Abstract
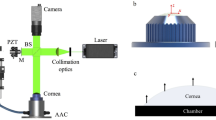
Cornea is a load-bearing tissue whose mechanical and viscoelastic characteristics are not well understood, due to the challenge associated with most of the measurements. A novel indentation technique has been developed for mechanical characterization of human and porcine corneal tissue, using a tailored depth-sensing microindentation instrument. During indentation, the corneas were suspended by clamping the edges of the cornea, thus allowing depth-sensing measurement free from the complication of the backing substrate. The deformation displacement and the amount of force applied by the indenter were used to obtain hysteresis and stress relaxation data for both human and porcine corneas. Optical coherence tomography was used to measure the thickness of the cornea. Simple theoretical analyses have been undertaken to explain the loading–unloading and the stress relaxation data. The effect of swelling on the mechanical properties of the cornea was also examined. Porcine corneas appeared to be less stiff and to demonstrate more linear response than human corneas under loading. More importantly, it is shown that swelling reduced the strength of the corneas. Our results demonstrate that this new indentation system can be used to characterize the mechanical and viscoelastic properties of corneas.








Similar content being viewed by others
References
Ahearne M., Yang Y., El Haj A. J., Then K. Y., Liu K. K. (2005) Characterizing the viscoelastic properties of thin hydrogel-based constructs for tissue engineering applications. J. R. Soc. Interface 2:455–463
Anderson K., El-Sheikh A., Newson T. (2004) Application of structural analysis to the mechanical behaviour of the cornea. J. R. Soc. Interface 1:3–15
Andreassen T. T., Simonsen A. H., Oxlund H. (1980) Biomechanical properties of keratoconus and normal corneas. Exp. Eye Res. 31:435–441
Begley M. R., Mackin T. J. (2004) Spherical indentation of freestanding circular thin films in the membrane regime. J. Mech. Phys. Solids 52:2005–2023
Borene M. L., Barocas V. H., Hubel A. (2004) Mechanical and cellular changes during compaction of a collagen-sponge-based corneal stromal equivalent. Ann. Biomed. Eng. 32:274–283
Fung Y. C. (1993) Biomechanics: Mechanical Properties of Living Tissues, 2nd. ed. Springer Verlag, New York
Gauthier O., Müller R., von Stechow D., Lamy B., Weiss P., Bouler J., Aguado E., Daculsi G. (2005) In vivo bone regeneration with injectable calcium phosphate biomaterial: A three-dimensional micro-computed tomographic, biomechanical and SEM study. Biomaterials 26:5444–5453
Grabner G., Eilmsteiner R., Steindl C., Ruckhofer J., Mattioli R., Husinsky W. (2005) Dynamic corneal imaging. J. Cataract Refract. Surg. 31:163–174
Hay, J. L. and G. M. Pharr. Instrumented indentation testing. In: ASM Handbook Volume 8, Mechanical Testing and Evaluation, edited by H. Kuhn and D. Medlin. 10th ed. Materials Park, OH: ASM International, 2000, pp. 232–243.
Hjortdal J. Ø., Møller-Pedersen T., Ivarsen A., Ehlers N. (2005) Corneal power, thickness, and stiffness: Results of a prospective randomized controlled trial of PRK and LASIK for myopia. J. Cataract Refract. Surg. 31:21–29
Hoeltzel D. A., Altman P., Buzard K., Choe K. (1992) Strip extensiometry for comparison of the mechanical response of bovine, rabbit, and human corneas. J. Biomech. Eng. 114:202–215
Ju B. F., Liu K. K., Ling S. F., Ng W. H. (2002) A novel technique for characterizing elastic properties of thin biological membrane. Mech. Mater. 34:749–754
Ju B. F., Wan K. T., Liu K. K. (2004) Indentation of a square elastomeric thin film by a flat-ended cylindrical punch in the presence of long-range intersurface forces. J. Appl. Phys. 96:6159–6163
Kampmeier J., Radt B., Birngruber R., Brinkmann R. (2000) Thermal and biomechanical parameters of porcine cornea. Cornea 19:355–363
Kasprzak H. T., Sultanova N. G. (1994) Simple holographic interferometric method of investigating the Poisson coefficient and elasticity moduli. Opt. Eng. 33:194–197
Kobayashi A. S., Staberg L. G., Schlegel W. A. (1973) Viscoelastic properties of human cornea. Exp. Mech. 13:497–503
Luce D. A. (2005) Determining in vivo biomechanical properties of the cornea with an ocular response analyzer. J. Cataract Refract. Surg. 31:156–162
Mandell R. B., Polse K. A., Brand R. J., Vastine D., Demartini D., Flom R. (1989) Corneal hydration control in Fuchs’ dystrophy. Invest Ophthalmol. Vis. Sci. 30:845–852
Maurice D. M. (1988) Mechanics of the Cornea. In: Cavanagh H. D. (ed) The Cornea: Transactions of the World Congress on the Cornea III. Raven Press, New York, pp. 187–193
Muller L. J., Pels E., Vrensen G. F. (2001) The effects of organ-culture on the density of keratocytes and collagen fibers in human corneas. Cornea 20:86–95
Muscat S., McKay N., Parks S., Kemp E., Keating D. (2002) Repeatability and reproducibility of corneal thickness measurements by optical coherence tomography. Invest. Ophthalmol. Vis. Sci. 43:1791–1795
Orwin E. J., Borene M. L., Hubel A. (2003) Biomechanical and optical characteristics of a corneal stromal equivalent. J. Biomech. Eng. 125:439–444
Reichlin T., Wild A., Dürrenberger M., Daniels A. U., Aebi U., Hunziker P. R., Stolz M. (2005) Investigating native coronary artery endothelium in situ and in cell culture by scanning force microscopy. J. Struct. Biol. 152:52–65
Shin T. J., Vito R. P., Johnson L. W., McCarey B. E. (1997) The distribution of strain in the human cornea. J. Biomech. 30:497–503
Seiler T., Matallana M., Sendler S., Bende T. (1992) Does Bowman’s layer determine the biomechanical properties of the cornea?. Refract. Corneal Surg. 8:139–142
Stewart W. C., Jenkins J. N., Stewart J. A. (2005) Corneal thickness after refractive surgery. Ophthalmology 112:1637
Storm C., Pastore J. J., MacKintosh F. C., Lubensky T. C., Janmey P. A. (2005) Nonlinear elasticity in biological gels. Nature 435:191–194
Walkenbach R. J., Boney F., Ye G. S. (1992) Corneal function after storage in dexsol or optisol. Invest. Ophthalmol. Vis. Sci. 33:2454–2458
Wan K. T. (1999) Fracture mechanics of a shaft loaded blister test-transition from a bending plate to a stretching membrane. J. Adhesion 70:209–219
Wan K. T., Liao K. (1999) Measuring mechanical properties of thin flexible films by a shaft-loaded blister test. Thin Solid Films 352:167–172
Wan K. T., Mai Y. W. (1995) Fracture mechanics of a shaft loaded blister of thin flexible membrane on rigid substrate. Int. J. Fract. 74:181–197
Wang H., Prendiville P. L., McDonnell P. J., Chang W. V. (1996) An ultrasonic technique for the measurement of the elastic moduli of human cornea. J. Biomech. 29:1633–1636
Yang F. (2003) Thickness effect on the indentation of an elastic layer. Mater. Sci. Eng. A 358:226–232
Zeng Y., Yang J., Huang K., Lee Z., Lee X. (2001) A comparison of biomechanical properties between human and porcine cornea. J. Biomech. 34:533–537
Acknowledgments
The authors are grateful for the finical support provided by the North Staffordshire R&D Consortium and to Dr Pierre Bagnaninchi for his help with OCT measurements.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
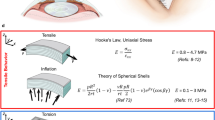
A diagram depicting the theoretical model of an indented cornea is shown in Fig. A1. In order to determine the relationship between the applied force and the strain, the cornea is assumed to be isotropic, parabolic in shape, and have a uniform thickness. The profile of the cornea can described as a line by the equation,
where a, b, and c represent constants. The slope at any point along the line can be found from the equation,
By substituting the points (r,0), and (0,w) into Eq. (A1) and (0,0) into Eq. (A2), the values for a, b, and c can be written as
where w is the vertical height of the clamped cornea and r is the radius at the clamped portion of the cornea. Equation (A1) can then be rewritten as follows,
The distance L from the clamped edge of the cornea to the center of the cornea before indentation, can be found from the equation
At an indentation depth of δ, the strain, ɛ, can be assumed to be
where L′ is the distance from the clamped edge of the cornea to the center of the cornea during indentation and can easily be determined by replacing w with w + δ in Eq. (A3). The force applied to the cornea by the indenter can be considered to be equivalent to a point load as the indenter radius is significantly smaller than the radius of the clamped portion. The force, F, applied to the cornea can be described as
where σ is the resulting stress around the clamped portion of the cornea, θ is the angle between the cornea edge and the horizontal, and h is the cornea thickness. θ can be determined from the equation
It is generally accepted that most biological materials including cornea exhibit a non-linear viscoelastic relationship between stress and strain and therefore a single value for the elastic modulus cannot be accurately obtained.27 Hoeltzel et al.11 used the equation
to define the relationship between tensile stress and strain where α represents a scaling factor, β represents the non-linear component between stress and strain and ɛs is the slack strain (the lowest strain to result in an increase in stress). By combining Eqs. (A9) and (A11), the relationship between force and strain can be described by the equation
which is equivalent to Eq. (1) in the text. This model has a number of limitations. One limitation of this model is the assumption that the cornea is isotropic rather than anisotropic. We would expect an anisotropic material to deform differently to an isotropic material and therefore assuming isotropy will lead to an error in our results. This error is dependent on the structure and deformation behavior of the cornea. Another limitation may be the assumption that the cornea undergoes stretching rather than bending. The relationship between bending and stretching for flat membranes during indentation has been previously examined.4,29 The relationship for a flat circular membrane can be described using the equation4
where λ is a dimensionless parameter to determine whether bending can be ignored, ν is the Poisson’s ratio, and E is the elastic modulus. The values obtained for λ using this equation ranged from 200 to 2000, which fall within the bending–stretching transition region for a flat circular membrane4 (85 < λ < 30,000). This suggests that both bending and stretching influence the deformation behavior of the cornea. However, since this equation has been derived for a flat material it does not accurately represent the deformation of the cornea, which should be less influenced from bending due to its already curved structure. Despite these limitations the present model provides a simple first order analysis of the material properties and serves the purpose of mechanical characterization. Further work to improve the model and predict the relationship between stretching and bending during indentation would be required to obtain more accurate results.
Rights and permissions
About this article
Cite this article
Ahearne, M., Yang, Y., Then, K.Y. et al. An Indentation Technique to Characterize the Mechanical and Viscoelastic Properties of Human and Porcine Corneas. Ann Biomed Eng 35, 1608–1616 (2007). https://doi.org/10.1007/s10439-007-9323-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-007-9323-9