Abstract
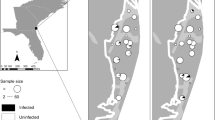
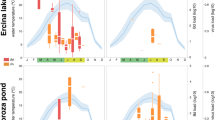
Three infectious pathogens Batrachochytrium dendrobatidis (Bd), Ranavirus (Rv) and Perkinsea (Pr) are associated with widespread and ongoing amphibian population declines. Although their geographic and host ranges vary widely, recent studies have suggested that the occurrence of these pathogens could be more common than previously thought, even in direct-developing terrestrial species traditionally considered less likely to harbor these largely aquatic pathogens. Here, we characterize Bd, Rv, and Pr infections in direct-developing terrestrial amphibians of the Pristimantis genus from the highland Ecuadorean Andes. We confirm the first detection of Pr in terrestrial-breeding amphibians and in the Andean region, present the first report of Rv in Ecuador, and we add to the handful of studies finding Bd infecting Pristimantis. Infection prevalence did not differ significantly among pathogens, but infection intensity was significantly higher for Bd compared to Pr. Neither prevalence nor intensity differed significantly across locality and elevation for Bd and Rv, although low prevalence in our dataset and lack of seasonal sampling could have prevented important epidemiological patterns from emerging. Our study highlights the importance of incorporating pathogen surveillance in biodiversity monitoring in the Andean region and serves as starting point to understand pathogen dynamics, transmission, and impacts in terrestrial-breeding frogs.




Similar content being viewed by others
References
Aguirre AA, Lampo M (2006) Protocolo de bioseguridad y cuarentena para prevenir la transmission de enfermedades en anfibios. In: Técnicas de Inventario y Monitoreo para los Anfibios de la Región Tropical Andina, Angulo A, Rueda-Almonacid JV, Rodriguez-Mahecha JV, LaMarca E (editors), Bogota, Colombia; Conservacion Internacional, pp 73–92
Allender MC, Bunick D, Mitchell MA (2013) Development and validation of TaqMan quantitative PCR for detection of frog virus 3-like virus in eastern box turtles (Terrapene carolina carolina). Journal of Virological Methods 188:121–125. https://doi.org/10.1016/j.jviromet.2012.12.012
Ayres C. Acevedo I, Monsalve-Carcaño C, Thumsova B, Bosch J (2020). Triple dermocystid-chytrid fungus-ranavirus co-infection in a Lissotriton helveticus. European Journal of Wildlife Research 66:41. https://doi.org/10.1007/s10344-020-01381-2
Becker CG, Bletz MC, Greenspan SE, Rodriguez D, Lambertini C, Jenkinson TS, et al. (2019). Low-load pathogen spillover predicts shifts in skin microbiome and survival of a terrestrial-breeding amphibian. Proceedings of the Royal Society B Biological Sciences 286:20191114.https://doi.org/10.1098/rspb.2019.1114
Bresciano JC, Salvador CA, Pazy Miño C, Parody-Merino AM, Bosch J, Woodhams DC (2015) Variation in the Presence of Anti-Batrachochytrium dendrobatidis Bacteria of Amphibians Across Life Stages and Elevations in Ecuador. EcoHealth 12:310–319. https://doi.org/10.1007/s10393-015-1010-y
Bolker B (2020). bbmle: Tools for General Maximum Likelihood Estimation. R package version 1.0.23.1. https://CRAN.R-project.org/package=bbmle.
Bosch J, Monsalve-Carcaño C, Price SJ, Bielby J (2020) Single infection with Batrachochytrium dendrobatidis or Ranavirus does not increase probability of co-infection in a montane community of amphibians. Scientific reports 10:21115.https://doi.org/10.1038/s41598-020-78196-3
Boyle DG, Boyle DB, Olsen V, Morgan JAT, Hyatt AD (2004) Rapid quantitative detection of chytridiomycosis (Batrachochytrium dendrobatidis) in amphibian samples using real-time Taqman PCR assay. Diseases of Aquatic Organisms 60:141–148. https://doi.org/10.3354/dao060141
Brannelly LA, Richards-Zawacki CL, Pessier AP (2012) Clinical trials with itraconazole as a treatment for chytrid fungal infections in amphibians. Diseases of Aquatic Organisms 101:95–104. https://doi.org/10.3354/dao02521
Burns TJ, Scheele BC, Brannelly LA, Clemann N, Gilbert D, Driscoll DA (2021). Indirect terrestrial transmission of amphibian chytrid fungus from reservoir to susceptible host species leads to fatal chytridiomycosis. Animal Conservation 24(4):602–612; https://doi.org/10.1111/acv.12665.
Caceres-Andrade JF (2014) Analisis exploratorio de datos para desarrollar propuestas de conservacion de la comunidad de Anfibios referents al Batrachochytrium dendrobatidis en el Parque Nacional Cajas, Cuenca-Ecuador. Thesis [Universidad del Azuay]
Candido M, Tavares LS, Alencar ALF, Ferreira CM, de Almeida Queiroz SR, Fernandes AM, et al.(2019) Genome analysis of Ranavirus frog virus 3 isolated from American Bullfrog (Lithobates catesbeianus) in South America. Scientific reports 9:17135.https://doi.org/10.1038/s41598-019-53626-z
Catenazzi A, Lehr E, Rodriguez LO, Vredenburg VT (2011) Batrachochytrium dendrobatidis and the collapse of anuran species richness and abundance in the upper Manu National Park, southeastern Peru. Conservation Biology 25:382–391. https://doi.org/10.1111/j.1523-1739.2010.01604.x
Catenazzi A, Lehr E, Vrendenburg VT (2013) Thermal Physiology, Disease, and Amphibian Declines on the Eastern Slopes of the Andes. Conservation Biology. https://doi.org/10.1111/cobi.12194
Campos-Cerqueira M, Aide TM (2017) Lowland extirpation of anuran populations on a tropical mountain. Peer J 5:e4059.https://doi.org/10.7717/peerj.4059
Chambouvet A, Gower DJ, Jirku M, Yabsley MJ, Davis AK, Leonard G, et al. (2015) Cryptic infection of a broad taxonomic and geographic diversity of tadpoles by Perkinsea protists. Proceedings of the National Academy of Sciences E4743–E4751.https://doi.org/10.1073/pnas.1500163112
Chinchar VG (2002) Ranaviruses (family Iridoviridae): emerging cold-blooded killers. Archives of virology 147(3):447–470.https://doi.org/10.1007/s007050200000
Cook JO (2008) Transmission and Occurence of Dermomycoides sp. in Rana sevosa and Other Ranids in the North Central Gulf of Mexico States. Master’s thesis (University of Southern Mississippi).
Coloma L, Lotters AS, Salas AW (2000) Taxonomy of the Atelopus ignescens complex (Anura: Bufonidae): designation of a neotype of Atelopus ignescens and recognition of Atelopus exiguus. Herpetologica 56:303–324
Crowl TA, Crist TO, Parmenter RR, Belovsky G, Lugo AE (2008) The spread of invasive species and infectious disease as drivers of ecosystem change. Frontiers in Ecology and the Environment 6:238–246. https://doi.org/10.1890/070151
Daszak P, Cunningham AA, Hyatt AD (2000) Emerging Infectious Diseases of Wildlife — Threats to biodiversity and human health. Science 287:443–449. https://doi.org/10.1126/science.287.5452.443
Davis AK, Yabsley MJ, Keel MK, Maerz JC (2007) Discovery of a novel alveolate pathogen affecting southern leopard frogs in Georgia: description of the disease and host effects. EcoHealth 4(3):310–317.https://doi.org/10.1007/s10393-007-0115-3
DiRenzo GV, Campbell Grant EH (2019) Overview of emerging amphibian pathogens and modeling advances for conservation-related decisions. Biological Conservation 236:474–483. https://doi.org/10.1016/j.biocon.2019.05.034
Fick SE, Hijmans RJ (2017) WorldClim 2: New 1-Km Spatial Resolution Climate Surfaces for Global Land Areas. International Journal of Climatology, 37:4302–4315; DOI:
Fox SF, Greer AL, Torres-Cervantes R, Collins JP (2006) First case of Ranavirus-associated morbidity and mortality in natural populations of the South American frog Atelognathus patagonicus. Diseases of aquatic organisms 72(1):87–92.https://doi.org/10.3354/dao072087
Galli L, Pereira A, Márquez A, Mazzoni R (2006) Ranavirus detection by PCR in cultured tadpoles (Rana catesbeiana Shaw, 1802) from South America. Aquaculture 257(1–4):78–82.https://doi.org/10.1016/j.aquaculture.2005.06.019
Galt N, Atkinson MS, Glorioso B, Waddle H, Litton M, Savage AE (2021) Widespread Ranavirus and Perkinsea infections in Cuban treefrogs (Osteopilus septentrionalis) invading New Orleans, USA. Herpetological Conservation and Biology 16(1):17–29
Gervasi S, Gondhalekar C, Olson DH, Blaustein AR (2013) Host Identity Matters in the Amphibian-Batrachochytrium dendrobatidis System: Fine-Scale Patterns of Variation in Responses to a Multi-Host Pathogen. PlosOne 8(1):e54490.https://doi.org/10.1371/journal.pone.0054490
Guayasamin JM, Mendoza AM, Longo AV, Zamudio KR, Bonaccorso E (2014) High prevalence of Batrachochytrium dendrobatidis in an Andean frog community (Reserva Las Gralarias, Ecuador). Amphibian and Reptile Conservation 8(1):33–44
Hedges SB, Duellman WE, Heinicke MP (2008) New World direct-developing frogs (Anura: Terrarana): molecular phylogeny, classification, biogeography, and conservation. Zootaxa 1737:1–182. https://doi.org/10.11646/zootaxa.3986.2.1
Hauselberger KF, Alford RA (2012) Prevalence of Batrachochytrium dendrobatidis infection is extremely low in direct-developing Australian microhylids. Diseases of Aquatic Organisms 100:191–200. https://doi.org/10.3354/dao02494
Hyatt AD, Boyle DG, Olsen V, Boyle DB et al (2007) Diagnostic assays and sampling protocols for the detection of Batrachochytrium dendrobatidis. Disseases of Aquatic Organisms 73:175–192
Isidoro-Ayza, M, Lorch JM, Grear DA, Winzeler M, Calhoun DL, Barichivich WJ (2017) Pathogenic lineage of Perkinsea associated with mass mortality of frogs across the United States. Scientific reports 7:10288.https://doi.org/10.1038/s41598-017-10456-1
Jancovich JK, Davidson EW, Parameswaran N, Mao J, Chinchar VG, Collins JP, Jacobs BL, Storfer A (2005) Evidence for emergence of an amphibian iridoviraldisease because of human-enhanced spread. Molecular Ecology 14:213–224. https://doi.org/10.1111/j.1365-294X.2004.02387.x
Karvemo S, Meurling S, Berger D, Laurila A (2018) Effects of host species and environmental factors on the prevalence of Batrachochytrium dendrobatidis in northern Europe. PLoS One 13(10):e0199852.https://doi.org/10.1371/journal.pone.0199852
Karwacki EE, Atkinson MS, Ossiboff RJ, Savage AE (2018) Novel quantitative PCR assay specific for the emerging Perkinsea amphibian pathogen reveals seasonal infection dynamics. Diseases of Aquatic Organisms 129:85–98. https://doi.org/10.3354/dao03239
Karwacki EE, Martin KR, Savage AE (2021) One hundred years of infection with three global pathogens in frog populations of Florida, USA. Biological Conservation 257:109088.https://doi.org/10.1016/j.biocon.2021.109088
Kinney VC, Heemeyer JL, Pessier AP, Lannoo MJ (2011) Seasonal Pattern of Batrachochytrium dendrobatidis Infection and Mortality in Lithobates areolatus: Affirmation of Vredenburg's “10,000 Zoospore Rule”. PloS One 6(3):e16708.https://doi.org/10.1371/journal.pone.0016708
Kriger KM, Hero JM (2007) The chytrid fungus Batrachochytrium dendrobatidis is non-randomly distributed across amphibian breeding habitats. Diversity and Ditributions 13:781–788. https://doi.org/10.1111/j.1472-4642.2007.00394.x
Kolby JE, Padgett-Flohr GE, Field R (2010) Amphibian chytrid fungus Batrachochytrium dendrobatidis in Cusuco National Park, Honduras. Diseases of Aquatic Organisms 92:245–251. https://doi.org/10.3354/dao02055
Kolby JE, Ramirez SD, Berger L, Richards-Hrdlicka KL, Jocque M, Skerratt LF (2015) Terrestrial dispersal and potential environ- mental transmission of the amphibian chytrid fungus (Batrachochytrium dendrobatidis). PLoS ONE 10:1–13. https://doi.org/10.1371/journal.pone.0125386
La Marca E, Lips KR, Lotters S, Puschendorf R, Ibanez R, Rueda-Almonacid JV et al (2005) Catastrophic population declines and extinctions in neotropical harlequin frogs (Bufonidae : Atelopus). Biotropica 37:190–201. https://doi.org/10.1111/j.1744-7429.2005.00026.x
Lips KR, Diffendorfer J, Mendelson III JR, Sears MW (2008) Riding the Wave: Reconciling the Roles of Disease and Climate Change in Amphibian Declines. PLoS Biology 6(3):e72.https://doi.org/10.1371/journal.pbio.0060072
Lips KR (2016) Overview of chytrid emergence and impacts on amphibians. Philosophical. Transactions Royal Society B Biological Sciences, 371:20150465.https://doi.org/10.1098/rstb.2015.0465
Longo AV, Burrowes PA, Joglar RL (2010) Seasonality of Batrachochytrium dendrobatidis infection in direct-developing frogs suggests a mechanism for persistence. Diseases of Aquatic Organisms 92:253–260. https://doi.org/10.3354/dao02054
Mao JH, Green DE, Fellers G, Chinchar VG (1999) Molecular characterization of iridoviruses isolated from sympatric amphibians and fish. Virus Res 63:45–52. https://doi.org/10.1016/S0168-1702(99)00057-X
Marsh IB, Whittington RJ, O’Rourke B, Hyatt AD, Chrisholm O (2002) Rapid differentiation of Australian, European and American Ranaviruses based on variation in major capsid protein gene sequence. Mol Cell Probes 16:137–151. https://doi.org/10.1006/mcpr.2001.0400
Martín-Torrijos L, Sandoval-Sierra JV, Muñoz J, Diéguez-Uribeondo J, Bosch J, Guayasamin JM (2016) Rainbow trout (Oncorhynchus mykiss) threaten Andean amphibians. Neotropical Biodiversity 2(1):26–36
Mesquita AFC, Lambertini C, Lyra M, Malagoli LR, James TY, Toledo LF, Haddad CFB, Becker CG (2017) Low resistance to chytridiomycosis in direct-developing amphibians. Scientific Reports 7:16605. https://doi.org/10.1038/s41598-017-16425-y
Ministerio de Ambiente del Ecuador (2012) Sistema de Clasificación de los Ecosistemas del Ecuador Continental. Subsecretaría de Patrimonio Natural, Quito, 143 pp.
Moss AS, Reddy NS, Dorta IM, Francisco MJS (2008) Chemotaxis of the amphibian pathogen Batrachochytrium dendrobatidis and its response to a variety of attractants. Mycologia 100:1–5. https://doi.org/10.1080/15572536.2008.11832493
Mouillet, C., Barta, B., Espinosa, R., Andino, P., Christoffersen, K.S. and Jacobsen, D(2018) Ecological effects of introduced rainbow trout (Oncorhynchus mykiss) in pristine Ecuadorian high Andean lakes. Fundamental and Applied Limnology 191(4):323–337. https://doi.org/10.1127/fal/2018/1154
Ortega-Andrade HM, Rodes Blanco M, Cisneros DF, Guerra Arevalo N, Lopez de Vargas-Machuca KM, Sanchez-Nivicela JC (2021) Red List assessment of amphibian species of Ecuador: A multidimensional approach for their conservation. PLoS ONE 16(5): e0251027. https://doi.org/10.1371/journal.pone.0251027
Paez NB, Ron SR (2019) Systematics of Huicundomantis, a new subgenus of Pristimantis (Anura, Strabomantidae) with extraordinary cryptic diversity and eleven new species. Zookeys 868:1–112. https://doi.org/10.3897/zookeys.868.26766
Piotrowski JS, Annis SL, Longcore JE (2004) Physiology of Batrachochytrium dendrobatidis, a chytrid pathogen of amphibians. Mycologia 96:9–15. https://doi.org/10.1080/15572536.2005.11832990
Price SJ, Ariel E, Maclaine A, Rosa GM, Gray MJ, Brunner JL et al (2017) From fish to frogs and beyond: Impact and host range of emergent ranaviruses. Virology 511:272–279. https://doi.org/10.1016/j.virol.2017.08.001
Price SJ, Garner TWJ, Nichols RA, Balloux F, Ayres C, Mora-Cabello de Alba A, Bosch J (2014) Collapse of amphibian communities due to an Introduced Ranavirus Current Biology 24:2586–2591. https://doi.org/10.1016/j.cub.2014.09.028
R Core Team (2019) R: A Language and environment for statistical computing (Version 3.6.2). R Foundation for Statistical Computing. http://www.R-project.org/
Retallick RWR, Miera V, Richards KL, Field K, Collins JP (2006) A non-lethal technique for detecting the chytrid fungus Batrachochytrium dendrobatidis on tadpoles. Diseases of Aquatic Organisms 72:77–85. https://doi.org/10.3354/dao072077
Rivera B, Cook K, Andrews K, Atkinson MS, Savage AE (2019) Pathogen Dynamics in an Invasive Frog Compared to Native Species. EcoHealth 16:222–234. https://doi.org/10.1007/s10393-019-01432-4
Ron SR, Merino-Viteri A (2000) Amphibian declines in Ecuador: overview and first report of chytridiomycosis from South America. Froglog 42:2–3
Ron SR, Merino-Viteri A, Ortiz DA (2019) Anfibios del Ecuador. Version 2019.0. Museo de Zoología, Pontificia Universidad Católica del Ecuador. https://bioweb.bio/faunaweb/amphibiaweb
Rosa G, Sabino-Pinto J, Laurentino T, Martel A, Pasmans F, Rebelo R, et al. (2017) Impact of asynchronous emergence of two lethal pathogens on amphibian assemblages. Science reports 7:43260. https://doi.org/10.1038/srep43260
Ruggeri J, Longo V, Gaiarsa MP, Alencar LRV, Lambertini C, Leite DS, et al. (2015) Seasonal Variation in Population Abundance and Chytrid Infection in Stream-Dwelling Frogs of the Brazilian Atlantic Forest. PloS One 10(7):e0130554. https://doi.org/10.1371/journal.pone.0130554
Savage AE, Mulder KP, Torres T, Wells S (2018) Lost but not forgotten: MHC genotypes predict overwinter survival despite depauperate MHC diversity in a declining frog. Conservation Genetics 19(2):309–322. https://doi.org/10.1007/s10592-017-1001-3
Scheele BC, Foster CN, Hunter DA, Lindenmayer DB, Heard Schmidt BR, GW, (2019) Living with the enemy: facilitating amphibian coexistence with disease. Biological Conservation 236:52–59. https://doi.org/10.1016/j.biocon.2019.05.032
Schock DM, Bollinger TK, Chinchar G, Jancovich JK, Collins JP (2008) Experimental Evidence that Amphibian Ranaviruses Are Multi-Host Pathogens. Ichtyology & Herpetology 1:133–143. https://doi.org/10.1643/CP-06-134
SIGAGRO Sistema de Informacion Geografica del agro (2002). Mapa de Zonas de Temperatua del Ecuador Continental. Ministerio de Agricultura, Ganaderia, Acuacultura y Pesca.
Smilansky V, Jirků M, Milner DS, Ibáñez R, Gratwicke B, Nicholls A, Lukeš J, Chambouvet A, Richards TA (2021) Expanded host and geographic range of tadpole associations with the Severe Perkinsea Infection group. Biology Letters 17: 20210166. https://doi.org/10.1098/rsbl.2021.0166
Sonn JM, Utz RM, Richards-Zawacki C (2019) Effects of latitudinal, seasonal, and daily temperature variations on chytrid fungal infections in a North American frog. Ecosphere 10(11):e02892. https://doi.org/10.1002/ecs2.2892
Soto-Azat C, Peñafiel-Ricaurte A, Price SJ, Sallaberry N, Garcia MP, Alvarado-Rybak M et al (2016) Xenopus laevis and Emerging Amphibian Pathogens in Chile. EcoHealth 13:775–783. https://doi.org/10.1007/s10393-016-1186-9
Talley BL, Muletz CR, Vredenburg VT, Fleischer RC, Lips KR (2015) A century of Batrachochytrium dendrobatidis in Illinois amphibians (1888–1989). Biological Conservation 182:254–261. https://doi.org/10.1016/j.biocon.2014.12.007
Urgiles VL, Székely P, Székely D, Christodoulides N, Sanchez-Nivicela JC, Savage AE (2019) Genetic delimitation of Pristimantis orestes (Lynch 1979) and P. saturninoi Brito et al., 2017 and the description of two new terrestrial frogs from the Pristimantis orestes species group (Anura, Strabomantidae). ZooKeys 864:111–146. https://doi.org/10.3897/zookeys.864.35102
Valencia-Aguilar A, Toledo LF, Vital MC, Mott T (2016) Seasonality, Environmental Factors, and Host Behavior Linked to Disease Risk in Stream-Dwelling Tadpoles. Herpetologica 72 (2): 98–106. https://doi.org/10.1655/HERPETOLOGICA-D-15-00013
vonMay R, Catenazzi A, Santa-Cruz R (2018) Microhabitat Temperatures and Prevalence of the Pathogenic Fungus Batrachochytrium dendrobatidis in Lowland Amazonian Frogs. Tropical Conservation Science 11:1–13. https://doi.org/10.1177/1940082918797057
Warne, R.W., LaBumbard, B., LaGrange, S., Vredenburg, V.T. and Catenazzi, A(2016). Co-infection by chytrid fungus and Ranaviruses in wild and harvested frogs in the tropical Andes. PLoS One 11(1):e0145864. https://doi.org/10.1371/journal.pone.0145864
Zimkus BM, Balaz V, Belasen AM, Bell RC, Channing A, Doumbia J, et al. (2020) Chytrid Pathogen (Batrachochytrium dendrobatidis) in African Amphibians: A Continental Analysis of Occurrences and Modeling of Its Potential Distribution 76(2):201–215. https://doi.org/10.1655/0018-0831-76.2.201
Acknowledgements
Our field expeditions in Ecuador were supported by Juan C. Sanchez-Nivicela, Paul Szekely, Diana Szekely, Amanda Quezada, Bruno Timbe and Valentina Posse. Boris Tinoco and Rodrigo Caroca provided suggestions for sampling and data management in Ecuador. Yanez Munoz from Instituto Nacional de Biodiversidad del Ecuador facilitated sample exportation to UCF. Emily Karwacki and Matthew Atkinson helped define protocols for laboratory analysis; Katherine Martin provided suggestions for data analysis. We are grateful to Molly C. Womack and Jackson Phillips for providing edits and suggestions in an early version of this manuscript. This study was funded by grants from the National Geographic Society and the Explorers Club awarded to VLU.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Urgiles, V.L., Ramírez, E.R., Villalta, C.I. et al. Three Pathogens Impact Terrestrial Frogs from a High-Elevation Tropical Hotspot. EcoHealth 18, 451–464 (2021). https://doi.org/10.1007/s10393-021-01570-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10393-021-01570-8