Abstract
The occurrence of azurocytes (AZ), a type of leukocyte unique to voles and previously described for three Microtus species, is now reported in Microtus agrestis. The goal of this study was to shed new light on the possible function and significance of these cells and on how they play a role in the natural history of rodent species. Individuals from three vole populations were sampled monthly for 2 years. A hemogram was produced for each individual, and AZ counts estimated. The counts of AZ were much higher in pregnant females, and these levels were higher the higher the past vole density. Males had low prevalences and counts, both for breeding and nonbreeding individuals, but they showed a seasonality that varied with age, body condition, and current and past vole density. Also, the occurrence of AZ in males was more likely after they had had low levels of indicators of condition, suggesting that azurocytes may result from a response to infection. Hence, overall our results suggest that, in females, these cells may be important for reproduction and may have a role in inducing abortion when conditions are not favorable, while in males they might be a response to infection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Leukocytes are the cell component of blood associated with inflammation and immunity. Their concentrations are routinely used as generic indices of health in human and veterinary medicine. Leukocytes of mammals have for long been known to be either polymorphonuclear granulocytes (neutrophils, eosinophils, and basophils) or mononuclear cells (monocytes and lymphocytes), but the nature of many subtypes is still unknown (Tizard, 2004). It has long been known that wild voles show a large seasonal variation in blood cell levels (e.g., Rewkiewicz-Dziarska, 1975), but large-scale studies investigating these variations thoroughly have been rare (e.g., Mihok and Schwartz, 1989).
More than two decades ago, a unique leukocyte of rodents in the genus Microtus was described and named an “azurocyte” (AZ), due to the outstanding purple granules present in its cytoplasm (Mihok et al., 1987). The cell is particularly common in late pregnancy (Mihok, 1987), and can be induced by progestins both in males and females (Mihok et al., 1987; Mihok and Schwartz,, 1991a). Based on similarities with other mammal cells with Natural Killer (NK) activity (Kurloff cells and large granular lymphocytes), it was suggested that these cells may be NK cells unique to the vole (Mihok and Schwartz, 1991b).
For almost 20 years, there has only been one mention of this new cell (Boonstra et al., 2005), until it was recently reported for another species, the field vole (Microtus agrestis) in a study that evaluated hematological dynamics in natural populations (Beldomenico et al., 2008a), also conforming to the pattern of breeding females having higher AZ counts.
The unique nature of the current dataset, with recorded blood levels of AZ in individuals from replicated natural populations monitored repeatedly over a 2-year period, thus provides an opportunity to investigate further the dynamics of these cells in natural populations and their relationship with factors intrinsic and extrinsic to the individuals that have them. The immediate aim is to shed new light on the possible function and significance of these cells and on how they play a role in the natural history of rodent species, but crucially, examining these cells in a natural population through a longitudinal study may throw light on their functional significance in a much wider range of medical, veterinary, and wildlife medicine settings.
The specific objectives of the present study were:
-
(i)
To describe seasonal and demographic patterns of blood AZ levels in wild field vole populations and to investigate the relationship between intrinsic and extrinsic factors and circulating levels of AZ in wild field voles.
-
(ii)
Because individuals in poor condition are more likely to show elevated indicators of infection when re-sampled a month later (Beldomenico et al., 2008b), we examined if the occurrence of AZ in males is dependent on preceding health status (condition) to evaluate if AZ may be a response to infection.
Materials and Methods
Hematology
As described in detail by Beldomenico et al. (2008a), a hemogram was produced with blood collected from the tip of the tail of live individuals to estimate the concentration of all blood cell types, including AZ. Briefly, 2 μl of noncoagulated blood were diluted 1:20 in 4% acetic acid with 1% crystal violet and 1:5000 in PBS, to count white blood cells (WBCs) and red blood cells (RBCs), respectively, using Kova Glasstic® slides (Hycor Biomedical Ltd., Penicuik, UK) with grids and hence to determine their concentration. The rest of the blood sample was used to produce blood smears for differential (relative) WBC counts, which allowed the proportion of each WBC (including AZ) to be estimated. At least 100 WBCs were examined on the smears. The large number of samples precluded a more exhaustive assessment of the proportion of WBCs (i.e., with more WBCs examined per sample). However, because we were particularly interested in determining the presence of elevated concentrations of AZ, we judged that it was more important to examine a large number of samples than to use a method that gave more reliable estimates when AZ concentrations were low. The concentration of AZ was then estimated as the product of the proportion WBCs that were AZ and the total WBC count. Note, therefore, that a failure to detect AZ should not be interpreted as “absence” but as “absence or low levels.” Hence we refer to “detectable levels” in the text. Smears were air-dried, fixed with methanol, and stained with Rapid Romanowsky Stain Pack—HS705 (HD Supplies, Aylesbury, UK).
Data Collection from Wild Field Voles
In Kielder Forest (Northumberland, UK), three sites with suitable habitat for field voles were sampled (“primary sessions”) every 4 weeks over a 2-year period (from April 2005 to March 2007) apart from 8-week gaps between November and February (Beldomenico et al., 2008a). At each site, a trapping grid measuring 50 × 50 m was established, with 100 Ugglan special live capture traps (Grahnab, Sweden), set at approximately 5-m intervals. In each primary session, the traps were checked for capture five times, at sunrise and before sunset (roughly 12-hour intervals) (Beldomenico et al., 2008a). Because we were especially concerned about the influence that confinement and handling may have on vole hematology through epinephrine and corticosteroids, a pilot study was conducted to assess whether different times of handling exerted a measurable effect on the hemogram and found no substantial impact (Beldomenico, 2007). Other studies have shown that a longer time in traps does not increase the stress levels in voles (Harper and Austad, 2001; Fletcher and Boonstra, 2006).
Individuals were uniquely and permanently marked on first capture with a small microchip transponder (Labtrac, AVID plc, Uckfield, UK). On first capture within a primary session, each vole was assessed for pelage (juvenile coat, first moult, adult coat), sex, body mass (to the nearest 0.5 g, using a spring balance), body condition, and reproductive status. Body condition (BODYCOND) was evaluated by estimating by palpation the degree of fat cover over the vertebral column and the pelvic bones, giving a score between 2 and 10 (Beldomenico et al., 2008a). For this study, reproductive status was only considered for females, which were classified as “non-gravid,” “gestating,” “possibly gestating,” or “lactating” (if they were both “lactating” and “gestating,” they were considered “gestating”). Females classified as “possibly gestating” were omitted from the analysis.
Statistical Approach
The general approach was that used in Beldomenico et al. (2008a, 2008b). The analysis had three parts: (i) an exploratory analysis focused on the description of seasonal and demographic patterns of AZ occurrence; (ii) generalized linear mixed models (GLMM) were used to investigate the relationship between intrinsic and extrinsic factors and circulating levels of AZ; (iii) to investigate if the occurrence of AZ in males was preceded by low levels of indicators of condition, RBCs, and lymphocytes (Beldomenico et al., 2008a), a longitudinal study at the individual level used GLMMs to determine whether males’ poor condition were more prone to show detectable levels of AZ when re-sampled 4 weeks later. Analyses were carried out in R (The R Foundation for Statistical Computing).
Hence, the first GLMM focused on the effect of reproductive status in adult females during the breeding season (from April to October), and a second included all months but only focused on males, to evaluate separately the fluctuations in AZ not associated with pregnancy. Explanatory variables were age, season, past and current vole densities, body condition, and whether individuals were recaptured (“R”) or newly captured (“N”) (R/N). Reproductive status (REPR) was also evaluated in the first model. Then, in a third analysis, the factors associated with the detection of AZ (yes/no) in a sample, mainly focusing on the relationship with RBC and lymphocyte levels one sample previously, were evaluated with observations from males that had no AZ detected in the first sample (n = 457), sampled from March to October (at 4-week intervals). In this analysis, the explanatory variables of interest were RBCs/μl (in millions) and lymphocytes/μl as estimated by the hemogram.
Individuals with a juvenile coat and ≤17 g in weight were not considered in the analysis of field data because AZ were rarely present in them (only 11% of juveniles in the wild). For post-juveniles, age was approximated using the dichotomous variable AGE_CLASS. “Adult” and “young” individuals were estimated on the basis of capture histories to be older and younger than 90 days, respectively. In the absence of enough trapping history, an individual was classified as adult if it weighed 22 g or more, and otherwise “young.” Once classified as an adult, individuals remained so for every successive capture.
SEASON was evaluated using cycles generated by two sinusoidal components to reflect seasonality (SEASON[sin] + SEASON[cos]) (Beldomenico et al., 2008a). Population sizes were estimated using Huggins’s closed capture models within a robust design (Huggins, 1989; Kendall and Nichols, 1997) conducted in program MARK (White and Burnham, 1999) using mixture models (Pledger, 2000) to allow heterogeneity in capture probabilities. The population densities investigated were current estimates (DENSITY-0; mean of the current and previous month’s population estimate), and densities at lags of 3 and 6 months. The third analysis also included the explanatory variables RBCs/μl and lymphocytes/μl.
As the distribution of AZ in the field data appeared to be negative binomial, general linear mixed models (GLMM) with a negative binomial response were used (Bliss and Fisher, 1953). The third analysis, however, evaluated the detection of AZ at a second sample as a binary response (yes/no). Vole identification number (VOLE_ID) was included as a random effect to account for correlated variation between individuals sampled repeatedly. As voles sampled at the same site in the same month shared the same population level covariates, the interaction between site and month as a random effect (SITE*SESSION) was also assessed (Telfer et al., 2005). However, the function used in the first two analyses (glmmPQL) does not allow more than one random effect. Hence, as “VOLE_ID” was consistently found to be the more important random effect, the variable “SITE” was included as a fixed effect in those analyses to control for the lack of independence of observations from the same site.
Model selection involved starting from a maximum model which was then restricted by stepwise elimination using the Akaike Information Criterion (AIC) (Akaike, 1974) to retain only the significant main effects and interactions. Terms were eliminated if they did not reduce the AIC by more than 2 units when included. However, because a ΔAIC of 2 may incorrectly select overly complex models when the true effect of a measured factor is relatively weak, data are highly overdispersed, or data are few (Richards, 2008), the ΔAIC resulting from the removal of each significant term is reported and discussion focused on those variables with higher ΔAIC values and stronger associations. Although the random effect was assessed in the maximum model, the model restriction was conducted without it, as the method employed does not allow determination of AIC values nor likelihood ratio tests when including random effects. Therefore, VOLE_ID was included in the model at the end of the restriction procedures.
Results
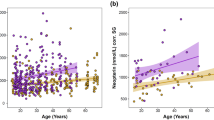
The occurrence of AZ had a strong seasonal pattern for both sexes, with a higher frequency of detectable levels of AZ during the breeding season (April–October; Fig. 1). Males showed a lower frequency of occurrence, and those with AZ generally had low counts. See Table 1 for a summary of the data by sex, age, season, and reproductive status.
(A) Seasonality of the proportions of non-juvenile field voles found to have AZ from March 2005 to March 2007. Darker gray indicates a differential count greater than 5% (i.e., more than 5% of the WBCs were AZ). (B) Proportion of adult females that were either gestating or lactating from March 2005 to March 2007. Darker gray indicates gestating individuals, lighter gray indicates lactating ones (eJun = early June; lJun = late June).
In adult females during the breeding season (Table 2) (number of observations = 658), AZ levels appeared to be higher for pregnant than for non-gravid and lactating animals, but when the density 6 months previously was low, pregnant and non-gravid females tended not to differ in spring (Fig. 2). Peak counts also arose earlier when density was lower 3 months previously.
Predicted AZ levels for adult females during the breeding season. Differences between reproductive statuses at different past densities (lag6 = density 6 months previously; densities 3 months previously fixed at 50). (Simulation for the site “ROB” in 2006.) Black lines indicate simulations with past density of 90, and gray lines indicate past density of 30. Solid line: pregnant; dashed line: lactating; dotted line: non-gravid.
Table 3 shows the final model describing the factors that influence AZ counts in post-juvenile males (number of observations = 1290). There was a marked seasonal pattern, but peaks occurred earlier in young individuals (broadly, April–June) than they did in adults (June–September) (Fig. 3). Indeed, in 2006, when seasonality itself was less marked, the adult peak roughly coincided with a trough in young individuals. Levels were higher in individuals with good body condition, and in such individuals higher AZ counts were associated with higher past densities (6 months previously). Adults also showed lower levels of AZ the higher the current population size, but this was not seen in young individuals.
Predicted AZ levels for post-juvenile males, by season, year, body condition scores, age (black adults, gray young), and varying current (lag0, average density between current and previous trapping session) and past (lag6, density 6 months previously) densities. Black lines indicate adult voles, and gray indicate young. Solid line: lag = 90; dashed line: lag = 60; dotted line: lag = 30.
The occurrence of detectable AZ levels in males was associated with preceding low levels of RBCs and lymphocytes (Table 4). In general, individuals with low lymphocyte counts had higher probabilities of showing detectable levels of AZ than those with higher lymphocyte counts (Fig. 4). Voles with low RBC counts, too, had higher probabilities of showing detectable levels of AZ among lighter individuals, but for heavy animals (>28 g) the lowest probabilities of showing detectable levels AZ were displayed by the anemic voles.
Predicted occurrence of AZ in male field voles by weight and year, for different levels of RBCs and in presence (a) and absence (b) of lymphopenia. In the simulation, anemic (- - -) are individuals with 3 million RBCs/μl, and “normal” (—) are voles with 8 million RBCs/μl. Vole weight: black line = 17 g; dark gray line = 22 g; light gray line = 35 g
Discussion
The general pattern described by Mihok et al. (1987) for Microtus pennsylvanicus was confirmed here for Microtus agrestis. We also observed high counts predominantly in pregnant females and low counts in other individuals. The main contributions of the present work to our knowledge of AZ biology are as follows.
-
The intriguing variation of AZ levels in males, which is dependent of season, age, body condition, and current and past vole density.
-
The observation that detectable AZ levels in males are more likely after they have had low levels of indicators of condition, an indication that azurocytes may result from a response to infection.
-
The observation that after high past vole densities, when condition is depressed due to lack of food (Huitu et al., 2007), pregnant females show higher azurocyte levels than following low densities.
Although AZ are yet to be characterized definitively, Mihok et al. (1987), employing histochemical and biochemical methods, determined that the AZ most closely resembles a lymphocyte. New World hystricomorph rodents also show a unique cell associated with pregnancy, the Kurloff cell, although these only have a single large intracytoplasmic granule (Eremin et al., 1980; Jara et al., 2005). Histochemically, azurocytes and Kurloff cells are nearly identical (Mihok et al., 1987). However, the Kurloff cell differs from the azurocyte in that it can be induced with estrogen (Ledingham, 1940), while AZ are induced by progestins (Mihok and Schwartz, 1991a). The Kurloff cell has NK activity (Eremin et al., 1980), as have other cells also associated with pregnancy in other mammals: large granular lymphocytes (LGLs) of humans (Iwatania et al., 1989) and uterine NK cells of rodents (Head, 1996). Based on similarities with LGLs and Kurloff cells, it was suggested that AZ are an NK cell unique to the vole (Mihok and Schwartz, 1991b), and it was proposed that its function could be maintaining immunocompetence during pregnancy or maintaining the survival of embryos/fetuses.
In humans, large granular lymphocytes of the NK lineage increase in peripheral blood during the first trimester of pregnancy (Iwatania et al., 1989), and similar cells also appear in the human endometrium at embryonic implantation (King et al., 1989; Bulmer et al., 1991). Also during pregnancy, murine and microtine rodents also present special NK cells (formerly known as Granulated Metrial Gland cells, and currently as uterine NK cells), but they have been found generally in the uterine glands, not circulating (Head, 1996; Stewart and Clarke, 1999), although their bone marrow origin has been confirmed (Stewart, 1991). In addition, the Kurloff cell (a cell with NK activity) shows higher levels in female hystricomorph rodents and increases during pregnancy (Revell, 1977; Jara et al., 2005). For all these reasons, NK cells are believed to be involved in maternal–embryonic interaction during normal mammalian pregnancy (Moffett-King, 2002).
Although it is true that uterine NK cells are larger than AZ and have different staining properties (Mihok and Schwartz, 1991b), the bone marrow origin of both (Stewart, 1991; Mihok and Schwartz, 1991b) and the coincidence in the timing of their occurrence during pregnancy suggest that they may be related. It has been suggested that the function of NK cells in the gravid uterus is to control the invasion of the trophoblast (Tizard, 2004). This is not supported, however, by the observation that levels of AZ in blood increase predominantly in the second half of gestation (Mihok, 1987) (also confirmed for M. agrestis [Beldomenico, 2007]). Studies in mice have shown that uterine NK cells occur in two waves during pregnancy, a small one soon after trophoblast implantation, and a later one in the metrial glands (Head, 1996). The function of the latter wave is unlikely to be related to uterine invasion control.
The role of the postimplantation wave of uterine NK cells in rodents, therefore, remains an open question. A plausible function might be aiding in the quick removal of dead material resulting from an abortion, as dead embryos cannot be eliminated via the vagina if there is another embryo blocking the cervix. This would explain why uterine NK cell granules contain large amounts of perforin, a cytolytic protein (Head, 1996). Consistent with the increase of AZ blood levels at the end of gestation (observed by Mihok, 1987), another possible function of NK cells might be aiding in the disengagement of the maternal–fetal unions.
In humans, it has been shown that high counts and activity of circulating NK cells were associated with first trimester embryo loss (Aoki et al., 1995; Emmer et al., 2000). Spontaneous embryo resorption is a common finding in rodents, and its cause has been attributed to an innate immune response (Baines et al., 1996) (NK cells are part of the innate immune system). Several studies in mice and rats have provided evidence that NK cells are the effectors of resorption (e.g., Baines et al., 1996; Arad et al., 2005). This suggests that embryo resorption might itself be an adaptive mechanism, not a misfiring of a function that has evolved to protect the uterus or disengage the placenta. It is likely that embryo resorption is a way of reducing the litter size during times of hardship. Our results here are consistent with this theory. When past densities were high, and therefore the condition of voles may be affected (Huitu et al., 2007; Beldomenico et al., 2008a), gestating females showed considerably higher AZ levels than non-gravid ones; whereas following low past densities, AZ counts of gestating and non-gravid females did not differ, particularly in spring (the highly demanding season [Beldomenico et al., 2008a]). A long-term study reported that the proportion of resorption cases in wild bank voles (Myodes [=Clethrionomys] glareolus) varied for different years, but did not relate this to variations in any proxies of “hardship” (Balèiauskas, 2005).
The density-dependent delay in the peak of AZ resembles the one observed for neutrophils in a study that used the same field data (Beldomenico et al., 2008a). The explanation offered for them is also suitable here: high densities in autumn/winter have an impact on resources (Huitu et al., 2007) and reproduction begins later (Ergon et al., 2001), and the increase in AZ levels is therefore postponed.
A fundamental question to ask is: If AZ cells are involved in gestation, why do males also have them at detectable levels? Interestingly, the seasonal pattern is very similar to that of females (Fig. 1). Treatment with estrogen causes elevation of Kurloff cell levels in male guinea pigs (Ledingham, 1940) and treatment with progestins causes elevation of AZ in male meadow voles (Mihok et al., 1987). It may be argued that AZ do not have a specific function in males, and that hormonal levels during the breeding season are secondarily causing their rise. However, breeding and nonbreeding adult males kept under controlled conditions did not differ in the occurrence of AZ, whereas they were significantly different in size (Beldomenico, 2007). Further, it has been shown that testosterone has a negative effect on NK cell proliferation (Page et al., 2006).
It has also been suggested that immunologic stimulation can raise Kurloff cells in guinea pigs (Revell, 1977), and experimental infection of M. pennsylvanicus with Reovirus-3 induced AZ maturation (Mihok and Schwartz, 1991a). Because the seasonal pattern of AZ in young males is similar to that observed for monocytes and neutrophils (Beldomenico et al., 2008a), stimulation by an antigen is suggested here, too. Older males, on the other hand, showed higher AZ counts, the better their body condition. In 2006 (a year when the metabolic demands of voles seemed not to be covered [Beldomenico et al., 2008a], only adult males in good condition showed a rise in AZ levels, especially at low current vole densities, and the peak counts occurred in summer, not spring. All this suggest that older males find it more difficult to present elevated levels of AZ, perhaps because of the negative effect of testosterone, referred to above (Page et al., 2006). The positive association with past densities in individuals with higher body condition scores might reflect higher incidences of infections following higher densities, which can only be translated into higher AZ levels by individuals in good condition.
Because poor condition is likely to predispose individuals to infection (Beldomenico et al., 2008b), the increased probability of occurrence of AZ in young males that were lymphopenic or anemic further supports the hypothesis that, in males, increases in AZ levels are a response to infection. However, the lowered probability of occurrence of detectable AZ levels in anemic males greater than 28 g in weight seems to contradict it. This, though, is also consistent with lower AZ levels being shown by adult males when conditions are less favorable (i.e., poor body condition, high densities, negative metabolic balance), linked to the negative effect of testosterone on NK cell proliferation (Page et al., 2006).
Further studies are needed to characterize field vole AZ in detail and, specifically, to determine whether they might include different subpopulations of cells, some related to gestation, others to responses to infection. Indeed, a better understanding of these cells should also contribute to the elucidation of related physiological and pathological processes in other species, including humans. Furthermore, given that in females these cells appear to be linked to the abortion of reproductive activity during unfavorable circumstances, measuring their levels could be a tool to assess the dynamics of health of natural populations.
References
Akaike H (1974) A new look at the statistical model identification. IEEE Transactions on Automatic Control AC-19:716–723
Aoki K, Kajiura S, Matsumoto Y, Ogasawara M, Okada S, Yagami Y, et al. (1995) Preconceptional natural killer cell activity as a predictor of miscarriage. Lancet 345:1340–1342
Arad M, Atzil S, Shakhar K, Adoni A, Ben-Eliyahu S (2005) Poly I-C induces early embryo loss in F344 rats: a potential role for NK cells. American Journal of Reproductive Immunology 54:49–53
Baines M, Duclos A, Fougerolles A, Gendron R (1996) Immunological prevention of spontaneous early embryo resorption is mediated by non-specific immunosimulation. American Journal of Reproductive Immunology 35:34–42
Balèiauskas L (2005) Results of the long-term monitoring of small mammal communities in the Ignalina nuclear power plant region (DRÛKÐIAI LTER site). Acta Zoologica Lituanica 15:79–83
Beldomenico P (2007) Infection and host population dynamics: the use and assessment of generic indices of health. PhD Thesis, University of Liverpool, Liverpool, UK
Beldomenico P, Telfer S, Gebert S, Lukomski L, Bennett M, Begon M (2008a) The dynamics of health of wild field vole (Microtus agrestis) populations: a haematological perspective. Journal of Animal Ecology; doi:10.1111/j.1365-2656.2008.01413.x
Beldomenico PM, Telfer S, Gebert S, Lukomski L, Bennett M, Begon M (2008b) Poor condition and infection: a vicious circle in natural populations. Proceedings of the Royal Society of London. Series B: Biological Sciences 275:1753–1759
Bliss C, Fisher R (1953) Fitting the negative binomial distribution to biological data. Biometrics 9:176–200
Boonstra R, Manzon RG, Mihok S, Helson JE (2005) Hormetic effects of gamma radiation on the stress axis of natural populations of meadow voles (Microtus pennsylvanicus). Environmental Toxicology and Chemistry 24:334–343
Bulmer J, Morrison L, Longfellow M, Ritson A, Pace D (1991) Granulated lymphocytes in human endometrium: histochemical and immunohistochemical studies. Human Reproduction 6:791–798
Emmer P, Nelen W, Steegers E, Hendriks J, Veerhoek M, Joosten I (2000) Peripheral natural killer cytotoxicity and CD56(pos)CD16(pos) cells increase during early pregnancy in women with a history of recurrent spontaneous abortion. Human Reproduction 15:1163–1169
Eremin O, Coombs R, Ashby J, Plumb D (1980) Natural cytotoxicity in the guinea-pig: the natural killer (NK) cell activity of the Kurloff cell. Immunology 41:367–378
Ergon T, MacKinnon JL, Stenseth NC, Boonstra R, Lambin X (2001) Mechanisms for delayed density-dependent reproductive traits in field voles, Microtus agrestis: the importance of inherited environmental effects. Oikos 95:185–197
Fletcher Q, Boonstra R (2006) Impact of live trapping on the stress response of the meadow vole (Microtus pennsylvanicus). Journal of Zoology 270:473–478
Harper J, Austad S (2001) Effect of capture and season on fecal glucocorticoid levels in deer mice (Peromyscus maniculatus) and red-backed voles (Clethrionomys gapperi). General and Comparative Endocrinology 123:337–344
Head JR (1996) Uterine natural killer cells during pregnancy in rodents. Natural Immunity 15:7–21
Huggins R (1989) On the statistical analysis of capture–recapture experiments. Biometrika 76:133–140
Huitu O, Jokinen I, Korpimäki E, Koskela E, Mappes T (2007) Phase dependence in winter physiological condition of cyclic voles. Oikos 116:565–577
Iwatania Y, Aminoa N, Kabutomoria O, Kanedaa T, Tanizawab O, Miyaia K (1989) Peripheral large granular lymphocytes in normal pregnant and postpartum women: decrease in late pregnancy and dynamic change in the puerperium. Journal of Reproductive Immunology 16:165–172
Jara LF, Sanchez JM, Alvarado H, Nassar-Montoya F (2005) Kurloff cells in peripheral blood and organs of wild capybaras. Journal of Wildlife Diseases 41:431–434
Kendall W, Nichols J (1997) Estimating temporary emigration using capture–recapture data with Pollock’s robust design. Ecology 78:563–578
King A, Wellings V, Gardner L, Loke Y (1989) Immunocytochemical characterization of the unusual large granular lymphocytes in human endometrium throughout the menstrual cycle. Human Immunology 24:195–205
Ledingham J (1940) Sex, hormones and the Foa-Kurloff cell. Journal of Pathology and Bacteriology 2:201–219
Mihok S (1987) Pregnancy test for meadow voles (Mycrotus pennsylvanicus) based on blood azurocyte counts. Canadian Journal of Zoology 65:2830–2832
Mihok S, Descôteaux J, Lawton T, Lobreau A, Schwartz B (1987) The azurocyte: a new kind of leukocyte from wild voles (Microtus). Canadian Journal of Zoology 65:54–62
Mihok S, Schwartz B (1989) Anemia at the onset of winter in the meadow vole (Microtus pennsylvanicus). Comparative Biochemistry and Physiology A 94:289–304
Mihok S, Schwartz B (1991a) Artificial induction of azurocytes in the meadow vole (Microtus pennsylvanicus). Comparative Biochemistry and Physiology C 99:213–218
Mihok S, Schwartz B (1991b) Origin and maturation patterns of azurocytes in the meadow vole (Microtus pennsylvanicus). Comparative Biochemistry and Physiology C 99:219–230
Moffett-King A (2002) Natural killer cells and pregnancy. Revue d’Immunologie 2:656–663
Page ST, Plymate SR, Bremner WJ, Matsumoto AM, Hess DL, Lin DW, et al. (2006) Effect of medical castration on CD4+ CD25+ T cells, CD8+ T cell IFN-gamma expression, and NK cells: a physiological role for testosterone and/or its metabolites. American Journal of Physiology—Endocrinology and Metabolism 290:E856–E863
Pledger S (2000) Unified maximum likelihood estimates for closed capture–recapture models using mixtures. Biometrics 56:434–442
Revell P (1977) The Kurloff cell. International Review of Cytology 51:275–314
Rewkiewicz-Dziarska A (1975) Seasonal changes in leukocyte indexes in Microtus arvalis (Pallas, 1779). Bulletin de l’Académie Polonaise des Sciences 23:475–480
Richards S (2008) Dealing with overdispersed count data in applied ecology. Journal of Applied Ecology 45:218–227
Stewart I (1991) Granulated metrial gland cells: pregnancy specific leukocytes? Journal of Leukocyte Biology 50:198–207
Stewart I, Clarke J (1999) Granulated metrial gland cells and interstitial trophoblast in the uterine wall of the bank vole, Clethrionomys glareolus, in early pregnancy. Journal of Anatomy 194:297–301
Telfer S, Bennett M, Bown K, Carslake D, Cavanagh R, Hazel S, et al. (2005) Infection with cowpox virus decreases female maturation rates in wild populations of woodland rodents. Oikos 109:317–322
Tizard IR (2004) Veterinary Immunology. An Introduction. Philadelphia: Saunders
White G, Burnham K (1999) Program MARK: survival estimation from populations of marked animals. Bird Study 46(Suppl):120–138
Acknowledgments
We thank Gill Telford, Roslyn Anderson, Jenny Rogers, and Gemma Chaloner for their assistance in the field, and Sue Jopson for her work in the animal house. This work was funded by Wellcome Trust grant 075202/Z/04/Z to M. Begon and a NERC Dorothy Hodgkin Award to P.M.B.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Beldomenico, P.M., Telfer, S., Gebert, S. et al. Azurocytes in Wild Field Voles: Factors Associated with Their Occurrence. EcoHealth 5, 317–327 (2008). https://doi.org/10.1007/s10393-008-0186-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10393-008-0186-9