Abstract
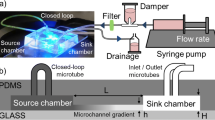
We demonstrate a compact Polydimethylsiloxane microfluidic chip which can quickly generate ten different chemical concentrations simultaneously. The concentration magnitude of each branch can be flexibly regulated based on the flow rate ratios of the two injecting streams. The temporal/pulsatile concentration gradients are achieved by integrating on-chip pneumatic actuated valves controlled by the external signals. The temporal concentration gradients can also be tuned precisely by varying applied frequency and duty cycle of the trigger signal. It is believed that such microdevice will be potentially used for some application areas of producing stable chemical gradients as well as allowing fast, pulsatile gradient transformation in seconds.








Similar content being viewed by others
References
Abhyankar VV, Lokuta MA et al (2006) Characterization of a membrane-based gradient generator for use in cell-signaling studies. Lab Chip 6:389–393
Abhyankar VV, Toepke MW et al (2008) A platform for assessing chemotactic migration within a spatiotemporally defined 3D microenvironment. Lab Chip 8(9):1507–1515
Ahmed D, Chan CY et al (2013) Tunable, pulsatile chemical gradient generation via acoustically driven oscillating bubbles. Lab Chip 13(3):328–331
Ainla A, Jansson ET et al (2010) A microfluidic pipette for single-cell pharmacology. Anal Chem 82(11):4529–4536
Atencia J, Morrow J et al (2009) The microfluidic palette: a diffusive gradient generator with spatio-temporal control. Lab Chip 9:2707–2714
Atencia J, Cooksey GA et al (2012) A robust diffusion-based gradient generator for dynamic cell assays. Lab Chip 12(2):309–316
Beta C, Wyatt D et al (2007) Flow photolysis for spatiotemporal stimulation of single cells. Anal Chem 79(10):3940–3944
Boyden S (1961) The chemotactic effect of mixtures of antibody and antigen on polymorphonuclear leucocytes. J Exp Med 115:453–466
Bruus H (2008) Theoretical microfluidics. Oxford University Press, Oxford
Carlo DD (2009) Inertial microfluidics. Lab Chip 9(21):3038–3046
Chen CY, Wo AM et al (2012) A microfluidic concentration generator for dose-response assays on ion channel pharmacology. Lab Chip 12(4):794–801
Chung BG, Flanagan LA et al (2005) Human neural stem cell growth and differentiation in a gradient-generating microfluidic device. Lab Chip 5(4):401–406
Cimetta E, Cannizzaro C et al (2010) Microfluidic device generating stable concentration gradients for long term cell culture: application to Wnt3a regulation of β-catenin signaling. Lab Chip 10(23):3277–3283
Dertinger SKW, Chiu DT et al (2001) Generation of gradients having complex shapes using microfluidic networks. Anal Chem 73(6):1240–1246
Estes MD, Hurth C et al (2013) A tuneable array of unique steady-state microfluidic gradients. Phys Chem Chem Phys 15(31):12805–12814
Haessler U, Pisano M et al (2011) Dendritic cell chemotaxis in 3D under defined chemokine gradients reveals differential response to ligands CCL21 and CCL19. PNAS 108(14):5614–5619
Holden MA, Kumar S et al (2003) Generating fixed concentration arrays in a microfluidic device. Sens Actuators B Chem 92(1–2):199–207
Horrocks MH, Rajah L et al (2013) Single-molecule measurements of transient biomolecular complexes through microfluidic dilution. Anal Chem 85(14):6855–6859
Hung PJ, Lee PJ et al (2005) Continuous perfusion microfluidic cell culture array for high-throughput cell-based assays. Biotechnol and Bioeng 89(1):1–8
Irimia D, Geba DA et al (2006a) Universal microfluidic gradient generator. Anal Chem 78(10):3472–3477
Irimia D, Liu SY et al (2006b) Microfluidic system for measuring neutrophil migratory responses to fast switches of chemical gradients. Lab Chip 6(2):191–198
Irimia D, Charras G et al (2007) Polar stimulation and constrained cell migration in microfluidic channels. Lab Chip 7(12):1783–1790
Jang YH, Hancock MJ et al (2011) An integrated microfluidic device for two-dimensional combinatorial dilution. Lab Chip 11(19):3277–3286
Jeon NL, Dertinger SKW et al (2000) Generation of solution and surface gradients using microfluidic systems. Langmuir 16(22):8311–8316
Jeon NL, Baskaran H et al (2002) Neutrophil chemotaxis in linear and complex gradients of interleukin-8 formed in a microfabricated device. Nat Biotechnol 20(8):826–830
Keenan TM, Folch A (2008) Biomolecular gradients in cell culture systems. Lab Chip 8(1):34–57
Kim C, Lee K et al (2008) A serial dilution microfluidic device using a ladder network generating logarithmic or linear concentrations. Lab Chip 8(3):473–479
Kim D, Lokuta MA et al (2009) Selective and tunable gradient device for cell culture and chemotaxis study. Lab Chip 9(12):1797–1800
Kress H, Park JG et al (2009) Cell stimulation with optically manipulated microsources. Nat Methods 6(12):905–909
Kuczenski B, Ruder WC et al (2009) Probing cellular dynamics with a chemical signal generator. PLoS ONE 4(3):e4847
Lam EW, Cooksey GA et al (2006) Microfluidic circuits with tunable flow resistances. Appl Phys Lett 89(16):164105
Lee K, Kim C et al (2010) Microfluidic network-based combinatorial dilution device for high throughput screening and optimization. Microfluid Nanofluid 8:677–685
Lee K, Kim C et al (2011) Microfluidic concentration-on-demand combinatorial dilutions. Microfluid Nanofluid 11:75–86
Li XJ, Chen YC et al (2011) A simple and fast microfluidic approach of same-single-cell analysis (SASCA) for the study of multidrug resistance modulation in cancer cells. Lab Chip 11(7):1378–1384
Lin F, Butcher EC (2006) T cell chemotaxis in a simple microfluidic device. Lab Chip 6(11):1462–1469
Martin P (1997) Wound healing–aiming for perfect skin regeneration. Science 276(75):75–81
Melin J, Quake SR (2007) Microfluidic large-scale integration: the evolution of design rules for biological automation. Annu Rev Biophys Biomol Struct 36:213–231
Ming GL, Wong ST et al (2002) Adaptation in the chemotactic guidance of nerve growth cones. Nature 417(6887):411–418
Morel M, Galas JC et al (2012) Concentration landscape generators for shear free dynamic chemical stimulation. Lab Chip 12(7):1340–1346
Nagai M, Ryu S et al (2010) Chemical control of Vorticella bioactuator using microfluidics. Lab Chip 10(12):1574–1578
Nandagopal S, Wu D et al (2011) Combinatorial guidance by CCR7 ligands for T lymphocytes migration in co-existing chemokine fields. PLoS ONE 6(3):e18183
Oh KW, Lee K et al (2012) Design of pressure-driven microfluidic networks using electric circuit analogy. Lab Chip 12(3):515–545
Park ES, DiFeo MA et al (2013) Sequentially pulsed fluid delivery to establish soluble gradients within a scalable microfluidic chamber array. Biomicrofluidics 7:011804
Rosa P, Tenreiro S et al (2012) High-throughput study of alpha-synuclein expression in yeast using microfluidics for control of local cellular microenvironment. Biomicrofluidics 6(1):014109
Saadi W, Rhee SW et al (2007) Generation of stable concentration gradients in 2D and 3D environments using a microfluidic ladder chamber. Biomed Microdevices 9:627–635
Toetsch S, Olwell P et al (2009) The evolution of chemotaxis assays from static models to physiologically relevant platforms. Integr Biol 1(2):170–181
Toh AGG, Wang ZP et al (2014) Engineering microfluidic concentration gradient generators for biological applications. Microfluid Nanofluid 16:1–18
Vandersarl JJ, Xu AM et al (2011) Rapid spatial and temporal controlled signal delivery over large cell culture areas. Lab Chip 11(18):3057–3063
Wang SJ, Saadi W et al (2004) Differential effects of EGF gradient profiles on MDA-MB-231 breast cancer cell chemotaxis. Exp Cell Res 300(1):180–189
Wang CJ, Li X et al (2008) A microfluidics-based turning assay reveals complex growth cone responses to integrated gradients of substrate-bound ECM molecules and diffusible guidance cues. Lab Chip 8(2):227–237
Yang CG, Wu YF et al (2011) A radial microfluidic concentration gradient generator with high-density channels for cell apoptosis assay. Lab Chip 11(19):3305–3312
Zicha D, Dunn GA et al (1991) A new direct-viewing chemotaxis chamber. J Cell Sci 99(4):769–775
Zigmond SH, Hirsch JG (1973) Leukocyte locomotion and chemotaxis. J Exp Med 137(2):387–410
Acknowledgments
We would like to thank Prof. Andrew W. O. Poon’s research group, of Department of Electronic and Computer Engineering in HKUST, for their assistance in COMSOL Multiphysics simulations. This publication is based on work partially supported by Award No. SA-C0040/UK-C0016, made by King Abdullah University of Science and Technology (KAUST), Hong Kong RGC Grants HKUST 604710 and 605411, and National Natural Science Foundation of China (Grant No. 11290165). The work is also partially supported by the Nanoscience and Nanotechnology Program at HKUST.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 2 (AVI 3012 kb)
Rights and permissions
About this article
Cite this article
Zhou, B., Xu, W., Wang, C. et al. Generation of tunable and pulsatile concentration gradients via microfluidic network. Microfluid Nanofluid 18, 175–184 (2015). https://doi.org/10.1007/s10404-014-1432-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10404-014-1432-9