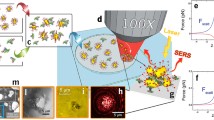
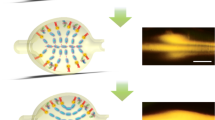
Abstract
An optofluidic device is reported in this paper that can highly improve the robustness of surface-enhanced Raman scattering (SERS) detection and provide fingerprint information of proteins with a concentration in the nanogram per liter range within minutes. Moreover, the conformational change of protein can also be obtained using this device. Fabricated by standard photolithography processes, the optofluidic device has a step microfluidic–nanofluidic structure, which provides robust SERS detection. The sensitivity of the device is investigated using insulin and albumin as target analytes at a concentration of 0.9 ng/L. The ability to detect conformational changes of proteins using this technology is also shown by probing these analytes before and after their denaturation.





Similar content being viewed by others
Abbreviations
- SERS:
-
Surface-enhanced Raman scattering
- A-Si:
-
Amorphous silicon
- PECVD:
-
Plasma-enhanced chemical vapor deposition
- HF:
-
Hydrofluoric
- CF4 :
-
Tetrafluoride
- BSA:
-
Bovine serum albumin
- RT:
-
Room temperature
References
Abe H, Manzel K, Schulze W et al (1981) Surface-enhanced Raman spectroscopy of CO adsorbed on colloidal silver particles. J Chem Phys 74:792–797
Campion A, Kambhampati P (1998) Surface-enhanced Raman scattering. Chem Soc Rev 27:241–250
Chaney SB, Shanmukh S, Dluhy RA et al (2005) Aligned silver nanorod arrays produce high sensitivity surface-enhanced Raman spectroscopy substrates. Appl Phys Lett 87(3):031908
Chou I, Benford M, Beier HT et al (2008) Nanofluidic biosensing for β-amyloid detection using surface enhanced Raman spectroscopy. Nano Lett 8:1729–1735
Dick LA, McFarland AD, Haynes CL et al (2002) Metal film over nanosphere (MFON) electrodes for surface-enhanced raman spectroscopy (SERS): improvements in surface nanostructure stability and suppression of irreversible loss. J Phys Chem B 106:853–860
Emory SR, Haskins WE, Nie S (1998) Direct observation of size-dependent optical enhancement in single metal nanoparticles. J Am Chem Soc 120:8009–8010
Félidj N, Aubard J, Lévi G et al (2003) Optimized surface-enhanced Raman scattering on gold nanoparticle arrays. Appl Phys Lett 82:3095–3097
Félidj N, Truong SL, Aubard J et al (2004) Gold particle interaction in regular arrays probed by surface enhanced Raman scattering. J Chem Phys 120:7141–7146
Fleischman M, Hendra PJ, McQuillan AJ (1974) Raman spectra of pyridine adsorbed at a silver electrode. Chem Phys Lett 26:163–166
Gersten J, Nitzan A (1980) Electromagnetic theory of enhanced Raman scattering by molecules adsorbed on rough surfaces. J Chem Phys 73:3023–3037
Gunnarsson L, Bjerneld EJ, Xu H et al (2001) Interparticle coupling effects in nanofabricated substrates for surface-enhanced Raman scattering. Appl Phys 78:802–804
Hering K, Cialla D, Ackermann K et al (2008) SERS: a versatile tool in chemical and biochemical diagnostics. Anal Bioanal Chem 390:113–124
Jiang C, Chang JY (2005) Unfolding and breakdown of insulin in the presence of endogenous thiols. FEBS Lett 579:3927–3931
Kneipp K, Wang Y, Kneipp H (1997) Single molecule detection using surface-enhanced Raman scattering (SERS). Phys Rev Lett 78:1667–1690
Kneipp K, Kneipp H, Kartha VB et al (1998) Detection and identification of a single DNA base molecule using surface-enhanced Raman scattering (SERS). Phys Rev E 57:R6281–R6284
Kneipp K, Kneipp H, Itzkan I et al (1999) Ultrasensitive chemical analysis by Raman spectroscopy. Chem Rev 99:2957–2976
Kneipp K, Kneipp H, Itzkan I et al (2002) Surface-enhanced Raman scattering and biophysics. J Phys Condens Matter 14:R597–R624
Kubo S, Gu Z, Tryk DA et al (2002) Metal-coated colloidal crystal film as surface-enhanced Raman scattering substrate. Langmuir 18:5043–5046
Lippert JL, Tyminski D, Desmueles PJ (1976) Determination of the secondary structure of proteins by laser Raman spectroscopy. J Am Chem Soc 98:7075–7080
Miura T, Thomas GJ (1995) Raman spectroscopy of proteins and their assemblies. Subcell Biochem 24:55–99
Miura T, Suzuki K, Naohio K et al (2000) Metal binding modes of alzheimer’s amyloid â-peptide in insoluble aggregates and soluble complexes. Biochemistry 39:7024–7031
Moskovits M (2005) Surface-enhanced Raman spectroscopy: a brief retrospective. J Raman Spectrosc 36:485–496
Nie S, Emory RS (1997) Probing single molecules and single nanoparticles by surface-enhanced Raman scattering. Science 275:1102–1106
Nikoobakht B, El-Sayed EA (2003) Surface-enhanced Raman scattering studies on aggregated gold nanorods. J Phys Chem A 107:3372–3378
Oldenburg SJ, Westcott SL, Averitt RD et al (1999) Surface enhanced Raman scattering in the near infrared using metal nanoshell substrates. J Chem Phys 111:4729–4735
Otto A (2005) The ‘chemical’ (electronic) contribution to surface-enhanced Raman scattering. J Raman Spectrosc 36:497–509
Pemberton JE, Buck RP (1981) Detection of low concentration of a colored adsorbate at silver by surface-enhanced and resonance-enhanced Raman spectrometry. Anal Chem 53:2263–2267
Pettinger B, Wenneng U, Wetzel H (1980) Surface plasmon enhanced Raman scattering frequency and angular resonance of Raman scattered light from pyridine on Au, Ag and Cu electrodes. Surf Sci 101:409–416
Podstawka E, Ozaki Y, Proniewicz LM (2004) Adsorption of S–S containing proteins on a colloidal silver surface studied by surface-enhanced Raman spectroscopy. Appl Spectrosc 58:1147–1156
Shanmugam G, Polavarapu PL (2004) Vibrational circular dichroism spectra of protein films: thermal denaturation of bovine serum albumin. Biophy Chem 111:73–77
Stevenson CL, Vo-Dinh T (1996) Signal expressions in Raman spectroscopy. In: Lasema JJ (ed) Modern techniques in Raman spectroscopy, 1st edn. Wiley, West Sussex, pp 1–39
Stewart S, Fredericks PM (1999) Surface-enhanced Raman spectroscopy of peptides and proteins adsorbed on an electrochemically prepared silver surface. Spectrochim Acta A 55:1615–1640
Su KH, Wei QH, Zhang X et al (2003) Interparticle coupling effects on plasmon resonances of nanogold particles. Nano Lett 3:1087–1090
Tu AT (1982) Raman spectroscopy in biology. Wiley, New York
Wetzel R, Becker M, Behlke J et al (1980) Temperature behaviour of human serum albumin. Eur J Biochem 104:469–478
Wang H, Levin CS, Halas NJ (2005) Nanosphere arrays with controlled sub-10-nm gaps as surface-enhanced Raman spectroscopy substrates. J Am Chem Soc 127:14992–14993
Wang M, Jing N, Chou I et al (2007) An optofluidic device for surface enhanced Raman spectroscopy. Lab Chip 7:630–632
Yu NT, Liu CS, O’Shea DC (1972) 34-laser Raman spectroscopy and the conformation of insulin and proinsulin. J Mol Biol 70:117–132
Zeman EJ, Schatz GC (1987) An accurate electromagnetic theory study of surface enhancement factors for silver, gold, copper, lithium, sodium, aluminum, gallium, indium, zinc, and cadmium. J Phys Chem 91:634–643
Zhao H, Yuan B, Dou X (2004) The effects of electrostatic interaction between biological molecules and nano-metal colloid on near-infrared surface-enhanced Raman scattering. J Opt A Pure Appl Opt 6:900–905
Acknowledgments
The fabrication work of this project was performed at the Cornell NanoScale Facility, a member of the National Nanotechnology Infrastructure Network, which is supported by the National Science Foundation (Grant ECS-0335765). The Texas A&M University Materials Characterization Facility was used to obtain the SERS spectra. The Raman spectra acquisition was supported by the National Science Foundation under Grant No. BES-0421409.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, M., Benford, M., Jing, N. et al. Optofluidic device for ultra-sensitive detection of proteins using surface-enhanced Raman spectroscopy. Microfluid Nanofluid 6, 411–417 (2009). https://doi.org/10.1007/s10404-008-0397-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10404-008-0397-y