Abstract
This study was designed to test the effect of recombinant tissue-type plasminogen activator (rt-PA) in reducing adhesion formation and to observe its influence on peritoneal neoangiogenesis. In 20 Wistar rats, a 4-cm midline incision was made, and a square piece of Silastic, 0.5×0.5 cm and 0.2 mm thick, was fixed on the right side of the peritoneum with two separate angular stitches of nylon 9/O. The rats were randomized into two groups of 10 animals each. In the first group we injected 0.2 mg of rt-PA intraperitoneally three times a day. The second group of 10 rats was used as a control group. Each rat was reoperated on day 12. Intraperitoneal injection of rt-PA seemed not to affect adhesion formation, as a 100% adhesion rate was reported in the treated group compared with 90% of the control group. The results showed that rt-PA acts on the neoangiogenesis involved in postsurgical adhesion formation by reducing the size and length of the vessels. This action seems to slow down peritoneal healing with a negative effect on postsurgical adhesion prevention.
Similar content being viewed by others
Introduction
The fibrinolytic activity of the peritoneum is related to plasminogen activator activity, which is present in both mesothelial and submesothelial blood vessels. Plasminogen activator activates plasmin to process fibrin, with release of split products and reabsorption of fibrous adhesions [1].
Massive adhesions occur when the fibrinolytic system is decreased by at least 50% [2]. Decreased plasminogen activator activity and a relatively impaired fibrinolytic [3, 4] system in peritoneal areas where there is a permanent ischemic stimulus induced by suturing does not succeed in removing fibrin. Fibrin acts as glue within the peritoneal cavity, inducing postsurgical adhesions [5].
Several preventive measures, such as using less invasive techniques and applying surgical adjuvants with the intent of separating peritoneal surfaces or improving the fibrinolytic system, have been employed by surgeons to minimize adhesion formation [6]. Various absorbable and nonabsorbable barrier adjuvants, such as Interceed, Preclude, Seprafilm, and Intergel [7–10], have been used to reduce postsurgical adhesion formation. Adept, a 4% icodextrin solution, has recently shown to be very promising in reducing postsurgical adhesions by means of a hydroflotation effect [11]. Despite progress made in preventing adhesion formation, the solution has yet to be reached.
Recombinant tissue-type plasminogen activator (rt-PA) has proven to be an effective inhibitor of adhesion formation when applied locally, in a gel formulation, in a rabbit experimental model [12]. The aim of the present study was to assess the influence of rt-PA on the mechanism that leads to postsurgical adhesion formation. We designed this study to evaluate whether rt-PA injected directly intraperitoneally can reduce adhesion formation, and to observe its influence on peritoneal neoangiogenesis.
Methods
Twenty Wistar [13] rats weighing 250–300 g were used. The rats were anesthetized with an intramuscular injection of 0.3 ml Hypnorm (fluanisone and phentanylcitrate). The abdominal cavity was opened through a 4-cm midline incision using a clean operative, but not strictly aseptic, technique. Using a steel spatula, we everted the abdominal wall, exposing the right side of the peritoneum. In this area, 1 cm lateral to the epigastric artery, we fixed a square piece of Silastic, 0.5×0.5 cm and 0.2 mm thick, with two separate angular stitches of nylon 9/O.
Each operation was performed using an operative microscope, the Zeiss OPMI 6 or 7 (Zeiss Belgium), fitted with a 200-mm focal length lens and ×12 eyepieces and 160-mm binocular tubes. This electrically-foot-controlled zoom microscope provided a magnification between 8× and 25×. The Silicon used was Silastic sheeting, nonreinforced 500–3 (Dow Corning Medical Products, Midland, MI, USA). The open peritoneum time was approximately 10 min for each animal. The abdominal wall was sutured in double layers, with separate stitches for the musculoperitoneum and a continuous Vicryl 3/0 stitch for the skin.
The rats were randomized into two groups of 10 animals each. In the first group, group A, we injected 0.2 mg of rt-PA intraperitoneally (Genentech, South San Francisco, CA, USA) three times a day (8 a.m., 4 p.m., and 12 p.m.) for 12 days to keep the drug blood levels high. The second group of 10 rats, group B, was used as a control group. Each rat was reoperated on day 12.
Inspection was performed through eversion of the abdominal wall exposing the peritoneal area where the piece of Silastic was fixed in order to observe the development of adhesions. Photographs were obtained at prefixed magnifications of 10×, 15×, 20×, and 25× using a tungsten film of 170 ASA pushed at 320 ASA during the development process. The camera automatically performed three exposures, one after the other, for each single shot. For the first slide, the shutter's opening time was automatically calculated by the camera according to the available light. The following two slides were respectively over- and underexposed automatically, avoiding any mistake. Instead of the direct light of the microscope, a special system of transillumination placed under the rat's everted skin was used. This kind of illumination allowed clear slides with no shining effect plus a good view of the vessels. On the side of each patch of Silastic, a millimetrated grid was placed. This allowed measurements to 0.1 mm. The degree of adhesions was scored by our modified classification of Diamond (Table 1) and DeCherney [14]. Omentoparietal adhesions to the piece of Silastic were scored according to their tenacity, type, and extent. The extent was measured by the percentage of Silastic surface covered by adhesions; this was possible by the two-dimensional structure of our model.Adhesions lysed with traction.
We studied the peritoneal neoangiogenesis by estimating the percentage of rats with a measurable vascularization in order to quantify the phenomenon. The selection was performed choosing the rats that showed rows of parallel vessels with measurable length and diameter on the peritoneum around the Silastic. The mean of each parameter was considered. Concerning the length of vessels, we observed the distance from the edge to the center of the Silastic and measured the longest vessel for each animal. The criteria for large vessels were a diameter of 50–100 μ, and for small vessels a diameter <50 μ.
Statistical analysis
Statistical analysis was performed using a two-tailed Fisher’s exact test and t-test. Differences between groups were considered statistically significant at p<0.05. The Statistica version 5.0 (StatSoft, Tulsa, OK, USA) statistical software package was used.
Results
Type, tenacity, and extent of adhesions
During the experiment three animals in group A (rt-PA) were excluded (on day 2) because of bleeding from the abdominal midline incision.
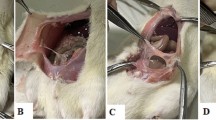
On day 12, 100% (seven) of the animals in group A (rt-PA) showed adhesions, and only 10% (one) of the animals in group B had no adhesions on the Silastic plate (Fig. 1). This difference is not statistically relevant (p=1).
Fifty-seven percent (four) of the animals in group A (rt-PA) and 100% (nine) of those in group B had dense, vascular adhesions with large vessels that required sharp dissection and that covered more than 50% of the Silastic surface (Figs. 2, 3 and 4). This difference showed borderline statistical significance (p=0.06).
Forty-three percent (three) of the animals in group A (rt-PA) had dense, vascular adhesions with small vessels that covered between 0 and 25% of the Silastic surface. In 28% (two) of the rats with dense vascular adhesions with small vessels, we found adhesions that essentially fell apart, and in 15% (one rat) we found adhesions that were lysed with traction.
No animal in group B showed the same type of adhesion. This difference is of borderline statistical relevance (p=0.06).
Peritoneal angiogenesis
Peritoneal neoangiogenesis was present in 100% (seven) of the animals in group A (rt-PA) and in 100% (nine) of those in group B (Figs. 5, 6 and 7).
Peritoneal neoangiogenesis in the rTPA group on day 12. Rows of straight parallels vessels going toward the center of the silastic patch are clearly visible over the silastic surface. The vessels do not reach the center of the silastic patch where is present a large avascularized area (Magnification x 15)
Peritoneal neoangiogenesis in the rTPA group on day 12. Same case as Fig. 5 at larger magnification (Magnification x 20)
Fifty-seven percent (four) of the animals in group A (rt-PA) and 77% (seven) of the animals in group B showed measurable vascular parameters (Fig. 8). This difference was not statistically significant (p=0.64).
The mean length of the vessels in group A (rt-PA) was 1.2 mm, compared with 2.5 mm in group B (Fig. 9).
Discussion
The bidimensional structure of the rat adhesion model that we used in the present study allows a quantitative evaluation of the mechanisms that take part in repairing a postsurgical trauma. While omentoparietal adhesions vascularize the Silastic surface, a new peritoneal tissue with its vascular network grows and covers the traumatized area [13].
Despite 30% of the animals treated with rt-PA being excluded from the study because of massive abdominal bleeding, accurate observation was possible for the rest of the group. Although the small number of rats in the treated group prevents us from reaching definitive conclusions, some interesting remarks and speculations can be made.
Intraperitoneal injection of rt-PA seems not to affect adhesion formation, as a 100% adhesion rate (seven rats) was reported in the treated group compared with 90% (nine rats) in the control group, although we noted a reduction of adhesions that required sharp dissection (100% in the control group vs. 57% in the rt-PA group; p=0.06).
It is remarkable that in 43% (three) of the treated rats in which adhesions did not require sharp dissection [adhesions were lysed with traction in one rat (15%), and adhesions spontaneously fell apart in two rats (28%)], we found small vessels, compared with the control group in which only dense adhesions with large vessels were reported (p=0.06).
In addition, in these 43% (three) of rats, we found a reduction of the Silastic surface that was covered by adhesions (between 0 and 25%), and it was possible to observe and study the rats' peritoneal healing. The mean length of the vessels measured in the treated animals was 1.2 mm, compared with 2.5 mm in the control group.
These data seem to indicate that rt-PA acts on the neoangiogenesis involved in postsurgical adhesion formation by reducing the size and length of the vessels. Unfortunately, this action is only partially effective regarding adhesion formation and seems to slow down peritoneal healing with a negative effect on postsurgical adhesion prevention. In fact, to have a perfect repair after a surgical trauma within the peritoneal cavity, we should find a medical treatment that can speed up peritoneal healing in order to prevent adhesion organization.
Our data do not confirm those of a previous study in a rabbit adhesion model in which rt-PA given intraperitoneally was shown to be effective in reducing adhesion formation at 7 days postoperatively [15]. Other studies have shown reduced adhesion formation when rt-PA was applied locally in a gel formulation, with no bleeding complications [5, 12].
The observation that comes out of our data is that rt-PA is probably effective in adhesion prevention when applied locally but not when given intraperitoneally. In addition, we must take into account our bleeding complications, which were not reported in the previous studies with rt-PA and that always must be considered when dealing with this type of drug. Further clinical trials need to be planned to test the real effect of this drug on peritoneal healing and adhesion prevention.
References
Buckman RF (1976) A unifying pathogenetic mechanism in the etiology of intraperitoneal adhesions. J Surg Res 20:1–5
Gervin AS (1973) Serosal hypofibrinolysis. A cause of post operative adhesions. Am J Surg 125:80–88
Buckman RF (1976) A physiologic basis for the adhesions free healing of deperitonealised surfaces. J Surg Res 21:67–76
Raftery AT (1981) Effect of peritoneal trauma on peritoneal fibrinolytic activity and intraperitoneal adhesion formation. Eur Surg Res 13:397–401
Doddy KJ (1989) Recombinant tissue plasminogen activator reduces adhesion formation in a rabbit uterine horn model. Fertil Steril 51:509–512
di Zerega GS et al (1997) Use of adhesion prevention barriers in pelvic reconstructive and gynaecologic surgery in pelvic surgery. Adhesion formation and prevention. Springer-Verlag, Berlin Heidelberg New York, pp 188–209
Interceed (TC7) Adhesion Barrier Study Group (1989) Prevention of postsurgical adhesion by interceed, an absorbable adhesion barrier: a prospective randomised multicentre clinical study. Fertil Steril 51:933–938
Surgical Membrane Study Group (1992) Prophylaxis of pelvic sidewall adhesion formation with Gore-Tex Surgical Membrane—a multicenter clinical investigation. Fertil Steril 57:921–923
Diamond MP, the Seprafilm Study Group (1992) Reduction of adhesions after uterine myomectomy by Seprafilm membrane (HAL-F): a blinded, prospective, randomised, multicentre clinical study. Fertil Steril 66:904–910
Johns DB, the INTERGEL International Adhesions Study Group (1999) Clinical evaluation of INTERGEL adhesion prevention solution for the reduction of adhesion following peritoneal cavity surgery. Fertil Steril 72(Suppl 1):S57
di Zerega GS et al (2002) A randomized controlled pilot study of the safety and efficacy of 4% icodextrin solution in the reduction of adhesion following laparoscopic gynaecological surgery. Hum Reprod 17:1031–1038
Menzies D, Ellis H (1989) Intra-abdominal adhesion and their prevention by topical tissue plasminogen activator. Royal Soc Med 82:534–535
Bigatti G, Boeckx W et al (1995) Experimental model for neoangiogenesis in adhesions formation. Hum Reprod 10:2290–2294
Diamond MP, DeCherney AH (1987) Pathogenesis of adhesion formation/reformation. Application to reproductive pelvic surgery. Microsurgery 8:103–107
Dorr PJ (1990) Prevention of postoperative adhesions by tissue-type plasminogen activator (t-PA) in the rabbit. Eur J Obstet Gynecol Reprod Biol 37:287–291
Acknowledgements
We would like to thank Prof. W. Costantini, who allowed cooperation between the University of Milan and the University of Leuven. In addition, we wish to thank Mr. I. Laermans, who, with his skill and experience, helped solve all practical problems.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bigatti, G., Segers, N., Boeckx, W. et al. Evaluation of recombinant tissue-type plasminogen activator in adhesion prevention and neoangiogenesis in a rat experimental adhesion model. Gynecol Surg 3, 175–179 (2006). https://doi.org/10.1007/s10397-005-0170-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10397-005-0170-0