Abstract
Background
In a global comparison, in 2006 Germany was the first in the world to switch naratriptan to an OTC medication but since then only almotriptan has been switched. Therefore, Germany has fallen behind recently because of the failure to carry out further switches.
Objectives
The aim of this extensive evaluation of various surveys among pharmacies, physicians, patients and stakeholders was to investigate whether there is an obvious need for an Rx-to-OTC switch of further triptans. In addition, reasons for or against such a switch should be evaluated.
Setting
The surveys were distributed in several ways. Most were based on online tools and were performed in 2017, 2018 and 2019.
Methods
Several surveys have been initiated using different methodologies. The pharmacist surveys were only conducted digitally. The physician surveys were first digitally based on an online platform. A paper form could also be edited manually and returned by fax. The respective questionnaires consisted of 5 to 17 questions on the topic of switching and could all be answered in about 5 to 10 min. BAH Health Monitor hosted the patient surveys and included telephone interviews. The stakeholder survey was performed by personal interviews. The surveys on economic calculations among patients were conducted digitally.
Main outcome measure
Sumatriptan and other Rx triptans are suitable and should be switched to OTC status.
Results
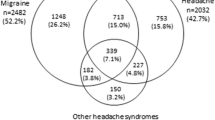
Nine hundred forty pharmacists and pharmaceutical technical assistants took part in the initial pharmacist survey. In the subsequent pharmacist surveys, the volume of participants was even higher and reached almost 4000. For the physician survey, 540 answers were received, and the BAH Health Monitor addressed about 1000 telephone interviews. Within the stakeholder survey, 24 interviews were carried out with 32 participants from a range of professional backgrounds; most are part of the German switch process. Finally, the patient survey related to economic calculations was based on 175 answers. Seventy percent of the participating physicians < 50 years old supported a switch of further triptans. For the question about how safe OTC triptans are, 41% of the 3568 participants from the Marpinion pharmacy survey voted that they are safe.
Conclusion
With a Rx-to-OTC switch of further triptans, patients would have an easier, faster and safe way to treat their migraine attacks.
Impact of findings on practice statements
The outcome of these surveys and interviews provides information for all participants in the German switch process about their decision-making behaviour. Furthermore, it allows deeper insights into patients’ needs and may help to fulfil them. The results can provide information about the German process and German needs worldwide and stimulate discussion about Rx-to-OTC switches.



Similar content being viewed by others
References
ABDA-Bundesvereinigung Deutscher Apothekerverbände e.V. (2019) Die Apotheke: Zahlen Daten Fakten 2019 [the pharmacy counts data facts 2019]
Bundesverband der Arzneimittel-Hersteller e.V. explanatory video “How medicines switch from prescription drug to OTC drug”. https://www.bah-bonn.de/presse/mediathek/videos/. Accessed 09 October 2018a
Bundesverband der Arzneimittel-Hersteller e.V. (2018b) Von der Verschreibungs-zur Apotheken-Pflicht [From prescription-only to pharmacy-only] https://www.bah-bonn.de/bah/?type=565&file=redakteur_filesystem/public/20180606_BAH_switches_D_web.pdf Accessed 09 October 2018
Colquhoun A, Darracott H, Mitra G, MaxDonald M (2009) Pharmacists’ perceptions of POM to P switches. http://www.selfcareforum.org/wp-content/uploads/2011/07/pharmacistswitchresearch2009.pdf. Accessed 08 October 2018
Diener H, Gaul C, Kropp P (2018) Therapie der Migräneattacke und Prophylaxe der Migräne [treatment of migraine attacks and prophylaxis of migraine]. Nervenheilkunde 37:689–715. https://doi.org/10.1055/s-0038-1673598
European Commission (2006) A guideline on changing the classification for the supply of a medicinal product for human use, 1–13. https://ec.europa.eu/health/sites/health/files/files/eudralex/vol-2/c/switchguide_160106_en.pdf. Accessed 08 October 2018
Gauld N (2019) Analysing the landscape for prescription to non-prescription reclassification (switch) in Germany: an interview study of committee members and stakeholders. BMC Health Serv Res 19:404. https://doi.org/10.1186/s12913-019-4219-6
IQVIA Germany https://www.iqvia.com/de-de/locations/germany Accessed 28 October 2019
Katsarava Z, Mania M, Lampl C, Herberhold J, Steiner T (2018) Poor medical care for people with migraine in Europe-evidence from the Eurolight study. J Headache Pain 19:10. https://doi.org/10.1186/s10194-018-0839-1
Kroth E (2018) Switch – the German process for moving medicines from prescription to non-prescription status. Gesundh Ökon Qual Manag 23:97–102. https://doi.org/10.1055/s-0043-115561
Lalonde L, Tsuyuki R, Landry E, Taylor J (2012) Results of a national survey on OTC medicines, part 2: do pharmacists support switching prescription agents to over-the-counter status? Can Pharm J 145:73–76
May U, Bauer C (2017) Apothekengestützte Selbstbehandlung bei leichteren Gesundheitsstörungen – Nutzen und Potenziale aus gesundheitsökonomischer Sicht [Pharmacy-Based Self-Care of Minor Ailments - a Health Economics Focused Perspective on Benefits and potentials]. Gesundh Ökon Qual Manag 22:S12–S22. https://doi.org/10.1055/s-0042-120487
May U, Bauer C, Schneider-Ziebe A (2019) Chancen und Herausforderungen innovativer switches: Teil 3: Triptane in der Migränebehandlung [opportunities and challenges of innovative switches: part 3: Triptans in migraine treatment]. Deutsche Apotheker Zeitung 159:54–59
Millier A, Cohen J, Toumi M (2013) Economic impact of a triptan Rx-to-OTC switch in six EU countries. PLoS One 8:e84088. https://doi.org/10.1371/journal.pone.0084088
Phan V, Wertheimer A (2016) Potential Rx-to-OTC switch drug candidates. iip, 7:1–8. https://doi.org/10.24926/iip.v7i1.414
Schneider-Ziebe A, May U (2019) The treatment of migraine patients with triptans – is there a need for further Rx-to-OTC switches? Gesundh Ökon Qual Manag. Advance online publication. https://doi.org/10.1055/a-0836-2852
Seddik A, Branner J, Ostwald D (2018) Krankheitslast und sozioökonomische Auswirkungen von Migräne in Deutschland [Illness burden and socio-economic effects of migraines in Germany]. Project report Wifor. https://www.wifor.com/uploads/2019/02/2018_Novartis_Socioeconomic-BoD-Migraine_Projektbericht_WifOR-1-1.pdf. Accessed 20 October 2019
Stippler A, Eckstein N, Kroth E (2019a) To switch or not to switch - German physicians' views on proposed new OTC medicines. SelfCare Journal 10:11–23
Stippler A, Eckstein N, Kroth E (2019b) To switch or not to switch—first Germany-wide study from the perspective of pharmacists in the European environment. J Public Health. https://doi.org/10.1007/s10389-019-01101-4
Stippler A, Kroth E, Eckstein N (2018) To switch or not to switch?: Erste deutschlandweite Umfrage zum OTC-Bedarf aus Sicht der Apothekerschaft [to switch or not to switch? First Germany-wide survey on demand of OTC from the pharmacy perspective]. Deutsche Apotheker Zeitung 158:74–81
Stippler A, Voltz A, Kroth E, Eckstein N (2018) OTC-Switches sind Option für viele Ärzte [OTC switches are an option for many doctors]. Ärzte Zeitung, pp 74–81
Sucker-Sket K (2019) Umfrage: Mehr Migräne-OTC erwünscht [Survey: More migraine OTC desired]. https://www.deutsche-apotheker-zeitung.de/news/artikel/2019/09/03-09-2019/mehr-migraene-otc-erwuenscht. Accessed 04 September 2019
Taylor J, Landry E, Lalonde L, Tsuyuki R (2012) Results of a national survey on over-the-counter medicines, part 1: pharmacist opinion on current scheduling status. Can Pharm J 145:40–44.e1. https://doi.org/10.3821/1913-701X-145.1.40
Funding
These studies were funded in full by the German Medicines Manufacturers’ Association (BAH) with a grant of about €80,000 (net).
Author information
Authors and Affiliations
Contributions
All three authors participated in the design, evaluation of results and writing of the paper.
Corresponding author
Ethics declarations
Conflicts of interest
Andrea Stippler, Elmar Kroth and Niels Eckstein declare that the design, evaluation and writing had no funding.
Elmar Kroth is a member (not entitled for vote) of the Expert Advisory Committee for Pharmacy-Only Issues in Germany and Managing Director of Scientific Affairs at BAH. Niels Eckstein is a professor at the University of Applied Sciences Kaiserslautern. Andrea Stippler is studying drug research at the University Bonn and is doing an internship at BAH.
Other than the above-mentioned conflicts, Andrea Stippler, Elmar Kroth and Niels Eckstein declare that they have no conflict of interest.
Ethics approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Results
Detailed information and results can be obtained from the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Stippler, A., Eckstein, N. & Kroth, E. Key results of a series of surveys among German pharmacies, physicians, patients and stakeholders regarding further triptans as potential OTC products. J Public Health (Berl.) 30, 409–415 (2022). https://doi.org/10.1007/s10389-020-01310-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10389-020-01310-2