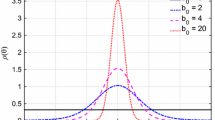
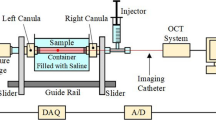
Abstract
We study whether an inverse modeling approach is applicable for characterizing vascular tissue subjected to various levels of internal pressure and axial stretch that approximate in-vivo conditions. To compensate for the limitation of axial-displacement/pressure/diameter data typical of clinical data, which does not provide information about axial force, we propose to constrain the ratio of axial to circumferential elastic moduli to a typical range. Vessel wall constitutive behavior is modeled with a transversely isotropic hyperelastic equation that accounts for dispersed collagen fibers. A single-layer and a bi-layer approximation to vessel ultrastructure are examined, as is the possibility of obtaining the fiber orientation as part of the optimization. Characterization is validated against independent pipette-aspiration biaxial data on the same samples. It was found that the single-layer model based on homogeneous wall assumption could not reproduce the validation data. In contrast, the constrained bi-layer model was in excellent agreement with both types of experimental data. Due to covariance, estimations of fiber angle were slightly outside of the normal range, which can be resolved by predefining the angles to normal values. Our approach is relatively invariant to a constant or a variable axial response. We believe that it is suitable for in-vivo characterization.







Similar content being viewed by others
References
Bathe K. J. (2005) ADINA System 8.3 Release Notes. ADINA R & D Inc, Watertown
Beller C. J., Labrosse M. R., Thubrikar M. J., Robicsek F. (2007) Finite element modeling of the thoracic aorta: including aortic root motion to evaluate the risk of aortic dissection. J. Med. Eng. Technol. 16:1–4
Beller C. J., Labrosse M. R., Thubrikar M. J., Robicsek F. (2004) Role of aortic root motion in the pathogenesis of aortic dissection. Circulation 109:763-769
Beller C. J., Labrosse M. R., Thubrikar M. J., Szabo G., Robicsek F., Hagl S. (2005) Increased aortic wall stress in aortic insufficiency: clinical data and computer model. Eur. J. Cardiothorac. Surg. 27:270–275
Beller C. J., Labrosse M. R., Thubrikar M. J., Szabo G., Robicsek F., Hagl S. (2005) Are there surgical implications to aortic root motion? J. Heart Valve Dis. 14:610–615
Billiar K. L., Sacks M. S. (1997) A method to quantify the fiber kinematics of planar tissues under biaxial stretch. J. Biomech. 30:753–756
Bogren H. G., Buonocore M. H. (1999) 4D magnetic resonance velocity mapping of blood flow patterns in the aorta in young vs. elderly normal subjects. J. Magn. Reson. Imaging 10:861–869
Cacho F., Doblaré M., Holzapfel G. A. (2007) A procedure to simulate coronary artery bypass graft surgery. Med. Biol. Eng. Comput. 45:819–827
Chen H. Y., Einstein D. R., Chen K., Vesely I. (2005) Computational modeling of vascular clamping: a step toward simulating surgery. Conf. Proc. IEEE Eng. Med. Biol. Soc. 1:302–303
Cook, R. D., Malkus, D. S., Plesha, M. E., and Witt, R. J. Basic elements. In: Concepts and Applications of Finite Element Analysis, 4th edn. New York: John Wiley & Sons, Inc., pp. 78–135.
Davis N. P., Han H. C., Wayman B., Vito R. (2005) Sustained axial loading lengthens arteries in organ culture. Ann. Biomed. Eng. 33:867–877
Ding Z., Friedman M. H. (2000) Dynamics of human coronary arterial motion and its potential role in coronary atherogenesis. J. Biomech. Eng. 122:488–492
Dobrin P. B. (1986) Biaxial anisotropy of dog carotid artery: estimation of circumferential elastic modulus. J. Biomech. 19:351–358
Draney M. T., Zarins C. K., Taylor C. A. (2005) Three-dimensional analysis of renal artery bending motion during respiration. J. Endovasc. Ther. 12:380–386
Einstein D. R., Freed A. D., Stander D., Stander N., Fata B., Vesely I. (2005) Inverse parameter fitting of biological tissues: a response surface approach. Ann. Biomed. Eng. 33:1819–1830
Freed A. D., Einstein D. R., Vesely I. (2005) Invariant formulation for dispersed transverse isotropy in aortic heart valves: an efficient means for modeling fiber splay. Biomech. Model. Mechanobiol. 4:100–117
Gasser T. C., Schulze-Bauer C. A., Holzapfel G. A. (2002) A three-dimensional finite element model for arterial clamping. J. Biomech. Eng. 124:355–363
Hassan T., Timofeev E. V., Saito T., Shimizu H., Ezura M., Tominaga T., Takahashi A., Takayama K. (2004) Computational replicas: anatomic reconstructions of cerebral vessels as volume numerical grids at three-dimensional angiography. AJNR Am. J. Neuroradiol. 25:1356–1365
Hayashi K. (2004) Mechanical properties of soft tissues and arterial walls. In: Holzapfel G. A., Ogden R. W. (eds) Biomechanics of Soft Tissue in Cardiovascular Systems. Springer, Wien, New York, New York, pp. 15–64
Holzapfel G. A. (2006) Determination of material models for arterial walls from uniaxial extension tests and histological structure. J. Theor. Biol. 238:290–302
Holzapfel G. A., Stadler M., Schulze-Bauer C. A. (2002) A layer-specific three-dimensional model for the simulation of balloon angioplasty using magnetic resonance imaging and mechanical testing. Ann. Biomed. Eng. 30:753–767
Honda T., Yano K., Matsuoka H., Hamada M., Hiwada K. (1994) Evaluation of aortic distensibility in patients with essential hypertension by using cine magnetic resonance imaging. Angiology 45:207–212
Humphrey J. D. (1995) Mechanics of the arterial wall: review and directions. Crit. Rev. Biomed. Eng. 23:1–162
Humphrey J. D., Kang T., Sakarda P., Anjanappa M. (1993) Computer-aided vascular experimentation: a new electromechanical test system. Ann. Biomed. Eng. 21:33–43
Kaw, A. K. Macromechanical analysis of lamina. In: Mechanics of Composite Materials. Boca Raton: CRC Press, pp. 53–148, 1997
Kozerke S., Scheidegger M. B., Pedersen E. M., Boesiger P. (1999) Heart motion adapted cine phase-contrast flow measurements through the aortic valve. Magn. Reson. Med. 42:970–978
L’Italien G. J., Chandrasekar N. R., Lamuraglia G. M., Pevec W. C., Dhara S., Warnock D. F., Abbott W. M. (1994) Biaxial elastic properties of rat arteries in vivo: influence of vascular wall cells on anisotropy. Am. J. Physiol. 267:574–579
Lanir Y., Fung Y. C. (1974) Two-dimensional mechanical properties of rabbit skin. I. Experimental system. J. Biomech. 7:29–34
Lanir Y., Fung Y.C. (1974) Two-dimensional mechanical properties of rabbit skin. II. Experimental results. J. Biomech. 7:171–182
Lu X., Pandit A., Kassab G. S. (2004) Biaxial incremental homeostatic elastic moduli of coronary artery: two-layer model. Am. J. Physiol. Heart Circ. Physiol. 287:1663–1669
Mercer J. L. (1969) Movement of the aortic annulus. Br. J. Radiol. 42:623–626
Migliavacca F., Pennati G., Di Martino E., Dubini G., Pietrabissa R. (2002) Pressure drops in a distensible model of end-to-side anastomosis in systemic-to-pulmonary shunts. Comput. Methods Biomech. Biomed. Engin. 5:243–248
Monos E., Raffai G., Contney S. J., Stekiel W. J., Cowley A. W. Jr. (2001) Axial stretching of extremity artery induces reversible hyperpolarization of smooth muscle cell membrane in vivo. Acta. Physiol. Hung. 88:197–206
Myers R. H., Montgomery D. C. (2002) Response Surface Methodology: Progress and Product Optimization Using Designed Experiments. Wiley, New York
Ohashi T., Abe H., Matsumoto T., Sato M. (2005) Pipette aspiration technique for the measurement of nonlinear and anisotropic mechanical properties of blood vessel walls under biaxial stretch. J. Biomech. 38:2248–2256
Roux W. J., Stander N., Haftka R. T. (1998) Response surface approximations for structural optimization. Int. J. Numer. Methods Eng. 42:517–534
Sacks M. S. (2000) Biaxial mechanical evaluation of planar biological materials. J. Elast. 61:199–246
Sacks M. S. (2003) Incorporation of experimentally-derived fiber orientation into a structural constitutive model for planar collagenous tissues. J. Biomech. Eng. 125:280–287
Sacks M. S., Sun W. (2003) Multiaxial mechanical behavior of biological materials. Annu. Rev. Biomed. Eng. 5:251–284
Schulze-Bauer C. A., Holzapfel G. A. (2003) Determination of constitutive equations for human arteries from clinical data. J. Biomech. 36:165–169
Silver, F. H. Mechanical properties of connective tissues. In: Biological Materials: Structure, Mechanical Properties, and Modeling of Soft Tissues. New York: New York University Press, 1987, pp. 164–195
Sipkema P., van der Linden P. J., Yin F. C. (2003) Effect of cyclic axial stretch of rat arteries on endothelial cytoskeletal morphology and vascular reactivity. J. Biomech. 36:653–659
Stander N., Craig K. J. (2002) On the robustness of a simple domain reduction scheme for simulation-based optimization. Eng. Comput. 19:431–450
Stander N., Craig K. J., Müllerschön H., Reichert R. (2005) Material identification in structural optimization using response surfaces. Struct. Multidisc. Optim. 29:93–102
Stander N., Roux W., Eggleston T., Craig K. (2004) LS-OPT User’s Manual: A Design Optimization and Probabilistic Analysis Tool for the Engineering Analyst. LSTC, Livermore
Steele B. N., Wan J., Ku J. P., Hughes T. J., Taylor C. A. (2003) In vivo validation of a one-dimensional finite-element method for predicting blood flow in cardiovascular bypass grafts. IEEE Trans. Biomed. Eng. 50:649–656
Stefanadis C., Stratos C., Vlachopoulos C., Marakas S., Boudoulas H., Kallikazaros I., Tsiamis E., Toutouzas K., Sioros L., Toutouzas P. (1995) Pressure–diameter relation of the human aorta. A new method of determination by the application of a special ultrasonic dimension catheter. Circulation 92:2210–2219
Sussman T., Bathe K. J. (1987) A finite element formulation for nonlinear incompressible elastic and inelastic analysis. Comput. Struct. 26:357–409
Takamizawa K., Hayashi K. (1987) Strain energy density function and uniform strain hypothesis for arterial mechanics. J. Biomech. 20:7–17
Tang D., Yang C., Kobayashi S., Ku D. N. (2001) Steady flow and wall compression in stenotic arteries: a three-dimensional thick-wall models with fluid-wall interactions. J. Biomech. Eng. 123:548–557
Tozzi P., Hayoz D., Oedman C., Mallabiabarrena I., Von Segesser L. K. (2001) Systolic axial artery length reduction: an overlooked phenomenon in vivo. Am. J. Physiol. Heart Circ. Physiol. 280:2300–2305
Van Loon P. (1977) Length-force and volume-pressure relationships of arteries. Biorheology 14:181–201
von Maltzahn W. W., Warriyar R. G., Keitzer W. F. (1984) Experimental measurements of elastic properties of media and adventitia of bovine carotid arteries. J. Biomech. 17:839–847
Vorp D. A., Severyn D. A. Steed D. L., Webster M. W. (1996) A device for the application of cyclic twist and extension on perfused vascular segments. Am. J. Physiol. 270:787–795
Weizsäcker H. W., Lambert H., Pascale K. (1983) Analysis of the passive mechanical properties of rat carotid arteries. J. Biomech. 16:703–715
Weizsäcker H. W., Pinto J. G. (1988) Isotropy and anisotropy of the arterial wall. J. Biomech. 21:477–487
Wikström J., Holmberg A., Johansson L., Löfberg A. M., Smedby O., Karacagil S., Ahlström H. (2000) Gadolinium-enhanced magnetic resonance angiography, digital subtraction angiography and duplex of the iliac arteries compared with intra-arterial pressure gradient measurements. Eur. J. Vasc. Endovasc. Surg. 19:516–523
Zhou J., Fung Y. C. (1997) The degree of nonlinearity and anisotropy of blood vessel elasticity. Proc. Natl. Acad. Sci. U.S.A. 94:14255–14260
Zhu H., Friedman M. H. (2003) Relationship between the dynamic geometry and wall thickness of a human coronary artery. Arterioscler. Thromb. Vasc. Biol. 23:2260–2265
Acknowledgments
The authors would like to thank Dr. Ivan Vesely and the Heart Valve Research Laboratory at Childrens Hospital Los Angeles for their financial support and vision. Dr. Einstein’s contribution was supported by NIH-NHLBI 1 R01 HL077921-01A2.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, K., Fata, B. & Einstein, D.R. Characterization of the Highly Nonlinear and Anisotropic Vascular Tissues from Experimental Inflation Data: A Validation Study Toward the Use of Clinical Data for In-Vivo Modeling and Analysis. Ann Biomed Eng 36, 1668–1680 (2008). https://doi.org/10.1007/s10439-008-9541-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-008-9541-9