratum corneum samples as a function of total surfactant concentration and change in SDS:C12TAB ratio in mixed micellar systems as determined by FT-IR. Each data bar represents the mean of four samples. Total surfactant concentrations investigated: 35 mM (black bars), 50 mM (upward striped bars), and 70 mM (empty bars). (b) Change in absorbance peak area at 2850 cm−1 for stratum corneum samples plotted against change in transepidermal current (r 2 = 0.88)
Since the SDS concentration outside the skin, that is, the total SDS concentration in the donor compartment, did not correlate with transepidermal current enhancement, we sought to determine whether the concentration of SDS inside the skin correlates with barrier perturbation. SDS concentration in the skin was determined using 14C-radiolabelled SDS. SDS penetration in the skin varied with the composition and concentration of SDS in the donor compartment (Fig. 3a). Further, this dependence was qualitatively similar to that of transepidermal current on the same parameters. Regardless of the concentration and composition, transepidermal current correlated well with SDS concentration inside the skin (Fig. 3b).
(a) Effect of total surfactant concentration and C12TAB co-surfactant on SDS penetration into porcine skin. 14C-radiolabeled SDS was used to determine concentration of SDS in skin. Skin was exposed to surfactant for 2.5 h. Solvent = PBS. Surfactant concentration fixed at 35 mM (black bars), 50 mM (upward striped bars), 70 mM (empty bars) or 100 mM (downward striped bars) in each sample. Each data bar represents the mean of four samples. (b) Relationship between SDS concentration in skin and change in transepidermal current (r 2 = 0.85)
Data in Figs. 1, 2, and 3 suggest that surfactant-induced skin effects correlate with the SDS concentration inside the skin but not in the donor compartment. A question then arises why does not the concentration of SDS inside the skin correlate with that in the donor compartment. One possibility is that the total SDS concentration in the donor compartment is not an accurate measure of SDS “available” for skin penetration. For example, if only SDS monomers and sub-micellar aggregates, but not micelles, are able to penetrate the skin, then the available SDS for penetration will vary greatly as a function of concentration and composition. In this regard, C12TAB may have a particularly significant role to play. Addition of C12TAB to SDS is known to increase the stability of micelles and sub-micellar aggregates, thereby reducing the concentration of monomeric SDS.18,35 To assess this possibility, concentration of monomers and sub-micellar aggregates in SDS:C12TAB mixtures was determined using ultrafiltration.18 A 10,000 MWCO filter was used since a typical SDS micelle has an approximate molecular weight of 18,720. Concentration of monomeric and sub-micellar SDS exhibited a strong dependence on concentration and composition (Fig. 4). It increased somewhat with increasing concentration for all compositions, although the increase was most prominent at 100 mM concentration. Regardless of the total surfactant concentration, the concentration of monomeric and sub-micellar SDS decreased with increasing fraction of C12TAB. This observation is in agreement with previous reports indicating that addition of C12TAB leads to stabilization of SDS micelles.18,35,36
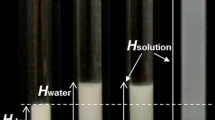
Filtrate concentration of SDS (i.e., monomer + sub-micellar aggregate concentration) for various SDS:C12TAB mixed micellar solutions at ratios varying from 100:0 to 90:10. The surfactant concentration in each sample was fixed at 35 mM (black bars), 50 mM (upward striped bars), 70 mM (empty bars) or 100 mM (downward striped bars). Each data bar represents the mean of three samples. Samples were filtered through 10,000 MWCO filters and centrifuged at 2,900g for 10 min. Less than 10% of the total volume was allowed to pass into the filtrate. Inset: Schematic of filtration process showing monomers and sub-micellar fragments in the filtrate
SDS concentration in the filtrate (i.e., monomer/sub-micellar SDS concentration) correlated well with SDS concentration inside the skin across all compositions tested in this study (Fig. 5a, r 2 = 0.8). For comparison, a plot of SDS concentration inside skin against donor SDS concentration is also shown (Fig. 5b, r 2 = 0.5). Comparison of Figs. 5a and 5b reveal that concentration of monomeric and sub-micellar SDS is a better determinant of SDS concentration inside the skin and hence, skin barrier perturbation, compared to SDS concentration in the donor.
Discussion
Transient disruption of SC allows for increase in skin permeability, thereby facilitating transdermal and topical drug delivery. While surfactants have been extensively studied as penetration enhancers, the effects of mixed surfactant systems on skin permeability have yet to be investigated in depth. Here, we have analyzed how mixing two commonly studied surfactants, SDS and C12TAB at various ratios affects the extent of enhancement. SDS and C12TAB were used as model surfactants since the thermodynamics of this system are relatively well studied.
Addition of C12TAB to SDS is known to induce significant changes in the micellar structure and stability. Patist et al. 35 have previously shown that SDS micellar stability may be tailored by the addition of oppositely charged surfactants such as alkyltrimethylammonium bromides (C n TABs). The long-chain TABs enhance SDS micellar stability, as measured by relaxation time, by up to 2000 times.36 Increased micellar stability, in turn, has been shown to reduce the monomer concentration.18 In the concentration range and the molar ratios studied here, there was little-to-no change in micelle size with the addition of C12TAB. The highest molar ratio of C12TAB was capped at 10% to limit the effects of viscosity and micelle size. The micelle sizes, as determined by dynamic light scattering, were in the range of 7–12 nm for all micelles studied.
In this study, the distribution of surfactants among micelles and monomers/sub-micellar population was determined using ultrafiltration.28 In this technique, a small portion (<10%) of the surfactant solution is passed through ultracentrifuge tubes containing a nanoporous membrane with a nominal MWCO that is smaller than that of the micelles.28 This methodology has been previously used to determine the presence of sub-micellar aggregates in surfactant solution (aggregates of surfactant containing less monomers than the aggregation number of the micelle; e.g., dimers, trimers, and other multimers).18 Of particular interest is the fact that this technique has been utilized to demonstrate that addition of C12TAB to SDS leads to stabilization of micelles and sub-micellar aggregates and such stabilization decreases and even virtually eliminates sub-micellar aggregates.18
Data in Fig. 4 show that addition of C12TAB indeed led to reduction of sub-micellar population of SDS. Further, the concentration of sub-micellar SDS correlated well with skin SDS concentration, transepidermal current and change in peak area at 2850 cm−1. None of these measured skin parameters correlated with SDS concentration in the donor. These observations lead to a key question: is surfactant-mediated barrier disruption induced by the penetration of micelles or monomer surfactants? In spite of its high relevance to drug delivery and personal care, this question has been relatively unexplored in the literature. Among a limited number of studies that directly address this questions, Moore et al. 31 have reported that transepidermal current in presence of SDS increases even for concentrations above the critical micelle concentration, based on which, they concluded that SDS micelles are able to penetrate into the skin. Addition of a nonionic surfactant, dodecyl hexa(ethylene oxide), to SDS led to decreased partitioning of SDS into skin,32 which was attributed to the larger hydrodynamic radius of the micelles which would prohibit the penetration of the micelles into the skin. Rhein et al. 41,42 have also investigated the addition of a milder co-surfactants (C12–C14 alkyl 6 and 7-ethoxy sulfate) to SDS to decrease irritation associated with the pure SDS system. They hypothesized that the decrease in irritation was due to the lowering of the cmc, which consequently results in a lowering of the monomer concentration of SDS.42 In other studies, a number of publications have reported on the possibility of penetration of intact lipid vesicles into the skin.4–6,9 It has also been shown that the addition of hydrophobically modified polymers to surfactant-based cleansers can serve to reduce their irritation potential.10
Our findings suggest that penetration of sub-micellar aggregates into skin represents an alternate hypothesis to the penetration of intact micelles into skin in terms of explaining the effect of surfactants on skin’s barrier properties. This finding would suggest that when forming systems for skin penetration, one must take into account micelle stability, which has been shown to relate directly to the concentration of sub-micellar aggregates (i.e., the more stable the micelle, the lower the sub-micellar aggregate concentration).35 We show here that the higher the sub-micellar concentration, the greater the penetration enhancement.
The strong correlation between sub-micellar surfactant population and barrier perturbation reported here suggests that such population is responsible for the effect, although this cannot be conclusively proved. Addition of C12TAB to SDS produces significant changes to the properties of surfactant mixtures, in addition to reducing sub-micellar population. Notably, addition of C12TAB increases micellar stability. Specifically, the lifetime of SDS micelles (~8 ms) is dramatically increased to about 2000 ms depending on the amount of added C12TAB.36 Addition of C12TAB also leads to tighter packing of surfactants compared to the loosely packed structure of SDS micelles owing to electrostatic interactions between oppositely charged head groups. The tight packing of surfactants could, in principle, reduce the penetration of micelles into the skin. Rigidity of lipid carriers has already been suggested to play a major role in skin penetration. In particular, flexible, elastic vesicles have been suggested to exhibit better penetration into skin compared to their rigid analogs.14,15
Conclusions
The results reported here indicate that the concentration of monomers and sub-micellar aggregates correlates with surfactant-mediated barrier perturbation, whereas there is no correlation to the total surfactant concentration. This strongly suggests that these species (monomers/sub-micellar aggregates) are responsible for perturbation of the skin barrier, although further experiments are necessary to reach a firm conclusion. Future studies should also focus on extending these findings to additional surfactants to assess the generality of these findings.
References
Ananthapadmanabhan, K. P., A. Lips, C. Vincent, F. Meyer, S. Caso, A. Johnson, K. Subramanyan, M. Vethamuthu, G. Rattinger, and D. J. Moore. pH-induced alterations in stratum corneum properties. Int. J. Cosmet. Sci. 25:103–112, 2003.
Barry, B. W. Novel mechanisms and devices to enable successful transdermal drug delivery. Eur. J. Pharm. Sci. 14(2):101–114, 2001.
Bommannan, D., H. Okuyama, P. Stauffer, and R. H. Guy. Sonophoresis. I. The use of high-frequency ultrasound to enhance transdermal drug delivery. J. Pharm. Res. 9(4):559–564, 1992.
Cevc, G., and G. Blume. New, highly efficient formulation of diclofenac for the topical, transdermal administration in ultradeformable drug carriers, Transfersomes. Biochim. Biophys. Acta 1514(2):191–205, 2001.
Cevc, G., and G. Blume. Biological activity and characteristics of triamcinolone-acetonide formulated with the self-regulating drug carriers, Transfersomes®. Biochim. Biophys. Acta 1614(2):156–164, 2003.
Cevc, G., and G. Blume. Hydrocortisone and dexamethasone in very deformable drug carriers have increased biological potency, prolonged effect, and reduced therapeutic dosage. Biochim. Biophys. Acta 1663(1-2):61–73, 2004.
Chien, Y. W., and A. K. Banga. Iontophoretic (transdermal) delivery of drugs: overview of historical development. J. Pharm. Sci. 78(5):353–354, 2006.
Denet, A.-R., R. Vanbever, and V. Préat. Skin electroporation for transdermal and topical delivery. Adv. Drug Deliv. Rev. 56(5):659–674, 2004.
Elsayed, M. M. A., O. Y. Abdallah, V. F. Naggar, and N. M. Khalafallah. Lipid vesicles for skin delivery of drugs: reviewing three decades of research. Int. J. Pharm. 332(1–2):1–16, 2007.
Fevola, M. J., J. J. LiBrizzi, and R. M. Walters. Reducing irritation potential of surfactant-based cleansers with hydrophobically-modified polymers. Polym. Prepr. 49(2):671–672, 2008.
Ghosh, S., and D. Blankschtein. The role of sodium dodecyl sulfate (SDS) micelles in inducing skin barrier perturbation in the presence of glycerol. J. Cosmet. Sci. 58:109–133, 2007.
Golden, G. M., J. E. McKie, and R. O. Potts. Role of stratum corneum lipid fluidity in transdermal drug flux. J. Pharm. Sci. 76(1):25–28, 2006.
Henry, S., D. V. McAllister, M. G. Allen, and M. R. Prausnitz. Microfabricated microneedles: a novel approach to transdermal drug delivery. J. Pharm. Sci. 87(8):922–925, 2000.
Honeywell-Nguyen, P. L., and J. A. Bouwstra. The in vitro transport of pergolide from surfactant-based elastic vesicles through human skin: a suggested mechanism of action. J. Control. Release 86(1):145–156, 2003.
Honeywell-Nguyen, P. L., P. M. Frederik, P. H. H. Bomans, H. E. Junginger, and J. A. Bouwstra. Transdermal delivery of pergolide from surfactant-based elastic and rigid vesicles: characterization and in vitro transport studies. Pharm. Res. 19(7):991–997, 2002.
Hsu, T., E. Jacobson, A. Hickey, E. Luo, and N. Gricenko. Transdermal delivery of hydrophilic drugs: the current status and potential for future drug delivery. Drug Deliv. Technol. 4(2):58–60, 2004.
Inoue, N., D. Kobayashi, M. Kimura, M. Toyama, I. Sugawara, S. Itoyama, M. Ogihara, K. Sugibayashi, and Y. Morimoto. Fundamental investigation of a novel drug delivery system, a transdermal delivery system with jet injection. Int. J. Pharm. 137(1):75–84, 1996.
James-Smith, M. A., D. Shekhawat, and D. O. Shah. Importance of micellar lifetime and sub-micellar aggregates in detergency processes. Tenside Surf. Deterg. 44(3):142–154, 2007.
Kamenka, N., M. Chorro, Y. Talmon, and R. Zana. Study of mixed aggregates in aqueous solutions of sodium dodecyl sulfate and dodecyltrimethylammonium bromide. Colloids Surf. 67:213–222, 1992.
Kanikkannan, N. Iontophoresis-based transdermal delivery systems. BioDrugs 16(5):339–347, 2002.
Kanikkannan, N., and M. Singh. Skin permeation enhancement effect and skin irritation of saturated fatty alcohols. Int. J. Pharm. 248(1–2):219–228, 2002.
Karande, P., A. Jain, and S. Mitragotri. Relationships between skin’s electrical impedance and permeability in the presence of chemical enhancers. J. Control. Release 110:307, 2006.
Karande, P., A. Jain, A. Arora, M. J. Ho, and S. Mitragotri. Synergistic effects of chemical enhancers on skin permeability: a case study of sodium lauroylsarcosinate and sorbitan monolaurate. Eur. J. Pharm. Sci. 31(1):1–7, 2007.
Kermode, M. Unsafe injections in low-income country health settings: need for injection safety promotion to prevent the spread of blood-borne viruses. Health Promot. Int. 19(1):95–103, 2004.
Kligman, A., and E. Christophers. Preparation of isolated sheets of human stratum corneum. Arch. Dermatol. 88(6):702–705, 1963.
Larsen, C. G., F. G. Larsen, and K. Thestruppedersen. Preparation of human epidermal tissue for functional immune studies. Acta Derm. Venereol. 68(6):474–479, 1988.
Mendelsohn, R., and D. J. Moore. Vibrational spectroscopic studies of lipid domains in biomembranes and model systems. Chem. Phys. Lipids 96(1–2):141–157, 1998.
Midura, R. J., and M. Yanagishita. Chaotropic solvents increase the critical micellar concentrations of detergents. Anal. Biochem. 228(2):318–322, 1995.
Mitragotri, S., D. Blankschtein, and R. Langer. Ultrasound-mediated transdermal protein delivery. Science 269(5225):850–853, 1995.
Mitragotri, S., D. Blankschtein, and R. Langer. Transdermal drug delivery using low-frequency sonophoresis. J. Pharm. Res. 13(3):411–420, 1996.
Moore, P. M., S. Puvvada, and D. Blankschtein. Challenging the surfactant monomer skin penetration model: penetration of sodium dodecyl sulfate micelles into the epidermis. J. Cosmet. Sci. 54:29–46, 2003.
Moore, P. N., A. Shiloach, S. Puvvada, and D. Blankschtein. Penetration of mixed micelles into the epidermis: effect of mixing sodium dodecyl sulfate with dodecyl hexa(ethylene oxide). J. Cosmet. Sci 54:143–159, 2003.
Naik, A., Y. N. Kalia, and R. H. Guy. Transdermal drug delivery: overcoming the skin’s barrier function. Pharm. Sci. Technol. Today 3(9):318–326, 2000.
Paruchuri, V. K., J. Nalaskowski, D. O. Shah, and J. D. Miller. The effects of cosurfactants on sodium dodecyl sulfate micellar structures at a graphite surface. Colloids Surf. A 272:157–163, 2006.
Patist, A., V. Chhabra, R. Pagidipati, R. Shah, and D. O. Shah. Effect of chain length compatibility on micellar stability in sodium dodecyl sulfate/alkyltrimethylammonium bromide solutions. Langmuir 13(3):432–434, 1997.
Patist, A., J. R. Kanicky, P. K. Shukla, and D. O. Shah. Importance of micellar kinetics in relation to technological processes. J. Colloid Interface Sci. 245(1):1–15, 2002.
Prausnitz, M. R. Microneedles for transdermal drug delivery. Adv. Drug Deliv. Rev. 56(5):581–587, 2004.
Prausnitz, M. R., and R. Langer. Transdermal drug delivery. Nat. Biotechnol. 26(11):1261–1268, 2008.
Prausnitz, M. R., S. Mitragotri, and R. Langer. Current status and future potential of transdermal drug delivery. Nat. Rev. Drug Discov. 3:115–124, 2004.
Ray, A., J. A. Reynolds, H. Polet, and J. Steinhar. Binding of large organic anions and neutral molecules by native bovine serum albumin. Biochemistry 5(8):2606, 1966.
Rhein, L. D., C. R. Robbins, K. Fernee, and R. Cantore. Surfactants structure effects on swelling of isolated human stratum corneum. J. Soc. Cos. Chem. 37:127–139, 1986.
Rhein, L. D., F. A. Simion, R. L. Hill, R. H. Cagan, J. Mattai, and H. I. Maibach. Human cutaneous response to a mixed surfactant system: role of solution phenomena in controlling surfactant irritation. Dermatologica 180:18–23, 1990.
Rosen, M. R. Delivery System Handbook for Personal Care and Cosmetic Products: Technology Applications and Formulations. Norwich, NY: William Andrew, Inc, 2005.
Schramm, J., and S. Mitragotri. Transdermal drug delivery by jet injectors: energetics of jet formation and penetration. J. Pharm. Res. 19(11):1673–1679, 2002.
Suhonen, T. M., J. A. Bouwstra, and A. Urtti. Chemical enhancement of percutaneous absorption in relation to stratum corneum structural alterations. J. Control. Release 59(2):149–161, 1999.
Walters, R. M., M. J. Fevola, J. J. LiBrizzi, and K. Martin. Designing cleansers for the unique needs of baby skin. Cosmet. Toilet. 123(12):53–60, 2008.
Williams, A. C., and B. W. Barry. Penetration enhancers. Adv. Drug Deliv. Rev. 56(5):603–618, 2004.
Zatz, J. L., and B. Lee. Skin penetration enhancement by surfactants. In: Surfactants in Cosmetics2nd, Vol. 68, edited by M. M. Rieger, and L. D. Rhein. New York, NY: Marcel Dekker, Inc, 1997, pp. 501–517.
Acknowledgment
The authors would like to acknowledge the financial support of the National Institute of Health Supplemental Post-doctoral Fellowship.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Daniel Takashi Kamei oversaw the review of this article.
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
James-Smith, M.A., Hellner, B., Annunziato, N. et al. Effect of Surfactant Mixtures on Skin Structure and Barrier Properties. Ann Biomed Eng 39, 1215–1223 (2011). https://doi.org/10.1007/s10439-010-0190-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-010-0190-4