Abstract
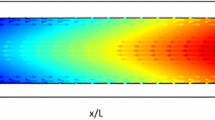
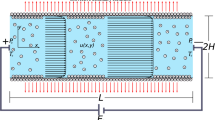
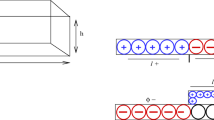
A comprehensive model of electrokinetic flow and transport of electrolytes in microchannels with conductivity gradients is developed. The electrical potential is modeled by a combination of an electrostatic and an electrodynamic approach. The fluid dynamics are described by the Navier–Stokes equations, extended by an electrical force term. The chemistry of the system is represented by source terms in the mass transport equations, derived from an equilibrium approach. Moreover, the interaction between ionic species concentration and physicochemical properties of the microchannel substrate (i.e. zeta-potential) is taken into consideration by an empirical approach. Approximate analytical solutions for all variables are found which are valid within the electrical double layer. By using the method of matched asymptotic expansions, these solutions provide boundary conditions for the numerical simulation of the bulk liquid. The models are implemented in a Finite-Element-Code. As an example, simulations of an electrophoretic injection/separation process in a micro-electrophoresis device are performed. The results of the simulations show the strong coupling between the involved physicochemical phenomena. Simulations with a constant and a concentration-depend zeta-potential clarify the importance of a proper modeling of the physicochemical substrate characteristics.











Similar content being viewed by others
References
Barz D, Ehrhard P (2005) Model and verification of electrokinetic flow and transport in a micro electrophoresis device. Lab Chip 5:949–958
Barz D, Vogel M, Steen P (2008) Determination of the zeta potential of porous substrates by droplet deflection: I. The influence of ionic strength and pH value of an aqueous electrolyte in contact with a borosilicate surface. Langmuir (in press)
Bhattacharyya S, Zheng Z, Conlisk A (2005) Electro-osmotic flow in two-dimensional charged micro- and nanochannels. J Fluid Mech 540:247–267
Conlisk A, McFerran J, Zheng Z, Hansford D (2002) Mass transfer and flow in electrically charged micro- and nanochannels. Anal Chem 74:2139–2150
Crambach A, Dunn M, Snyder R (1998) Theory of electrophoretic transport and separations. Verlag Chemie, Weinheim
Debye P, Hückel E (1923) Zur Theorie der Elektrolyte. I. Gefrierpunktserniedrigung und verwandte Erscheinungen. Phys Z 24:185–206
Erickson D, Li D (2002) Microchannel flow with patchwise and periodic surface heterogeneity. Langmuir 18:8949–8959
Fu L-M, Yang R-J, Lee G-B, Pan Y-J (2003) Multiple injection techniques for microfluidic sample handling. Electrophoresis 24:3026–3032
Gascoyne P, Vykoukal J (2002) Particle separation by dielectrophoresis. Electrophoresis 23:1973–1983
Grossman P, Colburn J (1992) Capillary electrophoresis: theory and practice. Academic Press, San Diego
Guber A, Heckele M, Herrmann D, Muslija A, Saile V (2004) Microfluidic lab-on-a-chip-systems based on polymers-fabrication and application. Chem Eng J 101:447–453
Hirokawa T, Kiso Y (1983) Complex-forming equilibria in isotachophoresis. 3. Estimation of optimum separation conditions of several organic acids by means of computer simulations. J Chromatogr 257:197–210
Hunter R (1981) Zeta potential in colloid science: principles and applications. Academic Press, London
Jung B, Zhu Y, Santiago J (2007) Detection of 100 am fluorophores using a high sensitivity on-chip ce system and transient isotachophoresis. Anal Chem 79:345–349
Kim M, Beskok A, Kihm K (2002) Electro-osmosis-driven micro-channel flows: a comparative study of microscopic particle image velocimetry measurements and numerical simulations. Exp Fluids 33:170–180
Kirby B, Hasselbrink E Jr (2004a) Zeta potential of microfluidic substrates: 1. Theory, experimental techniques and effects on separations. Electrophoresis 25:187–202
Kirby B, Hasselbrink E Jr (2004b) Zeta potential of microfluidic substrates: 2. Data for polymers. Electrophoresis 25:203–213
Kohlrausch F (1897) Über Concentrations-Verschiebungen durch Electrolyse von Lösungen und Lösungsgemischen. Ann Phys 62:209
Laser D, Santiago J (2004) A review of micropumps. J Micromech Microeng 14:R35–R64
Lide DE (1998) Handbook of chemistry and physics. CRC Press, Boca Raton
Lin H, Storey B, Oddy M, Chuan-Hua C, Santiago J (2004) Instability of electrokinetic flows with conductivity gradients. Phys Fluids 16:1922–1935
MacInnes J, Du X, Allen R (2003) Prediction of electrokinetic and pressure flow in a microchannel T-junction. Phys Fluids 15:1992–2005
Manz A, Graber N, Widmer H (1990) Miniaturized total chemical analysis systems: A novel concept for chemical sensing. Sens Actuators B 1:244–248
Meisel I, Ehrhard P (2006) Electrically-excited (electroosmotic) flows in microchannels for mixing applications. Eur J Mech B/Fluids 25:491–501
Mühlberger H, Hwang W, Guber A, Saile V, Hoffmann W (2008) Polymer lab-on-a-chip system with electrical detection. IEEE Sens 2008 (in press)
Osbourn D, Weiss D, Lunte C (2000) Online preconcentration methods for capillary electrophoresis. Electrophoresis 21:2768–2779
Patankar N, Hu H (1998) Numerical simulation of electroosmotic flow. Anal Chem 70:1870–1881
Ren C, Li D (2006) Sample transport control in a microchannel with spatial electrical conductivity gradients. J Colloid & Interface Sci 294:482–491
Saville D, Palusinski O (1986) Theory of electrophoretic separations. AIChE J 32:207–214
Scales P, Grieser F, Healy T (1992) Electrokinetics of the silica-solution interface: a flat plate streaming potential study. Langmuir 8:965–974
Sinton D, Ren L, Xuan X, Li D (2003) Liquid conductivity differences in microfluidic chips: injection, pumping and stacking. Lab Chip 3:173–179
Smoluchowski Mv (1903) Contribution à la théorie de l’endosmose électrique et de quelques phenoménes corrélatifs. Bull Int Acad Sci Cracovie 8:182–200
Tanyanyiwa J, Abad-Villar E, Fernández-Abedul T, Costa-Garcia A., Hoffmann W, Guber A, Herrmann D, Gerlach A, Gottschliche N, Hauser P (2003) High-voltage contactless conductivity-detection for lab-on-chip devices using external electrodes on the holder. Analyst 128:1019–1023
Tsai C-H, Yang R-J, Tai C, Fu L-M (2005) Numerical simulation of electrokinetic injection techniques in capillary electrophoresis microchips. Electrophoresis 26:674–686
van Dyke M (1975) Perturbation methods in fluid mechanics. The Parabolic Press, Stanford
Wainright A, Williams S, Ciambrone G, Harris D (2002) Sample pre-concentration by isotachophoresis in microfluidic devices. J Chromatogr A 979:69–80
Acknowledgments
The author would like to thank Brenton Cox and Leo Bühler for their useful suggestions. This work was generously supported by the Nano- and Microsystems program of the Helmholtz Association of German Research Centres.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
One result of the present article is that under certain conditions migration does not influence the concentration field of an electrolyte, which was first proposed by Kohlrausch (1897). Let us consider a channel filled with a liquid containing the positively and negatively charged species A+ and B−, respectively. The electrolytes in the left part (index l) of the channel have the concentration \(c_{{\rm A^+,l}}, c_{{\rm B^-,l}} ,\) whereas the electrolytes \( (c_{{\rm A^+},r}, c_{{\rm B}^-,r})\) in the right part are diluted with the factor n.
The migration of an electrolyte species can also be interpreted as a partial current density
The overall current density is the sum of the partial current densities, that is also expressed by Ohm’s law, i.e.
Hence, the electrical potential gradient can be expressed as
Relation (39) is inserted into the species transport equation 26. If the electrophoretic mobility is constant and charge conversation \(\nabla \cdot \vec{j}=0\) is accounted for, we obtain
When we neglect the concentration gradient between the undiluted and diluted liquid, the ratio of the concentrations (conductivities) within the left channel part to the concentrations (conductivities) within the right channel part corresponds everywhere to n, i.e.
Consequently, the ratio of species concentration to the conductivity is constant at a fixed location too, we have
Inserting this statement in Eq. 40 reveals that the migration term vanishes and the concentration field is influenced by convection and diffusion only. Referring to Sect. 4.1, the shown derivation explains that for every buffer electrolyte species the same qualitative concentration field is found.
Rights and permissions
About this article
Cite this article
Barz, D.P.J. Comprehensive model of electrokinetic flow and migration in microchannels with conductivity gradients. Microfluid Nanofluid 7, 249–265 (2009). https://doi.org/10.1007/s10404-008-0382-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10404-008-0382-5