Abstract
Aim
Identifying risk factors for low bone stiffness index (SI) might be one crucial strategy for osteoporosis prevention. Purpose was to characterize healthy schoolchildren across tertiles of SI.
Subject and methods
In 248 girls (13.4 ± 1.9 years, BMI: 20.2 ± 4.8 kg/m2) and 231 boys (13.6 ± 1.7 years, BMI: 19.3 ± 3.3 kg/m2), the following parameters were assessed: calcaneal SI (quantitative ultrasound), body composition (bioelectrical impedance analysis), Bone Healthy Eating Index (BoneHEI; food frequency questionnaire), and physical activity level (PAL; activity questionnaire). Participants were classified according to age- and sex-specific SI tertiles (low, medium, and high). Between-group comparisons were achieved by Kruskal–Wallis-H-tests (α = 0.05).
Results
Girls with low SI had significantly lower body mass (49.2 ± 16.7 vs 54.8 ± 12.2 kg; p < 0.01), BMI (19.6 ± 5.4 vs 21.3 ± 3.9 kg/m2; p < 0.0001), fat-free mass (36.3 ± 8.3 vs 39.5 ± 6.0 kg; p < 0.01), and fat mass (23.7 ± 9.1 vs 26.8 ± 7.2%; p < 0.05) compared to those with high SI. In boys, significant differences between low and high SI were obtained for PAL (1.49 ± 0.12 vs 1.56 ± 0.14; p < 0.01). BoneHEI was not significantly different between tertiles in both sexes.
Conclusion
Girls with low body mass and boys with low PAL have a higher risk for low SI. Schoolchildren should strive for normal body mass and perform regular physical activity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Skeletal growth and mineralization develop through the first 2 decades of life and reach a plateau (denoted as peak bone mass) in the late-teen or young-adult years (Faulkner and Bailey 2007). A high peak bone mass reduces the risk of fractures and osteoporosis later on (Heaney et al. 2001). Nearly 60% of the osteoporosis risk can be explained by the amount of bone mineral acquired by early adulthood (Hui et al. 1990). Therefore, an essential strategy for osteoporosis prevention is attaining a high peak bone mass during adolescence and young-adult life respectively.
In addition to genetic factors, several other factors, such as body weight and its composition, and lifestyle factors, influence adult peak bone mass (Weaver et al. 2016). Some of the most critical variables are anthropometric status and body composition. It has been shown that body mass (which is itself a composition of environmental and hereditary factors) is the largest single determinant of adult bone mass variability (Heaney et al. 2001), and a very low body mass index (BMI) is related to a higher risk of osteoporosis (Munhoz et al. 2018). In contrast, high fat-free mass levels are favorable for bone status due to the mechanical and biochemical coupling between muscle and bone (Ho-Pham et al. 2014).
Another crucial factor for bone health and bone mineral density is regular physical activity. Increased bone loading due to higher amounts and intensity of physical activity improves bone metabolism and leads to a higher bone density (Rubin and Lanyon 1984). Repeated activities with high intensity and rapid changes of direction, accelerations, and/or jumps are recommended to increase bone mineral density in children and adolescents (Witzke and Snow 2000).
Furthermore, the individual diet influences bone metabolism. It was shown that calcium and vitamin D deficiencies can decrease bone mineral density and lead to a predisposition to osteoporosis (Heaney et al. 2001). Finally, positive associations between sunlight exposure (Farrar et al. 2016; Alghadir et al. 2018), early-life breastfeeding (Jones et al. 2013), and bone status have been reported in adolescents. Other risk factors for osteoporosis include smoking, excessive alcohol intake, chronic diseases, and the use of medications (Ferrari 2005).
One strategy for prevention of osteoporosis in later life is the identification of children and adolescents with low bone mineral density, because of their high risk for low peak bone mass. In this context, it is important to point out risk factors for low bone mass in children and adolescents. The identification of these risk factors can help to develop prevention programs specifically targeted to the needs of girls and boys with low bone mass. In the literature, numerous risk factors for low bone parameters in children and adolescents are reported (Jones et al. 2013; Farrar et al. 2016; Alghadir et al. 2018; Heydenreich et al. 2020). However, the authors often used correlation analysis for the identification of a few risk factors. In contrast, characterization of numerous anthropometric and lifestyle factors of participants across tertiles of certain bone parameters has not yet been performed. Therefore, the purpose of this study was to characterize healthy girls and boys with low, medium, and high calcaneal stiffness index (SI), as a measure of bone mass, and to identify risk factors for low bone parameters of participants from the lowest tertile of SI.
Materials and methods
Participants
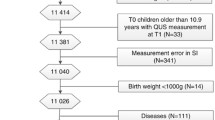
Four hundred and eighty-six schoolchildren from six secondary schools of different school types in or close by to Schwäbisch Gmünd, Germany, and their respective parents participated in the study. Further information about recruitment process can be found elsewhere (Heydenreich et al. 2020). Measurements were conducted between June 2010 and July 2011.
The participants were tested in a discrete room provided by the respective school. Measurements took place on one testing day in the following order: 1) anthropometry and body composition and 2) bone status (see details below). Furthermore, participants were asked to fill out a standardized questionnaire to assess their lifestyle habits and dietary intake (see details below). In addition, before investigations, parents of the students filled out a standardized questionnaire.
Anthropometric data and body composition
Body mass was measured to the nearest 0.1 kg using a calibrated beam scale (Seca 877, Seca, Hamburg, Germany), with participants in light clothing and without shoes. After that, depending on the estimated weight the clothing participants were wearing, examiners subtracted 0.5–1.0 kg from the measured body mass. Participants were standing in an upright position without wearing shoes and looking straight ahead when height was assessed to the nearest 0.5 cm using a measuring tape. The BMI was calculated and classified according to age- and sex-specific percentile curves for German children and adolescents (Kromeyer-Hauschild et al. 2001).
Body composition was assessed using an impedance analyzer (BIA 2000 – S, Data Input GmbH, Pöcking, Germany) by taking whole-body bioelectrical impedance measurements (resistance; R, and reactance; Xc, at 50 kHz and 800 μA). Participants were in light clothing, bladder-voided, and all metal artifacts were removed when measurements were performed in the morning. During the measurement, participants were in a supine position with legs and arms abducted 45° from the body. Two pairs of detector and injector electrodes were placed on the right alcohol-cleaned hand and foot. Participants were requested not to move during the measurement. Afterwards, the absolute fat-free mass was calculated (Plachta-Danielzik et al. 2012).
Bone status
Quantitative ultrasound (QUS) measurements with the Lunar Achilles InSight Ultrasonometer (GE Healthcare, Milwaukee, WI, USA) were performed to assess bone status. Obtained data were further analyzed using the accompanying software. QUS measurements use ultrasound waves to measure broadband ultrasound attenuation (BUA; dB ∙ MHz−1) and speed of sound (SOS; m ∙ s−1). The SI was then calculated using the following equation provided by the manufacturer:
The Z-score values of BUA and SOS were computed using the reference sample of a healthy, pediatric German sample stratified by age and sex (Wünsche et al. 2000) using the following equation: Z-score = (measured values – matched mean values)/matched SD. A Z-score of −2.0 standard deviations or lower was defined as “below the expected range for age” and a Z-score above −2.0 as “within the expected range for age” (Lewiecki et al. 2008).
Trained researchers performed and analyzed QUS measurements, and standardization was achieved according to the manufacturer’s recommendations. Participants were in a comfortable seated position directly in front of the Achilles device. They placed their left leg in the device so that foot, calf, and thigh aligned with the calf support and the positioner. Participants were requested not to move during the measurement. Calibration of the Achilles device was performed every week using a calibration phantom.
The precision of the QUS device which was used was reported to be < 2% for SI in children and adolescents (Xu et al. 2014). Furthermore, the SI measured by QUS significantly correlated with outcomes measured by dual energy X-ray absorptiometry (r = 0.69; p < 0.001) (Xu et al. 2014).
Participants were classified into age- and sex-specific tertiles (lowest, medium, and highest) according to their individual SI, where age was treated as a range (i.e., tertiles for 10- to < 11-year-old girls, tertiles for 10- to < 11-year-old boys, tertiles for 11- to < 12-year-old girls, etc.) (Online Resource 1). We assumed that children and adolescents in the lowest tertile are at higher risk for low peak bone mass than the other groups.
Lifestyle questionnaire and socio-demographic data
Participants were asked to fill out a standardized questionnaire in German, including questions about demographical data, physical activity, staying outside during summer/winter and weekdays/weekend (h ∙ d−1), and their smoking status (see Supplementary Material S1; translated into English). Participants documented their average time spent for low-, medium-, and high-intensity physical activities during 1 week (Krems et al. 2004). The average time spent for sporting activities was calculated as the sum of low-, medium-, and high-intensity sporting activities. By use of the equation of Müller et al. (Müller et al. 2004) the resting metabolic rate (RMR) was calculated:
For calculation of total energy expenditure, specific factors (coefficients) for each activity [World Health Organization (WHO) 1985] were multiplied by the estimated RMR (Müller et al. 2004). Lastly, PAL was calculated in accordance with the WHO’s approach (total energy expenditure/ estimated RMR) (World Health Organization (WHO) 1985).
Participants had to estimate their physical development using a validated questionnaire consisting of five items (growth in height, body hair, skin changes, facial hair, and voice changes for boys; breast development and menses for girls) (Carskadon and Acebo 1993). By use of an adapted 4-point-scale [1 = not yet started, 2 = barely started, 3 = definitely started, 4 = seems completed] each item had to be estimated (Petersen et al. 1988). Afterwards, puberty category scores were calculated with the three preeminent sexual maturation characteristics of the pubertal development scale (girls: menarche, breast and body hair growth, boys: deepening-voice, body hair and facial hair growth). By use of this score, the children were categorized into one of the five pubertal development stages (pre-, early, midpubescent, advanced, and postpubescent) designed to be similar to Tanner staging categories (Crockett 1988).
Parents of the schoolchildren were also asked to fill out a standardized questionnaire containing questions about the parents´ educational and migration status. The educational status of the parents was calculated according to a modified version of the international standard classification of education (ISCED) provided by UNESCO (Online Resource 2) (United Nations Educational Scientific and Cultural Organization (UNESCO) 2006; Schroedter et al., 2006). Since the education status was determined for both parents individually, the higher value was chosen to classify the children into low, medium, and high parental educational background. Participants were classified as having a migration background if they and at least one parent were not born in Germany or if both parents immigrated to Germany. Furthermore, parents were asked to respond to questions about their child (e.g., vitamin D supplementation in the first year of life, regular drug intake, and the history of fractures). The parental questionnaire was available for all school children.
Food intake
By using an adapted version of the standardized food frequency questionnaire “What do you eat?”(Mensink and Burger 2004) the intake of 13 food items was assessed. Participants estimated the average frequency and the usual quantity of food consumed within the last weeks. A Bone Healthy Eating Index (BoneHEI) was then computed based on the Healthy Nutrition Score for Kids and Youth (HuSKY) (Kleiser et al. 2009), taking into account the actual guidelines for an optimized mixed diet for children and adolescents (Alexy et al. 2008; Kersting and Alexy 2009). In short, the food items in the questionnaire were summarized into eight different food groups [fruits and vegetables, fish, bread, milk and dairy products, meat and sausages, tolerated food (sweets and snacks), soft drinks, and caffeinated beverages] (Schweter 2015). The individual intake was then related to the recommended intake of these food groups (Alexy et al. 2008; Kersting and Alexy 2009; Kleiser et al. 2009). A maximum score of 100 points could be reached for each category when the individual intake corresponded to the recommended intake. The BoneHEI was then calculated as the average of the points earned for each category [range 0–100 points]. A higher BoneHEI reflects a healthier diet for the bone. Detailed information about the calculation of the BoneHEI can be found elsewhere (Schweter 2015; Heydenreich et al. 2020).
Statistics
Statistical analyses were performed with SPSS statistics version 26 for MS-Windows (IBM Corp., Chicago, IL, USA). Data was checked for normality using the Shapiro–Wilk test, and mean values and standard deviations (SD) were calculated. Between-group comparisons were tested by Mann–Whitney U tests and Kruskal–Wallis tests for continuous variables and Fisher’s exact test for categorical variables. Cramér’s V was calculated to quantify the strength of association for the output of Fisher’s exact tests. The relationship between bone parameters and age were investigated using Spearman’s rank correlation analysis. The correlation coefficients (r) and Cramér’s V were classified according to Cohen (Cohen 1988). An r and V between 0.10–0.29 was considered a small, between 0.30–0.49 a moderate, and between 0.50–1.0 a strong association. The statistical significance was set at p < 0.05.
Results
Participants
Out of 486 initial participants, seven school children were excluded from the final analysis (two boys with missing questionnaire, one growth-restricted girl, two girls and one boy with implausible nutrition data, one girl with missing bone parameters). In total, data of 231 boys and 248 girls were analyzed. In Table 1, socio-demographic and anthropometric data and lifestyle factors, as well as further characteristics for all participants, are shown. Boys had significantly higher values for height, fat-free mass, absolute and relative RMR. In addition, boys spent more time on sports, had a higher PAL, and stayed longer outside than girls. The absolute and relative fat mass and the puberty category score were higher in girls than boys. Fisher’s exact test revealed that the puberty category score distribution significantly differed by sex. The association between puberty category score and sex was highly significant and can be regarded as “moderate” (Cramér’s V: 0.36, p < 0.001).
The mean bone status parameters were not significantly different between girls and boys (p > 0.05; Table 2). Whereas none of the children had SI and BUA values “below the expected range for age”, six individuals (five girls, one boy) had SOS values below this range. In both sexes, for BUA, SOS, and SI significant differences between age groups were obtained. There were significant positive relationships between BUA, SOS, SI, and age in both sexes (r = 0.38–0.57; p < 0.001), classified as moderate–strong associations. In the age group 13 to ≤ 14 years, girls had significantly higher values for BUA, SOS, and SI than boys, whereas boys ≥ 16 years had significantly higher values of SOS than girls.
Classification of SI tertiles
In Table 3, the characteristics of study participants across tertiles of SI are shown. Girls allocated in the highest tertile of SI had significantly higher body mass, BMI, fat-free mass, absolute and relative fat mass, absolute RMR, and a lower relative RMR than girls from the other two tertiles (p < 0.05). Furthermore, the classification of BMI was significantly different by SI tertiles (Fisher’s exact test). The association between BMI and SI was highly significant and can be classified as “strong” (Cramér’s V: 0.78, p < 0.001). In boys, the Fisher’s exact test revealed that the educational status of the parents significantly differed by SI tertiles. Parents of boys allocated in the lowest and medium tertile of SI had more often a lower educational status than those of boys from the highest tertile of SI. However, the association between parental educational status and SI was non-significant and can be classified as “small” (Cramér’s V: 0.14, p = 0.053). Also, the duration of sports and PAL were significantly different between tertiles, with lower values in the lowest compared to the highest tertile of SI (p < 0.05).
In Table 4, the food intake and the BoneHEI of included study participants across SI tertiles is shown. There were no significant group differences in either food intake or BoneHEI in girls and boys.
Discussion
The study aimed to characterize healthy German children and adolescents across tertiles of calcaneal SI, and to identify risk factors for low bone parameters of participants from the lowest tertile of SI. We assumed that children and adolescents in the lowest tertile are at higher risk for low peak bone mass, as well as for fractures and osteoporosis later in life.
In general, the bone status of participants of this study can be classified as relatively good. Compared to other studies, our research found a higher mean value of SI (girls and boys: SI ~94). For example, the SI of 177 school children (56 girls and 121 boys; age range 11–18 years) from a German college of physical education can be estimated to be ~69 when applying the equation used in the present study for calculation of SI (Mentzel et al. 2005). Furthermore, when the SI of our participants was compared with an age- and sex-matched German reference sample (1623 girls and 1676 boys; age range 6–18 years) (Wünsche et al. 2000), we found ~47% and ~60% higher values of SI for girls and boys of the present study respectively. One explanation for the observed differences of our participants´ bone parameters might be that there are methodological differences between the studies. Specifically, in the two mentioned studies, a different QUS device was used. It has been shown that the type of scanner used strongly influences the validity of QUS measurements (Wang et al. 2014). However, it also might be that participants of the present study have a more advantageous lifestyle for bone health than study participants of the above-mentioned studies. Unfortunately, there is no data available for either PAL or dietary intake in these studies. However, when we look in detail at our study participants´ characteristics, we do have to state that most of them were healthy, normal weight, breastfed, and vitamin D supplemented during infancy, and stayed outside on a regular basis. Their diet can be regarded as adequate [further details about their diet can be found elsewhere (Heydenreich et al. 2020)]. Therefore, it can be supposed that their lifestyle is favorable for bone health. This statement is supported by the fact that the mean SI of the lowest tertile of SI of our girls and boys were still ~22 and ~ 35% higher than the age- and sex-specific mean of the German reference sample (Wünsche et al. 2000). Nevertheless, we identified six individuals (five girls, one boy) with SOS values “below the expected range for age”.
Unsurprisingly, a positive association between age and bone parameters was obtained in the present study. This finding is in accordance with results published in the literature. For example, in 250 Egyptian children and adolescents (8–18 years), age was also significantly related to bone health (Alghadir et al. 2018). It is estimated that approximately 35% of the bone mineral content is acquired during the first 3 years of life, between 4 years of age and the start of puberty 20%, and during adolescence the remaining 45% (del Rio et al. 1994; Muñoz and Argente 2002). Since bone density and bone metabolism necessarily change during adolescence, it is essential to avoid disturbances in bone metabolism which interfere with peak bone mass achievement.
We found that the factors most associated with bone status in girls were anthropometric and body composition variables. Girls with low SI had a significantly lower body mass, BMI, fat-free mass, and absolute and relative fat mass than girls with high SI. These findings support the literature, where it is clearly stated that body weight is the largest single determinant of the variability in adult bone mass (Heaney et al. 2001). One explanation is that body mass imposes a greater mechanical load on the bone, and bone mass increases to accommodate the more significant load (Zhao et al. 2007). Therefore, it is obvious that higher levels of fat-free mass and fat mass, which are two components of body weight, are associated with higher bone mass. However, the impact of fat-free mass on bone health has also been attributed to the influence of biomechanical usage on bone development (Rauch and Schoenau 2001). In addition, hormones and nutrition influence the mechanical loads on bone; however, they cannot replace the guiding effect of mechanical strain on bone (Rauch and Schoenau 2001). Therefore, children and adolescents should perform regular physical activity to increase the mechanical loading on the bone and increase their amounts of fat-free mass. Also, very low body weight and BMI should be avoided, since this is associated with a higher risk for osteoporosis in later life (Munhoz et al. 2018). The finding of low body mass in relation to poor bone health is especially pertinent in adolescents, who may have distorted body image and therefore strive to achieve low body weight. For example, of 230 female and male German school children (8–12 years), 42% of the boys and 53% of the girls preferred a thin ideal body image, with 18% of the boys and 19% of the girls trying to lose body mass at the time of the investigations (Berger et al. 2005). The authors also found out that almost one third (32%) of the normal weight children expressed the wish to be thinner. However, distorted body image and weight-related factors are important individual-level risk factors for developing eating disorders. For example, in girls from the UK, body dissatisfaction in childhood was associated with later eating disorders (Micali et al. 2015). Eating disorders are associated with multiple neuroendocrine disruptions and low bone mineral density (Muñoz and Argente 2002). Long-term skeletal complications can result when excessive food restrictions, particularly during adolescence, can disrupt peak bone mass attainment (Muñoz and Argente 2002). It was shown that 50% of adolescents suffering from anorexia nervosa had bone mineral densities 1 SD below the mean at one or more skeletal sites (Misra et al. 2004). Therefore, not only with regard to better bone health, it is essential to improve body satisfaction in adolescents to reduce the prevalence of severe dietary restrictions and the risk of development of an eating disorder.
In girls with low SI, we also found that the absolute RMR was lower, and the relative RMR was higher than in those with high SI. However, RMR is mainly determined by anthropometric data. Since we observed a significant difference in body mass between groups, it is not surprising that the group with the highest body mass (highest tertile of SI) also has a higher absolute RMR than the other groups. The finding that the relative RMR was higher in girls with low SI is not contradictory; indeed, it results from of the low body mass in the denominator.
In boys, the duration of sports and PAL were significantly different between SI tertiles, with lower values in boys with low compared to those with high SI. This finding supports the theory that increased bone loading due to higher amounts and intensity of physical activity enhances bone metabolism and leads to a higher bone density (Rubin and Lanyon 1984). It was also shown that in very active German children and adolescents, higher values of bone factors were found compared to a normal-active reference sample (Mentzel et al. 2005). Again, these results support the importance of regular physical activity for optimal bone development during childhood and adolescence.
We also obtained the finding that in boys, parental educational status significantly differed across SI tertiles. One explanation is that higher education typically correlates with higher socioeconomic status and better living conditions. In adolescents, a positive association between higher socioeconomic status and physical activity is reported (Hanson and Chen 2007). This might be because adolescents with low socioeconomic backgrounds spend more time indoors due to unsafe neighborhoods or lack of green spaces, and lack of financial resources to participate in sports clubs or fitness centers (Estabrooks et al. 2003). Also, adolescents with low socioeconomic background usually report inadequate nutritional habits (Hanson and Chen 2007). Reasons might be that nutritious food cannot be afforded and nutrition knowledge is lower (Nabhani-Zeidan et al. 2011). In the present study, we also found that girls and boys with low educational background had a significantly lower BoneHEI than those with high or medium educational background (p < 0.05; data not shown). However, the duration of sports and PAL did not differ depending on educational background.
Surprisingly, in the present study, we did not observe significant differences in food intake and BoneHEI between SI tertiles. In contrast, a recent systematic review found that dietary patterns do relate to bone mineral density across different age groups (Denova-Gutiérrez et al. 2018). The authors found that in children and adolescents, the lowest category of a “prudent/healthy” dietary pattern (i.e., poorest diet) was associated with low bone mineral density. Furthermore, Korean adolescents in the highest tertile of “milk and cereal” dietary pattern score had a significantly reduced likelihood of having a low bone mineral density compared with those from the lowest tertile (Shin et al. 2013). One explanation for the missing association between dietary intake and bone status in participants of the present study might be that their dietary intake, in general, can be regarded as adequate. Therefore, dietary intake may not add substantially to other determinants of bone status at this age. It also might be that the selected food items may not capture the variables of the diet that are truly important for the determination of adolescent bone quality.
Limitations and strengths
The following limitations of the present study have to be discussed. Firstly, QUS measurements were applied to assess bone parameters in the study population, which is not the gold standard for assessing bone quality. Nevertheless, QUS measurements can represent an acceptable alternative method compared to the gold standard dual-energy X-ray absorptiometry to assess bone health in adolescents (Torres-Costoso et al. 2018). However, it has been shown that bone mineral density varies between measuring sites (Arlot et al. 1997). In the present study, the bone status was determined at the calcaneal, which is obviously a weight-bearing site, and thus demonstrates probably higher bone mineral densities than at other sites. Therefore, when comparing the results of the present study with those from studies assessing bone mineral densities at other sites, one should take into account the variation of bone parameters across different measuring sites. We classified the participants into low, moderate, and high SI using age- and sex-specific tertiles. Using another method for SI classification might have led to different results.
Secondly, we have to admit that a direct link between low levels of SI (and other bone parameters) during childhood and adolescence, and a higher risk for osteoporosis in later life is not reported in the literature, since there do not exist longitudinal studies studying bone parameters of participants across a wide life-span. However, there is a universal consensus that the amount of peak bone mass during early adulthood is an essential factor with regard to osteoporosis development in later life (Heaney et al. 2001). Therefore, we wanted to characterize healthy children and adolescents across SI tertiles and identify risk factors for low bone parameters in participants allocated in the lowest SI tertile.
Another limitation of the results of our study is that the relative importance of SI compared to other factors influencing peak bone mass cannot be estimated. To our knowledge, in the literature there exists only one longitudinal study aiming to assess bone parameters during childhood, adolescence, and young-adult life to determine peak bone mass (Baxter-Jones et al. 2011). Unfortunately, in this study neither food intake nor physical activity behavior was reported. In addition, bone parameters were assessed by dual-energy X-ray absorptiometry, limiting the transferability of these results to our study. Therefore, at this moment it is impossible to quantify the impact of SI in relation to other factors on peak bone mass.
The assessment of physical activity represents another limitation. Physical activity was estimated based on a structured questionnaire (Krems et al. 2004). However, self-reported physical activity measures demonstrate a greater level of variability than objective measures. Also, we used estimated values to calculate PAL (Müller et al. 2004). It was shown that equation formulas often under- or overestimate real energy costs at rest at an individual level (Heydenreich et al. 2019). We tried to minimize the effect of false estimation of RMR by using an equation developed explicitly for German children and adolescents aged 5–17 years (Müller et al. 2004).
In addition, to evaluate the relationship of food intake and bone health, an adapted version of a standardized food frequency questionnaire was applied, where intake of 13 food items was questioned (Mensink and Burger 2004). However, it might be that other food items also linked to bone health were not considered.
Lastly, we have to address the limitation that further information, apart from education and immigration status, about the parents were not assessed. Therefore, it is impossible to evaluate the relationship between further parent-related risk factors (e.g., prevalence of fractures, body composition status) and bone mass in girls and boys from the present study.
In addition to the limitations described above, the present study has several strengths worth noting. First of all, we assessed and analyzed multiple parameters in many girls and boys (n = 479). Also, we questioned numerous lifestyle factors, such as physical activity habits, dietary factors, habitual outdoor physical activity, use of medication, history of fractures, and breastfeeding during infancy. In comparison to other studies, where mostly correlation analysis was performed for the detection of risk factors for low bone status in adolescents (Jones et al. 2013; Farrar et al. 2016; Alghadir et al. 2018; Heydenreich et al. 2020), we differentiated our participants into tertiles of SI. Thereby, we were able to characterize healthy girls and boys with a lower SI (i.e., lowest tertile of SI) in comparison to those from the other tertiles, and to identify risk factors for low bone parameters of participants from the lowest tertile of SI.
Conclusions
The present study results show that girls and boys from the lowest SI tertile had a lower body mass and a lower level of physical activity than those in the other tertiles. Therefore, we recommend girls to strive for a normal body mass and boys to increase the level of physical activity to improve bone strength and reduce the risk for fractures and osteoporosis in later life. Adequate prevention programs should consider these findings.
References
Alexy U, Clausen K, Kersting M (2008) Die Ernährung gesunder Kinder und Jugendlicher nach dem Konzept der Optimierten Mischkost. Ernährungs Umschau 3:168–177
Alghadir AH, Gabr SA, Rizk AA (2018) Physical fitness, adiposity, and diets as surrogate measures of bone health in schoolchildren: a biochemical and cross-sectional survey analysis. J Clin Densitom 21:406–419. https://doi.org/10.1016/j.jocd.2017.12.006
Arlot ME, Sornay-Rendu E, Garnero P et al (1997) Apparent pre- and postmenopausal bone loss evaluated by DXA at different skeletal sites in women: the OFELY cohort. J Bone Miner Res 12:683–690. https://doi.org/10.1359/jbmr.1997.12.4.683
Baxter-Jones ADG, Faulkner RA, Forwood MR et al (2011) Bone mineral accrual from 8 to 30 years of age: an estimation of peak bone mass. J Bone Miner Res 26:1729–1739. https://doi.org/10.1002/jbmr.412
Berger U, Schilke C, Strauß B (2005) Gewichtssorgen und Diätverhalten bei Kindern in der 3. und 4. Klasse. Psychother Psychosom Med Psychol 55:331–338. https://doi.org/10.1055/s-2004-834745
Carskadon MA, Acebo C (1993) A self-administered rating scale for pubertal development. J Adolesc Health 14:190–195. https://doi.org/10.1016/1054-139x(93)90004-9
Cohen J (1988) Statistical power analysis for behavioral sciences. Lawrence Erlbaum Associates, Hillsdale, NJ
Crockett LJ (1988) Pubertal development scale: pubertal categories. Unpublished manuscript. Pennsylvania State University, Department of Human Development and Family Studies, University Park
del Rio L, Carrascosa A, Pons F et al (1994) Bone mineral density of the lumbar spine in white Mediterranean Spanish children and adolescents: changes related to age, sex, and puberty. Pediatr Res 35:362–366. https://doi.org/10.1203/00006450-199403000-00018
Denova-Gutiérrez E, Méndez-Sánchez L, Muñoz-Aguirre P et al (2018) Dietary patterns, bone mineral density, and risk of fractures: a systematic review and meta-analysis. Nutrients 10:1922. https://doi.org/10.3390/nu10121922
Estabrooks PA, Lee RE, Gyurcsik NC (2003) Resources for physical activity participation: does availability and accessibility differ by neighborhood socioeconomic status? Ann Behav Med 25:100–104. https://doi.org/10.1207/S15324796ABM2502_05
Farrar MD, Mughal MZ, Adams JE et al (2016) Sun exposure behavior, seasonal vitamin D deficiency, and relationship to bone health in adolescents. J Clin Endocrinol Metab 101:3105–3113. https://doi.org/10.1210/jc.2016-1559
Faulkner RA, Bailey DA (2007) Osteoporosis: a pediatric concern? Med Sport Sci 51:1–12. https://doi.org/10.1159/000102993
Ferrari SL (2005) Osteoporosis: a complex disorder of aging with multiple genetic and environmental determinants. In: Simopoulos AP (ed) World review of nutrition and dietetics. Karger, Basel, pp 35–51
Hanson MD, Chen E (2007) Socioeconomic status and health behaviors in adolescence: a review of the literature. J Behav Med 30:263–285. https://doi.org/10.1007/s10865-007-9098-3
Heaney RP, Abrams S, Dawson-Hughes B et al (2001) Peak Bone Mass. Osteoporos Int 11:985–1009. https://doi.org/10.1007/s001980070020
Heydenreich J, Schutz Y, Melzer K, Kayser B (2019) Comparison of conventional and individualized 1-MET values for expressing maximum aerobic metabolic rate and habitual activity related energy expenditure. Nutrients 11:458. https://doi.org/10.3390/nu11020458
Heydenreich J, Schweter A, Lührmann P (2020) Association between body composition, physical activity, food intake and bone status in German children and adolescents. Int J Environ Res Public Health 17:7294. https://doi.org/10.3390/ijerph17197294
Ho-Pham LT, Nguyen UDT, Nguyen TV (2014) Association between lean mass, fat mass, and bone mineral density: a meta-analysis. J Clin Endocrinol Metab 99:30–38. https://doi.org/10.1210/jc.2014-v99i12-30A
Hui SL, Slemenda CW, Johnston CC (1990) The contribution of bone loss to postmenopausal osteoporosis. Osteoporos Int 1:30–34. https://doi.org/10.1007/bf01880413
Jones G, Hynes KL, Dwyer T (2013) The association between breastfeeding, maternal smoking in utero, and birth weight with bone mass and fractures in adolescents: a 16-year longitudinal study. Osteoporos Int 24:1605–1611. https://doi.org/10.1007/s00198-012-2207-3
Kersting M, Alexy U (2009) Empfehlungen für die Ernährung von Kindern und Jugendlichen. Die Optimierte Mischkost optimiX ®, 2. überarb. Auflage. Forschungsinstitut für Kinderernährung (FKE), Dortmund
Kleiser C, Mensink GBM, Scheidt-Nave C, Kurth B-M (2009) HuSKY: a healthy nutrition score based on food intake of children and adolescents in Germany. Br J Nutr 102:610. https://doi.org/10.1017/S0007114509222689
Krems C, Lührmann PM, Neuhäuser-Berthold M (2004) Physical activity in young and elderly subjects. J Sports Med Phys Fitness 44:71–76
Kromeyer-Hauschild K, Wabitsch M, Kunze D et al (2001) Perzentile für den Body-mass-Index für das Kindes- und Jugendalter unter Heranziehung verschiedener deutscher Stichproben. Monatsschr Kinderheilkd 149:807–818. https://doi.org/10.1007/s001120170107
Lewiecki EM, Gordon CM, Baim S et al (2008) International Society for Clinical Densitometry 2007 adult and pediatric official positions. Bone 43:1115–1121. https://doi.org/10.1016/j.bone.2008.08.106
Mensink GBM, Burger M (2004) Was isst du? Bundesgesundheitsbl Gesundheitsforsch Gesundheitsschutz 47:219–226. https://doi.org/10.1007/s00103-003-0794-z
Mentzel H-J, Wünsche K, Malich A et al (2005) Einfluss sportlicher Aktivität von Kindern und Jugendlichen auf den Kalkaneus — Eine Untersuchung mit quantitativem Ultraschall. RöFo - Fortschritte Auf Dem Geb Röntgenstrahlen Bildgeb Verfahr 177:524–529. https://doi.org/10.1055/s-2005-857969
Micali N, De Stavola B, Ploubidis G et al (2015) Adolescent eating disorder behaviours and cognitions: gender-specific effects of child, maternal and family risk factors. Br J Psychiatry J Ment Sci 207:320–327. https://doi.org/10.1192/bjp.bp.114.152371
Misra M, Aggarwal A, Miller KK et al (2004) Effects of anorexia nervosa on clinical, hematologic, biochemical, and bone density parameters in community-dwelling adolescent girls. Pediatrics 114:1574–1583. https://doi.org/10.1542/peds.2004-0540
Müller MJ, Bosy-Westphal A, Klaus S et al (2004) World Health Organization equations have shortcomings for predicting resting energy expenditure in persons from a modern, affluent population: generation of a new reference standard from a retrospective analysis of a German database of resting energy expenditure. Am J Clin Nutr 80:1379–1390. https://doi.org/10.1093/ajcn/80.5.1379
Munhoz L, Aoki EM, Cortes ARG et al (2018) Osteoporotic alterations in a group of different ethnicity Brazilian postmenopausal women: an observational study. Gerodontology 35:101–109. https://doi.org/10.1111/ger.12322
Muñoz MT, Argente J (2002) Anorexia nervosa in female adolescents: endocrine and bone mineral density disturbances. Eur J Endocrinol 147:275–286. https://doi.org/10.1530/eje.0.1470275
Nabhani-Zeidan M, Naja F, Nasreddine L (2011) Dietary intake and nutrition-related knowledge in a sample of Lebanese adolescents of contrasting socioeconomic status. Food Nutr Bull 32:75–83. https://doi.org/10.1177/156482651103200201
Petersen AC, Crockett L, Richards M, Boxer A (1988) A self-report measure of pubertal status: reliability, validity, and initial norms. J Youth Adolesc 17:117–133. https://doi.org/10.1007/BF01537962
Plachta-Danielzik S, Gehrke MI, Kehden B et al (2012) Body fat percentiles for German children and adolescents. Obes Facts 5:77–90. https://doi.org/10.1159/000336780
Rauch F, Schoenau E (2001) The developing bone: slave or master of its cells and molecules? Pediatr Res 50:309–314. https://doi.org/10.1203/00006450-200109000-00003
Rubin CT, Lanyon LE (1984) Regulation of bone formation by applied dynamic loads. J Bone Joint Surg Am 66:397–402
Schroedter J, Lechert Y, Lüttinger P (2006) Die Umsetzung der Bildungsskala ISCED-1997 für die Volkszählung 1970, die Mikrozensus-Zusatzerhebung 1971 und die Mikrozensen 1976–2004 (Version 1). ZUMA-Methodenbericht 2006/08. ZUMA, Mannheim
Schweter A (2015) Einfluss verschiedener Lebensstilfaktoren auf die Knochenmasse von Kindern und Jugendlichen: Möglichkeiten der Gesundheitsförderung im Setting Schule [Internet]. [Schwäbisch Gmünd, Germany]: University of Education, Schwäbisch Gmünd. [cited 2020 May 15]. Available from: https://phsg.bsz-bw.de/frontdoor/index/index/docId/27
Shin S, Hong K, Kang SW, Joung H (2013) A milk and cereal dietary pattern is associated with a reduced likelihood of having a low bone mineral density of the lumbar spine in Korean adolescents. Nutr Res 33:59–66. https://doi.org/10.1016/j.nutres.2012.11.003
Torres-Costoso A, Vlachopoulos D, Ubago-Guisado E et al (2018) Agreement between dual-energy X-ray absorptiometry and quantitative ultrasound to evaluate bone health in adolescents: the PRO-BONE study. Pediatr Exerc Sci 30:466–473. https://doi.org/10.1123/pes.2017-0217
United Nations Educational Scientific and Cultural Organization (UNESCO) (2006) International Standard Classification of Education (ISCED 1997), Re-edition. UNESCO, Paris. [cited 2020 Oct 29]. Available from: http://uis.unesco.org/en/topic/international-standard-classification-education-isced
Wang KC, Wang KC, Amirabadi A et al (2014) Evidence-based outcomes on diagnostic accuracy of quantitative ultrasound for assessment of pediatric osteoporosis — a systematic review. Pediatr Radiol 44:1573–1587. https://doi.org/10.1007/s00247-014-3041-x
Weaver CM, Gordon CM, Janz KF et al (2016) The National Osteoporosis Foundation’s position statement on peak bone mass development and lifestyle factors: a systematic review and implementation recommendations. Osteoporos Int 27:1281–1386. https://doi.org/10.1007/s00198-015-3440-3
Witzke KA, Snow CM (2000) Effects of plyometric jump training on bone mass in adolescent girls. Med Sci Sports Exerc 32:1051–1057. https://doi.org/10.1097/00005768-200006000-00003
World Health Organization (WHO) (1985) Energy and protein requirement. Report of a Joint FAO/WHO/UNU Expert Consultation. Technical Report Series 724. WHO, Geneva
Wünsche K, Wünsche B, Fähnrich H et al (2000) Ultrasound bone densitometry of the os calcis in children and adolescents. Calcif Tissue Int 67:349–355. https://doi.org/10.1007/s002230001143
Xu Y, Guo B, Gong J et al (2014) The correlation between calcaneus stiffness index calculated by QUS and total body BMD assessed by DXA in Chinese children and adolescents. J Bone Miner Metab 32:159–166. https://doi.org/10.1007/s00774-013-0474-5
Zhao L-J, Liu Y-J, Liu P-Y et al (2007) Relationship of obesity with osteoporosis. J Clin Endocrinol Metab 92:1640–1646. https://doi.org/10.1210/jc.2006-0572
Acknowledgements
The authors would like to thank all volunteers who participated in this study.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Heydenreich, J., Schweter, A. & Lührmann, P. Characteristics of healthy German children and adolescents across tertiles of calcaneal stiffness index. J Public Health (Berl.) 30, 2461–2471 (2022). https://doi.org/10.1007/s10389-021-01682-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10389-021-01682-z