Abstract
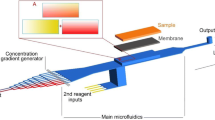
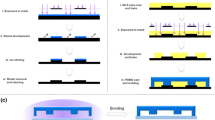
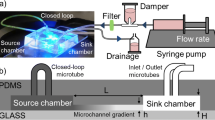
We successfully determined a suitable glucose concentration for endothelial cells (ECs) using a gradient-generating microfluidic chip and a micro-stamper that were fabricated using micro-electro-mechanical systems (MEMS) technology. Our strategy was to generate a stable concentration gradient in the observation area based on a microfluidic network and micro-mixers, which produced a concentration gradient under various flow rates. The areas for cell adhesion were delineated on a glass slide with a micro-stamper using the micro-contact printing (μCP) method. We also discuss which glucose concentration gradients are suitable for cell viability test (i.e., 0–0.2%, 0.05–0.15%, and 0.06–0.17%). After examining various concentration gradients, the suitable glucose concentration for EC’s viability test was determined to range from 0.077% (4.2 mM) to 0.147% (8.16 mM). Higher or lower concentrations caused the ECs to atrophy or die. In this study, we describe a gradient-generating microfluidic chip that can be used to produce various drug concentrations for multi-concentration tests.






Similar content being viewed by others
References
Baumgartner-Parzer SM, Wagner L, Pettermann M, Grillari J, Gessl A, Waldhausl W (1995) High-glucose-triggered apoptosis in cultured endothelial cells. Am Diabetes Assoc 44:1323–1327
Boyden SV (1962) Rapid quantitation of neutrophil chemotaxis: use of a polyvinylpyrrolidone-free polycarbonate membrane in a multiwell assembly. J Immunol Methods 115:453–466
Branch DW, Corey JM, Weyhenmeyer JA, Brewer GJ, Wheeler BC (1998) Microstamp patterns of biomolecules for high-resolution neuronal networks. Med Biol Eng Comput 36:135–141
Chang JC, Brewer GJ, Wheeler BC (2003) A modified microstamping technique enhances polylysine transfer and neuronal cell patterning. Biomaterials 24:2863–2870
Chung BG, Park JW, Hu JS, Huang C, Monuki ES, Jeon NL (2007) A hybrid microfluidic-vacuum device for direct interfacing with conventional cell culture methods. BMC Biotechnol 7:60–67
Das T, Mallick SK, Paul D, Bhutia SK, Bhattacharyya TK, Maiti TK (2007) Microcontact printing of concanavalin a and its effect on mammalian cell morphology. J Colloid Interface Sci 314:71–79
DCCT Research Group (1993) The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 329:977–986
Dertinger KWS, Chiu DT, Jeon NL, Whitesides GM (2001) Generation of gradients having complex shapes using microfluidic networks. Anal Chem 73:1240–1246
Dewey CF, Bussolari SR, Gimbrone MA, Davies PF (1981) The dynamic response of vascular endothelial cells to fluid shear stress. J Biomech Eng 103:177–185
Folch A, Toner M (2000) Microengineering of cellular interactions. Annu Rev Biomed Eng 2:227–256
Folch A, Jo BH, Hurtado O, Beebe DJ, Toner M (2000) Microfabricated elastomeric stencils for micropatterning cell cultures. J Biomed Mater Res 52:346–353
Jeon NL, Dertinger SKW, Chiu DT, Choi IS, Stroock AD, Whitesides GM (2000) Generation of solution and surface gradients using microfluidic systems. Langmuir 16:8311–8316
Katanosaka Y, Bao JH, Komatsu T, Suemori T, Yamada A, Mohri S, Naruse K (2008) Analysis of cyclic-stretching responses using cell-adhesion-patterned cell. J Biotechnol 133:82–89
Kim YD, Park CB, Clark DS (2001) Stable sol-gel microstructured and microfluidic networks for protein patterning. Biotechnol Bioeng 73:331–337
Kim L, Vahey MD, Lee HY, Voldman J (2006) Microfluidic arrays for logarithmically perfused embryonic stem cell culture. Lab Chip 6:394–406
Li Y, Yuan B, Ji H, Han D, Chen S, Tian F, Jiang X (2007) A method for patterning multiple types of cells by using electrochemical desorption of self-assembled monolayers within microfluidic channels. Angew Chem Int Ed 46:1094–1096
McGinn S, Poronnik P, King M, Gallery EDM, Pollock CA (2003) High glucose and endothelial cell growth: novel effects independent of autocrine TGF-beta 1 and hyperosmolarity. Am J Physiol Cell Physiol 284:1374–1386
Millioni R, Puricelli L, Iori E, Arrigoni G, Tessari P (2010) The effects of rosiglitazone and high glucose on protein expression in endothelial cells. J Proteome Res 9:578–584
Nelson RD, Quie PG, Simmons RL (1975) Chemotaxis under agarose: a new and simple method for measuring chemotaxis and spontaneous migration of human polymorphonuclear leukocytes and monocytes. J Immunol 115:1650–1656
Park TH, Shuler ML (2003) Integration of cell culture and microfabrication technology. Biotechnol Prog 19:243–253
Rossetti L, Giaccari A, DeFronzo RA (1990) Glucose toxicity. Am Diabetes Assoc 13:610–630
Somersalo K, Salob OP, Bjorkstenb F, Mustakallioc KK (1990) A simplified Boyden chamber assay for neutrophil chemotaxis based on quantitation of myeloperoxidase. Anal Biochem 185:238–242
Song H, Poo MM (1999) Signal transduction underlying growth cone guidance by diffusible factors. Curr Opin Neurobiol 9:355–363
Takayama S, McDonald JC, Ostuni E, Liang MN, Kenis PJA, Ismagilov RF, Whitesides GM (1999) Patterning cells and their environments using multiple laminar fluid flows in capillary networks. Proc Natl Acad Sci USA 96:5545–5548
Takayama S, Ostuni E, Qian XP, McDonald JC, Jiang XY, LeDuc P, Wu MH, Ingber DE, Whitesides GM (2001a) Topographical micropatterning of poly(dimethylsiloxane) using laminar flows of liquids in capillaries. Adv Mater 13:570–574
Takayama S, Ostuni E, LeDuc P, Naruse K, Ingber DE, Whitesides GM (2001b) Laminar flows-subcellular positioning of small molecules. Nature 411:1016
Wang S, Yue F, Zhang L, Wang J, Wang Y, Jiang L, Lin B, Wang Q (2009) Simultaneous quantification of active components in the herbs and products of Si-Wu-Tang by high performance liquid chromatography-mass spectrometry. J Pharm Biomed Anal 49:806–810
Wechezak AR, Viggers RF, Sauvage LR (1985) Fibronectin and F-actin redistribution in cultured endothelial cells exposed to shear stress. Lab Invest 53:639–647
Wyart C, Ybert C, Bourdieu L, Herr C, Prinz C, Chatenay D (2002) Constrained synaptic connectivity in functional mammalian neuronal networks grown on patterned surfaces. J Neurosci Methods 117:123–131
Yki-Järvinen H, Helve E, Koivisto VA (1987) Hyperglycemia decreases glucose uptake in type I diabetes. Am Diabetes Assoc 36:892–896
Acknowledgments
The authors would like to thank the Center for Micro/Nano Technology, National Cheng Kung University, Tainan, Taiwan, R.O.C., for access to equipment and technical support. Funding from the Ministry of Education and the National Science Council of Taiwan, R.O.C. under Grants NSC 97-2221-E-006-222-MY3 and NSC 99-2221-E-006-203-MY3 are gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yeh, CH., Chen, CH. & Lin, YC. Use of a gradient-generating microfluidic device to rapidly determine a suitable glucose concentration for cell viability test. Microfluid Nanofluid 10, 1011–1018 (2011). https://doi.org/10.1007/s10404-010-0730-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10404-010-0730-0