Abstract
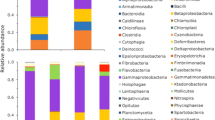
Amphibian populations are decreasing worldwide due to a variety of factors. In South America, the chytrid fungus Batrachochytrium dendrobatidis (Bd) is linked to many population declines. The pathogenic effect of Bd on amphibians can be inhibited by specific bacteria present on host skin. This symbiotic association allows some amphibians to resist the development of the disease chytridiomycosis. Here, we aimed (1) to determine for the first time if specific anti-Bd bacteria are present on amphibians in the Andes of Ecuador, (2) to monitor anti-Bd bacteria across developmental stages in a focal amphibian, the Andean marsupial tree frog, Gastrotheca riobambae, that deposits larvae in aquatic habitats, and (3) to compare the Bd presence associated with host assemblages including 10 species at sites ranging in biogeography from Amazonian rainforest (450 masl) to Andes montane rainforest (3200 masl). We sampled and identified skin-associated bacteria of frogs in the field using swabs and a novel methodology of aerobic counting plates, and a combination of morphological, biochemical, and molecular identification techniques. The following anti-Bd bacteria were identified and found to be shared among several hosts at high-elevation sites where Bd was present at a prevalence of 32.5%: Janthinobacterium lividum, Pseudomonas fluorescens, and Serratia sp. Bd were detected in Gastrotheca spp. and not detected in the lowlands (sites below 1000 masl). In G. riobambae, recognized Bd-resistant bacteria start to be present at the metamorphic stage. Overall bacterial abundance was significantly higher post-metamorphosis and on species sampled at lower elevations. Further metagenomic studies are needed to evaluate the roles of host identity, life-history stage, and biogeography of the microbiota and their function in disease resistance.



Similar content being viewed by others
References
Aranda S, Montes-Borrego M, Landa BB (2011) Purple-pigmented violacein-producing Duganella spp. Inhabit the rhizosphere of wild and cultivated olives in southern Spain. Microbial Ecology 62(2):446–459.
Becker MH, Brucker RM, Schwantes CR, Harris RN, Minbiole KPC (2009) The bacterially produced metabolite violacein is associated with survival of amphibians infected with a lethal fungus. Applied Environmental Microbiology 75:6635–6638.
Becker MH, Harris RN (2010) Cutaneous Bacteria of the Redback Salamander Prevent Morbidity Associated with a Lethal Disease. PLoS One 5:e10957.
Becker MH, Harris RN, Minbiole KP, Schwantes CR, Rollins-Smith LA, Reinert LK, et al. (2011) Towards a better understanding of the use of probiotics for preventing chytridiomycosis in Panamanian golden frogs. Ecohealth 8:501–506.
Benson D, Boguski M, Lipman D, Ostell J, Ouellette B, Rapp B, Wheeler D (1999) Genbank. Nucleic Acids Research 27:12–7.
Berger L, Speare R, Daszak P, Green DE, Cunninham A, Goggin CL, et al. (1998) Chytridiomycosis causes amphibian mortality associated with population declines in the rain forests of Australia and Central America. Proceedings of the National Academy of Sciences USA 95:9031–9036.
Blaustein AR, Romansic JR, Scheessele EA, Han BA, Pessier AP, Longcore JE (2005) Interspecific variation in susceptibility of frog tadpoles to the pathogenic fungus, Batrachochytrium dendrobatidis. Conserv Biology 19:1460–1468.
Bletz M, Loudon A, Becker M, Bell S, Woodhams DC, Minbiole K, Harris R (2013) Mitigating amphibian chytridiomycosis with bioaugmentation: characteristics of effective probiotics and strategies for their selection and use. Ecology letters. doi:10.1111/ele.12099.
Bosch J, Martínez-Solano I, García-París M (2001) Evidence of a chytrid fungus infection involved in the decline of the common midwife toad (Alytes obstretricans) in protected areas of central Spain. Biological Conservation 97:331–337.
Bosch J, Carrascal LM, Durán L, Walker S, Fisher MC (2007) Climate change and outbreaks of amphibian chytridiomycosis in a montane area of Central Spain; is there a link? Proceedings of the Royal Society B: Biological Sciences 274:253–260
Boyle DG, Boyle DB, Olsen V, Morgan, JAT, Hyatt AD (2004) Rapid quantitative detection of chytridiomycosis (Batrachochytrium dendrobatidis) in amphibian samples using the realtime Taqman PCR assay. Diseases of Aquatic Organisms 60:141–148.
Brucker RM, Baylor CM, Walters RL, Lauer A, Harris RN, Minbiole KPC (2008a) The identification of 2,4-diacetylphloroglucinol as an antifungal metabolite produced by cutaneous bacteria of the salamander Plethodon cinereus. Journal of Chemical Ecology 34:39–43.
Brucker RM, Harris RN, Schwantes CR, Gallaher TN, Flaherty DC, Lam BA, Minbiole KPC (2008b) Amphibian chemical defense: antifungal metabolites of the microsymbiont Janthinobacterium lividum on the salamander Plethodon cinereus. Journal of Chemical Ecology 34:1422–1429.
Buchan A, LeCleir GR, Gulvik CA, González JM (2014) Masters recyclers: features and functions associated with phytoplankton blooms. Nature Reviews Microbiology 12:686–698.
Bustamante M, Ron S, Coloma L (2005) Cambios en la diversidad en siete comunidades de anuros en los Andes de Ecuador. Biotropica 37:180–189.
Cole ME, Bustamante MR, Almeida-Reinoso D, Funk WC (2014) Spatial and temporal variation in population dynamics of Andean frogs: Effects of forest disturbances and population declines. Global Ecology and Conservation 1:60–70.
Conlon JM, (2011) The contribution of skin antimicrobial peptides to the system of innate immunity in anurans. Cell Tissue Research 343:201–212.
Crawford AJ, Lips KR, Bermingham E (2010) Epidemic disease decimates amphibian abundance, species diversity, and evolutionary history in the highlands of central Panama. Proceedings of the National Academy of Sciences USA 107:13777–13782.
Crump ML, Scott NJ (1994) Visual encounter surveys. In: Measuring and Monitoring Biological Diversity. Standard Methods for Amphibians, Meyer W, Donnelley MA, McDiarmid RW, Hayek LAC, Foster MS (editors). Washington, DC: Smithsonian Institution Press, pp 84–92.
Fisher MC, Henk DA, Briggs CJ, et al. (2012) Emerging fungal threats to animal, plant and ecosystem health. Nature 484:186–194.
Fierer N, Bradford MA, Jackson RB (2007) Toward an ecological classification of soil bacteria. Ecology 88:1354–1364.
Flechas SV, Sarmiento C, Cárdenas ME, Medina EM, Restrepo S, Amezquita A (2012) A. Surviving chytridiomycosis: differential anti-Batrachochytrium dendrobatidis activity in bacterial isolates from three lowland species of Atelopus. Plos ONE 7(9):e44832. doi:10.1371/journal.pone.0044832.
Funk WC, Caminer M, Ron SR (2012) High levels of cryptic species diversity uncovered in Amazonian frogs. Proceedings of the Royal Society B-Biological Sciences 279:1806–1814. doi:10.1098/rspb.2011.1653.
Gillis M, De Ley J (2006) The genera Chromobacterium and Janthinobacterium. Prokaryotes 5:737–746.
Green DE, Converse KA, Schrader AK (2002) Epizootiology of sixty-four Amphibian morbidity and mortality events in the USA, 1996–2001. Annals of the New York Academy of Sciences 969:323–339.
Hakvåg, S, Fjærvik E, Klinkenberg G, Even FS, Borgos KD, Josefsen, TE, Trond EE, Zotchev ES (2009) Violacein-producing Collimonas sp. from the sea surface microlayer of costal waters in Trøndelag, Norway. Marine Drugs 7:576–588.
Harris RN, James TY, Lauer A, Simon MA, Patel A (2006) Amphibian pathogen Batrachochytrium dendrobatidisis inhibited by the cutaneous bacteria of amphibian species. EcoHealth 3:53–56.
Harris RN, Brucker RM, Walke JB, Becker MH, Schwantes CR, Flaherty DC, et al. (2009) Skin microbes on frogs prevent morbidity and mortality caused by a lethal skin fungus. Journal of International Society for Microbial Ecology 3:818–824.
Jiang P, Wang H, Zhang C, Lou K, Xing X (2010) Reconstruction of the violacein biosynthetic pathway from Duganella sp. B2 in different heterologous hosts. Applied Microbiology and Biotechnology 86(4):1077–1088.
Kiesecker JM, Blaustein AR, Belden LK (2001) Complex causes of amphibian population declines. Nature 410:681–684.
Kilburn VL, Ibáñez R, Sanjur O, Bermingham E, Suraci JP, Green DM (2010) Ubiquity of the pathogenic chytrid fungus, Batrachochytrium dendrobatidis, in anuran communities in Panama. EcoHealth 7(4):537–548.
Kilpatrick AM, Briggs CJ, Daszak P (2010) The ecology and impact of chytridiomycosis: an emerging disease of amphibians. Trends Ecology and Evolution 25:109–118.
Kueneman JG, Parfrey LW, Woodhams DC, Archer HM, Knight R, McKenzie VJ (2013) The amphibian skin-associated microbiome across species, space and life history stages. Molecular Ecology 2014 23(6):1238–1250.
Kriger KM, Hines HB, Hyatt HD, Boyle DG, Hero JM (2006) Techniques for detecting chytridiomycosis in wild frogs: comparing histological with real-time taqman PCR. Disease of Aquatic Organisms 71:141–148.
Manzano Pazquel AL (2010) Prevalencia de quitridiomicosis en la población larvaria de Gastrotheca riobambae del parque Metropolitano de Quito. Facultad de Ingeniería en Biotecnología. ESPE. Sede Sangolquí. Quito, pp. 43–51.
Meyer EA, Cramp RL, Bernal MH, Frankling EC (2012) Changes in cutaneous microbial abundance with sloughing: possible implications for infection and disease in amphibians. Disease of Aquatic Organisms 101:235–242.
Muletz CR, Myers JM, Domangue RJ, Herrick JB, Harris RN (2012) Soil bioaugmentation with amphibian cutaneous bacteria protects amphibian hosts from infection by Batrachochytrium dendrobatidis. Biological Conservation 152:119–126.
Lam BA, Walke JB, Vredenburg VT, Harris RN (2010) Proportion of individual with anti-Batrachochytrium dendrobatidis skin bacteria is associated with population persistence in the frog Rana muscosa. Biological Conservation 143:529–531.
La Marca E, Lips KR, Lötters S, Puschendorf R, Ibáñez R, Rueda-Almonacid JV, et al. (2005) Catastrophic population declines and extinctions in Neotropical harlequin frogs (Bufonidae: Atelopus). Biotropica 37:190–201.
Lane DJ (1991) 16S/23S rRNA sequencing. In: Nucleic Acid Techniques in Bacterial Systematics, Stackebrandt E, Goodfellow M (editors). Chichester, England: John Wiley and Sons, pp. 115–175.
Lauer A, Simon MA, Banning JL, Andre E, Duncan K, Harris RN (2007) Common cutaneous bacteria from the eastern red-backed salamander can inhibit pathogenic fungi. Copeia 3:630–640.
Lauer A, Simon MA, Banning JL, Lam BA, Harris RN (2008) Diversity of cutaneous bacteria with antifungal activity isolated from female four-toed salamanders. Journal of International Society for Microbial Ecology 2:145–157.
Lips KR, Burrowes PA, Mendelson JR, Parra-Olea G (2005) Amphibian declines in Latin America: a synthesis. Biotropica 37:222–228.
Logan NA (1989) Numerical taxonomy of violet-pigmented, gram-negative bacteria and description of Iodobacter fluviatile gen. nov., comb. nov. International Journal of Systematic Bacteriology 39:450–456.
Ramirez S, Rodriguez B (2012) Estado poblacional y relaciones ecológicas de Gastrotheca riobambae (Anura: Hemiphractidae) en dos localidades del volcán Pasochoa, Pichincha—Ecuador. Grupo de biodiversidad IASA Bol. Téc. 10 Serie Zoologica 7:69–97.
Retallick RWR, Miera V, Richards KL, Field KJ, Collins JP (2006) A non-lethal technique for detecting the chytrid fungus Batrachochytrium dendrobatidis on tadpoles. Diseases of Aquatic Organisms 72:77–85
Rollins-Smith LA, Woodhams DC (2012) Amphibian immunity: staying in tune with the environment. In: Ecoimmunology, Demas GE, Nelson RJ (editors), New York: Oxford University Press, Chap. 4, pp. 92–143
Ron SR, Merino-Vitteri A (2000) Amphibian declines in Ecuador: overview and first report of chytridiomycosis from South America. FrogLog 42:2–3.
Ron SR (2005) Predicting the distribution of the amphibian pathogen Batrachochytrium dendrobatidis in the New World. Biotropica 37(2):209–221.
Rosenburg E, Koren O, Reshef L, Efrony R, Zilber-Rosenberg I (2007) The role of microorganisms in coral health, disease and evolution. Nature Review Microbiology 5:355–362.
Russell DM, Goldberg CS, Lisette PW, Rosenblum EB (2010) Batrachochytrium dendrobatidis infection dynamics in the Columbia spotted frog Rana luteiventris in north Idaho, USA. Disease of Aquatic Organisms 92:223–230.
Skerratt LF, Berger L, Speare R, Cashins S, McDonald KR, Phillott AD, Hines HB, Kenyon N (2007) Spread of chytridiomycosis has caused the rapid global decline and extinction of frogs. Ecohealth 4:125–134.
Stuart SN, Chanson JS, Cox NA, Young BE, Rodrigues AS, Fischman DL, Waller RW (2004) Status and trends of amphibian declines and extinctions worldwide. Science 306:1783–1786.
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Molecular Biology and Evolution 28:2731–2739.
Tobler U, Schmidt BR (2010) Within-and among-population variation in chytridiomycosis-induced mortality in the toad Alytes obstetricans. PLoS ONE 5:e10927.
Piotrowski JS, Annis SL, Longcore JE (2004) Physiology of Batrachochytrium dendrobatidis, a chytrid pathogen of amphibians. Mycologia 96:9–15.
Stevenson LA, Roznik EA, Alford RA, Pike DA (2014) Host-specific thermal profiles affect fitness of a widespread pathogen. Ecology and Evolution. doi:10.1002/ece3.1271
Walke JB, Harris RN, Reinert LK, Rollins-Smith LA, Woodhams DC (2011) Social immunity in amphibians: evidence for vertical transmission of innate defences. Biotropica 43:396–400.
Wiggins PJ, Smith JM, Harris RN, Kevin PC, Minbiole PC (2011) Gut of red-backed salamanders (Plethodon cinereus) may serve as a reservoir for an antifungal cutaneous bacterium. Journal of Herpetology 45(3):329–332.
Woodhams DC, Ardipradja K, Alford RA, Harris RN, Marantelli G, Reinert LK, Rollins-Smith LA (2007a) Resistance to chytridiomycosis varies by amphibian species and is correlated with skin peptide defenses. Animal Conservation 10:409–508
Woodhams DC, Rollins-Smith LA, Briggs CJ, Vredenburg VT, Simon MA, Billheimer D, Shakhtour B, Shyr Y, Harris RN (2007b) Symbiotic bacteria contribute to innate immune defenses of the threatened mountain yellow-legged frog, Rana muscosa. Biological Conservation 138:390–398
Woodhams DC, Alford RA, Briggs CJ, Johnson M, Rollins-Smith LA (2008) Life-history trade-offs influence disease in changing climates: strategies of an amphibian pathogen. Ecology 89:1627–1639.
Woodhams DC, Bosch J, Briggs CJ, Cashins S, Davis LR, Lauer A, Muths E, Puschendorf R, Schmidt BR, Sheafor B, Voyles J (2011) Mitigating amphibian disease: strategies to maintain wild populations and control chytridiomycosis. Frontiers in Zoology 8(1):8. doi:10.1186/1742-9994-8-8.
Yada S, Wang Y, Zou Y, Nagasaki K, Hosokawa K, Osaka I, Arakawa R, Enomoto K (2008) Isolation and characterization of two groups of novel marine bacteria producing violacein. Marine Biotechnology 10:128–132.
Acknowledgments
We would like to thank Universidad de las Americas (Quito, Ecuador), and Banco Santander and Fundación General CSIC for partial funding, and Universidad Inodamerica (Quito, Ecuador) for support in Ecuador. JCB and AMP-M were funded by master scholarships from CSIC-Spain. JCB would like to mention special thanks to Georgina Bresciano and Veronica Maino for invaluable support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bresciano, J.C., Salvador, C.A., Paz-y-Miño, C. et al. Variation in the Presence of Anti-Batrachochytrium dendrobatidis Bacteria of Amphibians Across Life Stages and Elevations in Ecuador. EcoHealth 12, 310–319 (2015). https://doi.org/10.1007/s10393-015-1010-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10393-015-1010-y