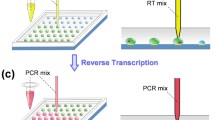
Abstract
This study presents an optical microfluidic platform and method for performing real-time polymerase chain reactions of MDA-MB-231 breast cancer cell DNA within droplet-in-oil micro-reactors. Illumination of the droplets using a low-power (20–40 mW) infrared (IR) laser at 1,460 nm provides a simple approach for droplet manipulation and rapid thermal cycling. The nanoliter droplet volumes allow for extremely fast amplification times, from cell lysis to assay completion in 15 min or less. Droplets containing lysis buffer and subsequently master mix solutions are optically positioned in mineral oil to coalesce with droplets containing live cells on a Petri dish surface for reverse-transcription polymerase chain reactions (RT-PCR). The optical PCR setup is also shown to amplify DNA in droplets containing single or multiple cells and distinguish between methylated and unmethylated BRCA1 promoters in microdroplets containing sample at the single-cell level. Melting curves generated using IR heating indicates a melting temperature of 86 °C for the 255-bp amplicon. The results are consistent with standard PCR and methylation-specific protocols performed in a commercial system. The simplicity of the droplet-in-oil Petri dish platform provides an easy and efficient tool for DNA analysis from live cells, and can be integrated with other microfluidic technologies for complex and large-scale assays.






Similar content being viewed by others
References
Abate AR, Chen CH, Agresti JJ, Weitz DA (2009) Beating Poisson encapsulation statistics using close-packed ordering. Lab Chip 9:2628–2631
Abràmoff MD, Magalhães PJ, Ram SJ (2004) Image processing with ImageJ. Biophotonics Int 11:36–42
Basu AS, Gianchandani YB (2008) Virtual microfluidic traps, filters, channels and pumps using Marangoni flows. J Micromech Microeng 18:115031
Beer NR, Hindson BJ, Wheeler EK, Hall SB, Rose KA, Kennedy IM, Colston BW (2007) On-chip, real-time, single-copy polymerase chain reaction in picoliter droplets. Anal Chem 79:8471–8475
Bianco T, Chenevix-Trench G, Walsh DC, Cooper JE, Dobrovic A (2000) Tumour-specific distribution of BRCA1 promoter region methylation supports a pathogenetic role in breast and ovarian cancer. Carcinogenesis 21:147–151
Bird AP (1980) DNA methylation and the frequency of CpG in animal DNA. Nucleic Acids Res 8:1499–1504
Dixit SS, Kim H, Vasilyev A, Eid A, Faris GW (2010) Light-driven formation and rupture of droplet bilayers. Langmuir 26:6193–6200
Dixit SS, Pincus A, Guo B, Faris GW (2012) Droplet shape analysis and permeability studies in droplet lipid bilayers. Langmuir 28:7442–7451
Dorfman KD, Chabert M, Codarbox J-H, Rousseau G, de Cremoux P, Viovy J-L (2005) Contamination-free continuous flow microfluidic polymerase chain reaction for quantitative and clinical applications. Anal Chem 77:3700–3704
Edd JF, Di Carlo D, Humphry KJ, Koster S, Irimia D, Weitz DA, Toner M (2008) Controlled encapsulation of single-cells into monodisperse picolitre drops. Lab Chip 8:1262–1264
Erlich HA, Gelfand D, Sninsky JJ (1991) Recent advances in the polymerase chain reaction. Science 252:1643–1651
Hatch AC, Fisher JS, Pentoney SL, Yang DL, Lee AP (2011) Tunable 3D droplet self-assembly for ultra-high-density digital micro-reactor arrays. Lab Chip 11:2509–2517
Herman JG, Graff JR, Myöhänen S, Nelkin BD, Baylin SB (1996) Methylation-specific PCR: a novel PCR assay for methylation status of CpG islands. Proc Natl Acad Sci USA 93:9821–9826
Hu W, Ohta A (2011) Aqueous droplet manipulation by optically induced Marangoni circulation. Microfluid Nanofluid 11:307–316
Hühmer AFR, Landers JP (2000) Noncontact infrared-mediated thermocycling for effective polymerase chain reaction amplification of DNA in nanoliter volumes. Anal Chem 72:5507–5512
Hunt HC, Wilkinson JS (2008) Optofluidic integration for microanalysis. Microfluid Nanofluid 4:53–79
Kim H, Dixit S, Green CJ, Faris GW (2009a) Nanodroplet real-time PCR system with laser assisted heating. Opt Express 17:218–227
Kim H, Vishniakou S, Faris GW (2009b) Petri dish PCR: laser-heated reactions in nanoliter droplet arrays. Lab Chip 9:1230–1235
Kiss MM, Ortoleva-Donnelly L, Beer NR, Warner J, Bailey CG, Colston BW, Rothberg JM, Link DR, Leamon JH (2008) High-throughput quantitative polymerase chain reaction in picoliter droplets. Anal Chem 80:8975–8981
Kotz KT, Noble KA, Faris GW (2004) Optical microfluidics. Appl Phys Lett 85:2658–2660
Kotz KT, Gu Y, Faris GW (2005) Optically addressed droplet-based protein assay. J Am Chem Soc 127:5736–5737
Kumaresan P, Yang CJ, Cronier SA, Blazej RG, Mathies RA (2008) High-throughput single copy DNA amplification and cell analysis in engineered nanoliter droplets. Anal Chem 80:3522–3529
Lee D, Chen P-J, Lee G-B (2010) The evolution of real-time PCR machines to real-time PCR chips. Biosens Bioelectron 25:1820–1824
Liu RH, Yang J, Lenigk R, Bonanno J, Grodzinski P (2004) Self-contained, fully integrated biochip for sample preparation, polymerase chain reaction amplification, and DNA microarray detection. Anal Chem 76:1824–1831
Marcus JS, Anderson WF, Quake SR (2006) Microfluidic single-cell mRNA isolation and analysis. Anal Chem 78:3084–3089
Mazutis L, Araghi AF, Miller OJ, Baret J-C, Frenz L, Janoshazi A, Taly V, Miller BJ, Hutchison JB, Link D, Griffiths AD, Ryckelynck M (2009) Droplet-based microfluidic systems for high-throughput single DNA molecule isothermal amplification and analysis. Anal Chem 81:4813–4821
Morimoto Y, Tan W-H, Tsuda Y, Takeuchi S (2009) Monodisperse semi-permeable microcapsules for continuous observation of cells. Lab Chip 9:2217–2223
Nagrath S, Sequist LV, Maheswaran S, Bell DW, Irimia D, Ulkus L, Smith MR, Kwak EL, Digumarthy S, Muzikansky A, Ryan P, Balis UJ, Tompkins RG, Haber DA, Toner M (2007) Isolation of rare circulating tumour cells in cancer patients by microchip technology. Nature 450:1235–1239
Nakano M, Komatsu J, Matsuura S-I, Takashima K, Katsura S, Mizuno A (2003) Single-molecule PCR using water-in-oil emulsion. J Biotechnol 102:117–124
Nishimura T, Ogura Y, Tanida J (2012) Optofluidic DNA computation based on optically manipulated microdroplets. Microfluid Nanofluid. doi:10.1007/s10404-012-0934-6
Northrup MA, Benett B, Hadley D, Landre P, Lehew S, Richards J, Stratton P (1998) A miniature analytical instrument for nucleic acids based on micromachined silicon reaction chambers. Anal Chem 70:918–922
Oda RP, Strausbauch MA, Huhmer AFR, Borson N, Jurrens SR, Craighead J, Wettstein PJ, Eckloff B, Kline B, Landers JP (1998) Infrared-mediated thermocycling for ultrafast polymerase chain reaction amplification of DNA. Anal Chem 70:4361–4368
Ohashi T, Kuyama H, Hanafusa N, Togawa Y (2007) A simple device using magnetic transportation for droplet-based PCR. Biomed Microdevices 9:695–702
Roper MG, Easley CJ, Landers JP (2005) Advances in polymerase chain reaction on microfluidic chips. Anal Chem 77:3887–3893
Schaerli Y, Wootton RC, Robinson T, Stein V, Dunsby C, Neil MAA, French PMW, Demello AJ, Abell C, Hollfelder F (2009) Continuous-flow polymerase chain reaction of single-copy DNA in microfluidic microdroplets. Anal Chem 81:302–306
Shi W, Qin J, Ye N, Lin B (2008) Droplet-based microfluidic system for individual Caenorhabditis elegans assay. Lab Chip 8:1432–1435
Sundberg SO, Wittwer CT, Gao C, Gale BK (2010) Spinning disk platform for microfluidic digital polymerase chain reaction. Anal Chem 82:1546–1550
Tan Y-C, Hettiarachchi K, Siu M, Pan Y-R, Lee AP (2006) Controlled microfluidic encapsulation of cells, proteins, and microbeads in lipid vesicles. J Am Chem Soc 128:5656–5658
Teh S-Y, Lin R, Hung L-H, Lee AP (2008) Droplet microfluidics. Lab Chip 8:198–220
Terazono H, Hattori A, Takei H, Takeda K, Yasuda K (2008) Development of 1,480 nm photothermal high-speed real-time polymerase chain reaction system for rapid nucleotide recognition. Jpn J Appl Phys 47:5212–5216
Tewhey R, Warner JB, Nakano M, Libby B, Medkova M, David PH, Kotsopoulos SK, Samuels ML, Hutchison JB, Larson JW, Topol EJ, Weiner MP, Harismendy O, Olson J, Link DR, Frazer KA (2009) Microdroplet-based PCR enrichment for large-scale targeted sequencing. Nat Biotechnol 27:1025–1031
Thakur S, Nakamura T, Calin G, Russo A, Tamburrino JF, Shimizu M, Baldassarre G, Battista S, Fusco A, Wassell RP, Dubois G, Alder H, Croce CM (2003) Regulation of BRCA1 transcription by specific single-stranded DNA binding factors. Mol Cell Biol 23:3774–3787
Wang F, Burns MA (2009) Performance of nanoliter-sized droplet-based microfluidic PCR. Biomed Microdev 11:1071–1080
Wei M, Grushko TA, Dignam J, Hagos F, Nanda R, Sveen L, Xu J, Fackenthal J, Tretiakova M, Das S, Olopade OI (2005) BRCA1 promoter methylation in sporadic breast cancer is associated with reduced BRCA1 copy number and chromosome 17 aneusomy. Cancer Res 65:10692–10699
Weis JH, Tan SS, Martin BK, Wittwer CT (1992) Detection of rare mRNAs via quantitative RT-PCR. Trends Genet 8:263–264
Yang X, Yan L, Davidson NE (2001) DNA methylation in breast cancer. Endocr-Relat Cancer 8:115–127
Yu Y, Li B, Baker CA, Zhang X, Roper MG (2012) Quantitative polymerase chain reaction using infrared heating on a microfluidic chip. Anal Chem 84:2825–2829
Zamorano PL, Mahesh VB, Brann DW (1996) Quantitative RT-PCR for neuroendocrine studies: A mini review. Neuroendocrinology 63:397–407
Zhang Y, Bailey V, Puleo CM, Easwaran H, Griffiths E, Herman JG, Baylin SB, Wang T-H (2009) DNA methylation analysis on a droplet-in-oil PCR array. Lab Chip 9:1059–1064
Acknowledgments
We thank Nahid Waleh for technical assistance, and acknowledge the thoughtful discussions with Keith Laderoute, Christopher Green, and Sanhita Dixit. This work was supported by the DOD Breast Cancer Research Program, Grant Number W81XWH-09-1-0298. The US Army Medical Research Acquisition Activity, 820 Chandler Street, Fort Detrick MD 21702-5014 is the awarding and administering acquisition office. The content of this paper does not necessarily reflect the position or the policy of the government, and no official endorsement should be inferred.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hettiarachchi, K., Kim, H. & Faris, G.W. Optical manipulation and control of real-time PCR in cell encapsulating microdroplets by IR laser. Microfluid Nanofluid 13, 967–975 (2012). https://doi.org/10.1007/s10404-012-1016-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10404-012-1016-5