Abstract
Progression of the physical weakness during neoadjuvant therapy (NAT) in patients with esophageal or gastroesophageal junction cancer is a serious problem; however, prehabilitation during NAT has the potential to overcome the unmet need. Nevertheless, systematic reviews on this topic have not been summarized. Therefore, this systematic review aimed to determine prehabilitation’s effectiveness, acceptability, and safety during NAT for patients with esophageal or gastroesophageal junction cancer. An electronic search was performed in the MEDLINE, Web of Science, CENTRAL, CINAHL, and PEDro databases. A meta-analysis was conducted to assess the effectiveness of prehabilitation during NAT, along with a descriptive analysis of acceptance and safety. This study analyzed data from three randomized controlled trials (RCTs) and nine non-RCTs involving 664 patients. The meta-analysis of two RCTs demonstrated that prehabilitation during NAT may be more effective than usual care in enhancing tolerance to NAT and grip strength; moreover, one RCT and three non-RCTs revealed that prehabilitation may reduce the risk of postoperative complications. The adherence rates for exercise programs in two RCTs and seven non-RCTs were 55–76%. Additionally, two studies reported a 76% adherence rate for multimodal prehabilitation programs, including exercise, dietary, and psychological care. Six studies reported no serious prehabilitation-related adverse events during NAT. Prehabilitation during NAT may be a safe and beneficial intervention strategy for patients with esophageal or gastroesophageal junction cancer. However, the investigation of strategies to enhance adherence is essential. Furthermore, additional high-quality RCTs are needed to examine the effect of prehabilitation during NAT.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Esophageal cancer is a common and lethal tumor globally [1]. The standard treatment for locally advanced esophageal or gastroesophageal junction cancers is neoadjuvant therapy (NAT) followed by surgery [2, 3]. Although NAT improves survival compared with surgery alone [2,3,4], it causes severe adverse events and significantly reduces physical fitness and skeletal muscle mass [5, 6]. In surgeries for esophageal or gastroesophageal junction cancers with significant physical stress, preoperative physical weakness is a severe problem associated with postoperative complications, poor prognosis, and decreased quality of life [7, 8]. Furthermore, the latest studies have shown that themselves, such as reduced physical fitness and skeletal muscle loss during the NAT has a negative impact on postoperative prognosis [6, 9,10,11]. Therefore, to improve postoperative outcomes, the prevention of physical weakness during NAT is essential, and strategies to achieve this should be prioritized.
Prehabilitation, consisting mainly of aerobic exercise, inspiratory muscle training, and nutritional management, might be feasible and effective for preoperative fitness and clinical outcomes of esophagectomy in the systematic review [12]. Meanwhile, there is a lack of information regarding the effectiveness and feasibility of prehabilitation during NAT in a previous review [12]. Various adverse events and symptoms develop during NAT [3]. For patients undergoing surgery after NAT, a prehabilitation program during NAT tailored to the changes in disease status and physical condition is necessary, distinct from common prehabilitation programs. Several recent studies regarding prehabilitation during NAT for patients with esophageal or gastroesophageal junction cancer have demonstrated the feasibility and effectiveness of prehabilitation on physical fitness, skeletal muscle mass, and tolerance to NAT [13,14,15]. However, although there are systematic reviews on prehabilitation during NAT in patients with breast and rectal cancer [16, 17], information on clinical practice and evidence gaps in patients with esophageal or gastroesophageal junction cancer have not been summarized.
This systematic review aimed to determine the effectiveness, acceptability, and safety of prehabilitation during NAT for patients with esophageal or gastroesophageal junction cancer.
Materials and methods
Search strategy
A literature search was performed on March 10, 2023, to identify relevant studies assessing prehabilitation regimens including exercise in patients with esophageal or gastroesophageal junction cancer during NAT, in the MEDLINE (PubMed), Web of Science, CENTRAL, CINAHL, and Physiotherapy Evidence (PEDro) databases. Unpublished literature was searched using the ClinicalTrials.gov and OpenGrey databases. The search included the following keywords: “esophagus,” “gastric,” “neoadjuvant,” “physical therapy,” “prehabilitation,” and “respiratory training.” The references of the included articles were screened, and a manual search was performed to identify missing articles. The complete electronic search strategy is available in the electronic supplementary material (Online Resource 1). Two reviewers (TI and ST) independently assessed titles and abstracts to include relevant references. Subsequently, the full-text articles selected by title and abstract screening were assessed for eligibility. Data extraction from the included studies was performed independently by two reviewers (TI and ST). In cases of disagreement regarding the inclusion or data extraction, a third author (TK) was consulted. The authors of the included studies were contacted to obtain unpublished data. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [18] were followed (Online Resource 2). The searched references were uploaded to Rayyan [19] (Qatar Computing Research Institute, Ar Rayyan, Qatar), and duplicate references were removed. The protocol for this systematic review was registered with PROSPERO (https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42023395998).
Study selection
We included randomized controlled trials (RCTs) and quasi-RCTs evaluating the feasibility or efficacy of prehabilitation during NAT. Retrospective cohort and single-arm studies were included to investigate the feasibility of this approach. There were no restrictions on the country or language. All published and unpublished papers, including conference abstracts, were eligible. No animal studies were included. No exclusions were made on the basis of the observation period. Systematic reviews, meta-analyses, narrative reviews, cluster RCTs, cross-sectional studies, crossover trials, case series, case reports, and letters were excluded. Studies that met the following criteria were included: those involving patients with esophageal or gastroesophageal junction cancer receiving NAT; those incorporating prehabilitation including aerobic exercise, resistance training, and respiratory training during NAT; and those assessing preoperative physical fitness. Studies that met the following criteria were excluded: participants with unstable comorbidities that precluded exercise therapy or exercise function testing, such as severe cardiac or respiratory dysfunction or orthopedic disease; cognitive disorders (Mini-Mental State Examination score less than 24); and nonradical surgery.
Assessment outcomes and other variables
The primary outcomes were exercise capacity (cardiopulmonary exercise test and 6-min walk test) and postoperative complications (postoperative pulmonary complications and surgical site infections, etc.). The secondary outcomes included an assessment of physical fitness other than exercise capacity, tolerance to NAT (completion rate of scheduled treatment), the adherence of prehabilitation (attendance/session*100), and adverse events of prehabilitation.
To understand the characteristics of the included studies, we collected information on participants’ characteristics (age, sex, body mass index [BMI], diagnosis, and NAT regimen) and prehabilitation protocols (type, intensity, time, and frequency).
Assessment for risk of bias and quality of evidence
We assessed the risk of bias in the RCTs using the revised Cochrane risk-of-bias tool for randomized trials (RoB 2) [20]. The RoB2 tool categorizes the risk of bias into “low,” “some concerns,” or “high” groups. The risk of bias in non-randomized comparative studies was assessed using the Newcastle–Ottawa Scale (NOS) [21]. For NOS scores ranging from 0 to 9, we considered scores of 0–3, 4–6, and 7–9 as low, moderate, and high-quality studies, respectively. Single-arm studies were assessed using the National Heart, Lung, and Blood Institute Quality Assessment Tool for before-after (pre- and post-intervention) studies [22]. Based on the 12-item rating, “Good,” “Fair,” and “Poor” scores were determined. All risk-of-bias assessments were conducted in duplicate by two well-trained reviewers (TI and ST). Disagreements between the reviewers were resolved through discussion or by inviting an additional reviewer (TK). The quality of evidence was determined by one researcher (TI) and confirmed and finalized by another researcher (ST). The GRADE (Grading of Recommendations, Assessment, Development, and Evaluation) method was used to check the quality of the evidence [23]. Summary of the findings, prepared according to the Cochrane Handbook is presented in Table 1. Table 1 shows the relative effects per outcome, number of participants, quality of evidence, and additional comments. The standard mean difference (SMD) and risk ratio (RR) were used as relative effects.
Statistical analysis
A meta-analysis of the studies comparing the two groups was performed if at least two published RCTs reported the same outcome within the same timeframe. Published non-RCTs and unpublished studies were included in the meta-analysis only when one or fewer published RCTs examined for each outcome. We pooled the mean differences and 95% confidence intervals (CIs) for the continuous variables (reporting mean and standard deviation or standard error of the mean) for each trial. For cases in which the outcome units were different, we attempted to integrate the data by calculating SMD. RRs with 95% CIs were selected as summary statistics for dichotomized outcomes. The meta-analysis was performed using the Review Manager software (RevMan 5.4, Cochrane) [24]. Missing data were referred to the principal investigator, and in cases where they were unavailable, the substitution method was not used. When a meta-analysis was not possible, results were described narratively. In cases with I2 > 50%, we assessed substantial heterogeneity, and subgroup analysis was performed by age when we have gathered sufficient data. The Cochran chi-squared test (Q test) was used for the I2 statistic, and a p-value of < 0.10 was considered statistically significant. Considering that the intervention effects varied owing to setting differences across studies, we applied a random-effects model. If the mean differences and 95% CIs were not reported, the study was excluded from the meta-analysis. To confirm the robustness of the results, a meta-analysis was performed using non-RCTs for sensitivity analysis. We assessed the possibility of reporting bias using funnel plots.
Results
Study identification
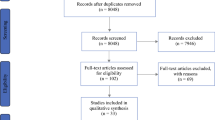
A systematic search of the databases yielded 7342 results. Duplicates were removed, leaving 3,902 records. In the primary screening, 3440 articles were reviewed by title and abstract, and in the secondary screening, 27 articles were evaluated for eligibility using the full text. Ultimately, 12 articles [13,14,15, 25,26,27,28,29,30,31,32,33] were eligible for inclusion. A systematic search of these databases is shown in Fig. 1.
Study characteristics
The study characteristics are summarized in Table 2. Three studies were included in each category: RCTs [13, 14, 25], quasi-RCTs [15, 26, 27], retrospective cohort studies [28,29,30], and prospective single-arm intervention studies [31,32,33]. The literature consisted of nine original articles [13,14,15, 26, 28,29,30, 32, 33] and three unpublished studies [25, 27, 31]. The total number of participants in the 12 included studies was 664. The mean age of the participants ranged from 59.2 to 67.0 years, 74.7 to 100% were men, and the mean BMI ranged from 21.1 to 28.9 kg/m2. Three studies [13, 26, 33] used neoadjuvant chemoradiotherapy, five [15, 27, 29, 30, 32] used neoadjuvant chemotherapy, and three [14, 28, 31] used neoadjuvant chemoradiotherapy or chemotherapy.
Characteristics of the prehabilitation intervention
The characteristics of prehabilitation during NAT are presented in Table 3. Prehabilitation programs mainly consisted of aerobic exercise and resistance training, and the duration ranged 4–16 weeks, with one to seven sessions per week. Aerobic exercise duration ranged 15–60 min, performed at low to moderate intensity [13, 14, 29, 32, 33] moderate/vigorous-intensity activity [28, 30], or high-intensity interval training [15, 26, 31]. Aerobic exercise included programs with expert instruction [13,14,15, 26, 29, 31], self-training [28,29,30,31,32,33], and remote communication using phone calls or applications [28, 30,31,32,33]. Resistance training was performed at moderate to high intensity with expert instruction [15, 26, 29] or self-training [14, 28,29,30,31,32]. Additionally, some studies incorporated nutritional interventions [13, 14, 28,29,30,31, 33] and medical counseling [14, 29,30,31] within their prehabilitation programs.
Assessment of risk of bias
The risk of bias was assessed only for original articles. We presented the risk of bias assessment for exercise capacity as the primary outcome of the published two RCTs (Fig. 2). The bias risks for the two RCTs [13, 14] were categorized as “some concerns” and “high,” respectively. The bias in the outcome measurement was judged to be “high” for one RCT [13]. Three comparative studies, excluding RCTs, were judged to be of “high” [15, 28] or “moderate” quality [26] using the NOS (Online Resource 3). In comparison, one non-RCT [26] had a risk of bias. Two non-RCTs [15, 28] had a risk of bias in the outcome assessment. Four single-arm studies were judged to be of good [29] or fair [30, 32, 33] quality (Online Resource 4). Three studies [30, 32, 33] did not report the blinding of outcome assessments, suggesting the possibility of lower quality.
Effect of prehabilitation
Forest plots using two RCTs [13, 14] are shown for the effects of prehabilitation during NAT on exercise capacity (Fig. 3), grip strength (Fig. 4), skeletal muscle mass (Fig. 5), and tolerance to NAT (Fig. 6). Only one RCT [14] assessed the postoperative complications; hence, meta-analysis was performed using non-RCTs [15, 26, 28] (Fig. 7). In all four studies [14, 15, 26, 28], complications were assessed with Clavien–Dindo classification of 3 or higher. A summary of the effects of prehabilitation during NAT is shown in Table 4. Prehabilitation affected completion rate of NAT (RR [95% CI], 1.51 [1.10, 2.08]) and the change in grip strength during NAT (SMD [95% CI], 1.22 [0.53, 1.90]). However, prehabilitation have no significant effect on exercise capacity (SMD [95% CI], 0.93 [-0.30, 2.17]) and skeletal muscle mass (SMD [95% CI], 0.27 [ – 0.11, 0.65]). Prehabilitation during NAT reduced the risk of postoperative complications (RR [95% CI], 0.61 [0.39, 0.94]). In the sensitivity analysis, including RCTs, non-RCTs, and unpublished literature [13,14,15, 25], prehabilitation during NAT had a significant effect on the exercise capacity (SMD [95% CI], 1.63 [0.11, 3.14]; Online Resource 5) and skeletal muscle mass (SMD [95% CI], 0.36 [0.02, 0.70]; Online Resource 6). Due to the small number of RCTs, a subgroup analysis could not be performed.
Effect of prehabilitation during NAT on exercise capacity. Forest plot shows effect on exercise capacity after NAT. Forest plot using only randomized controlled trials. Std. standard; 95% CI 95% confidence interval; NAT neoadjuvant therapy. Cases with I2 > 50% are considered substantially heterogeneous
Effect of prehabilitation during NAT on skeletal muscle mass. Forest plot shows effect on skeletal muscle mass after NAT. Forest plot using only randomized controlled trials. Std. standard; 95% CI 95% confidence interval; NAT neoadjuvant therapy. Cases with I2 > 50% are considered substantially heterogeneous
Feasibility of the prehabilitation
Feasibility of the prehabilitation during NAT is presented in Table 4. Five studies [13, 14, 26, 29, 31] reported adherence (attendance/sessions*100) to supervised exercise during NAT, ranging 59.6–76.1%. Adherence to low- to moderate- intensity supervised exercise included in multimodal prehabilitation during NAT was 76.0% or 76.1% [14, 29]. Six studies [14, 28, 30,31,32,33] reported 55.0–69.4% adherence to home-based exercises. Adherence to home-based exercises using the application was relatively high (69.4%) [33]. Two studies [13, 31] reported 80.8–100% adherence to dietary interventions. Two studies [14, 32] reported 82.0–100% adherence to psychological care. Nine studies [13, 14, 26, 28,29,30,31,32,33] reported 0–14.3% dropout rates of participants with no disease association in the experimental group. Dropout rates from low- to moderate-intensity supervised exercise included in multimodal prehabilitation during NAT were relatively low (0–7.7%). The characteristics of dropouts from prehabilitation included health-related and non-health concerns (travel, lack of time, and lack of energy) [14, 26, 32, 33]. Six studies [13, 14, 26, 29, 31, 32] reported no serious adverse events (0%).
Discussion
Summary of main results
This systematic review, encompassing data from 12 articles involving 664 participants, aimed to assess the effectiveness, acceptability, and safety of prehabilitation during NAT for patients with esophageal or gastroesophageal junction cancer. The meta-analysis, including two RCTs, demonstrated that prehabilitation was more effective than usual care regarding tolerance to NAT and grip strength. Moreover, an extensive meta-analysis including RCTs, non-RCTs, and unpublished literature showed that prehabilitation during NAT might contribute to a decrease in the risk of postoperative complications and the maintenance of exercise capacity and skeletal muscle mass. Multimodal prehabilitation approaches, including supervised exercise and dietary and psychological care, were well accepted. Serious adverse events related to prehabilitation were not reported.
Implications of the present review
To our knowledge, this study is the first systematic review of the effectiveness, acceptability, and safety of prehabilitation for patients with esophageal or gastroesophageal junction cancer during NAT. Although prehabilitation is considered a standard approach before major surgeries and several reviews have reported its efficacy, acceptability, and safety information on NAT was lacking [12]. Furthermore, a previous review [34] of prehabilitation during NAT for all types of cancer has not focused on important RCTs in patients with esophageal cancer [13, 14, 25]. During NAT, patients with esophageal or gastroesophageal junction cancer experience medical problems such as malnutrition [35] and mental illness [36] due to treatment toxicity. Therefore, we conducted a systematic review of prehabilitation during NAT to summarize the current evidence to develop prehabilitation program specifically for patients during NAT and presented three main findings. First, a meta-analysis of two RCTs [13, 14] showed an effect of prehabilitation on tolerance to NAT and a change in grip strength during NAT. Previous reviews [37, 38] have shown that frailty, namely reduced physiological reserve, leads to vulnerability to the stress of cancer treatment. Therefore, prehabilitation during NAT might contribute to preventing frailty for NAT and surgery against esophageal or gastroesophageal junction as part of multimodal cancer treatment. Second, one RCT and three non-RCTs [14, 15, 26, 28] showed that prehabilitation may reduce the risk of postoperative complications. This finding is essential because postoperative complications after esophagectomy are recognized as one of the most critical concerns. Third, prehabilitation during NAT may be considered safe, showing no significant adverse events related to prehabilitation, irrespective of its frequency, intensity, time, or type. Nevertheless, investigating strategies to enhance adherence to prehabilitation programs is essential. The sample size and number of RCTs are insufficient to make specific recommendations for prehabilitation of patients with esophageal or gastroesophageal junction cancer during NAT; however, these findings will guide the development of future prehabilitation programs.
Effect of prehabilitation during NAT
Physical fitness
Our meta-analysis using only RCTs demonstrated a preventive effect of prehabilitation on the grip strength loss during NAT (SMD [95% CI], 1.22 [0.53, 1.90]). Furthermore, meta-analyses that included RCTs, non-RCTs, and unpublished literature demonstrated preventive effects on the exercise capacity (SMD [95% CI], 1.63 [0.11, 3.14]) and skeletal muscle mass loss (SMD [95% CI], 0.36 [0.02, 0.70]). A meta-analysis using only RCTs showed an SMD [95% CI] of 0.93 [ – 0.30, 2.17] for exercise capacity and 0.27 [ – 0.11, 0.65] for skeletal muscle mass, indicating that a prevention effect could be determined in the future with a sufficient sample size. The results are consistent with the evidence in patients with breast cancer [16] and are considered valid. Since physical fitness indices are key surrogate markers of postoperative clinical outcomes, our findings regarding the effects of prehabilitation during NAT are valuable.
Tolerance to NAT
Interestingly, a meta-analysis of the present review showed that prehabilitation during NAT might be effective in completion rate of scheduled NAT (RR [95% CI], 1.51 [1.10, 2.08]). Xu et al. [13] showed a trend of higher completion rate of NAT in the prehabilitation group than in the usual care group (71% vs. 50%), and Allen et al. [14] found higher completion rates of NAT at full dose (75% vs. 46%). Previous studies have shown that sarcopenia, namely skeletal muscle loss and muscle weakness/physical function decline, in patients with cancer may impact treatment discontinuation and dose reduction [39,40,41]. Furthermore, our meta-analysis suggests that prehabilitation during NAT may have a positive impact on grip strength, and sensitivity analysis showed that prehabilitation affected exercise capacity and skeletal muscle mass. Therefore, prehabilitation may have enhanced the tolerability of NAT by preventing sarcopenia. The effect of prehabilitation on tolerance to NAT may contribute to improving dysphagia, minimizing surgical invasion through tumor regression, reducing postoperative recurrence by completing a standard course of NAT, and maximizing the therapeutic benefit [2, 3]. Therefore, evaluation of the impact of prehabilitation during NAT on tolerance to NAT and long-term outcomes should be a priority for future clinical trials.
Postoperative complications
The meta-analysis including one RCT and three non-RCTs suggested that prehabilitation during NAT may reduce the risk of postoperative complications. Prehabilitation during NAT may have contributed to the reduction of postoperative complications through the improvement in exercise capacity, grip, and skeletal muscle mass, which are risk factors [6, 9,10,11]. The results of our meta-analysis on the potential effectiveness of prehabilitation during NAT on physical fitness indices support this hypothesis. This finding is essential because postoperative complications after esophagectomy are one of the most acknowledged critical concerns. However, the evidence is insufficient, and further RCTs should be conducted to clarify the impact of prehabilitation during NAT on the postoperative complications.
Feasibility of prehabilitation during NAT
A previous study [42] demonstrated that prehabilitation was effective in enhancing exercise capacity for patients with rectal cancer during NAT. This effective program was characterized by an excellent adherence rate of 96% [42]. Consequently, this review focused on identifying a prehabilitation program that would be acceptable for patients with esophageal cancer during NAT as a secondary outcome. Considering most reports conventionally consider ≥ 75% adherence as acceptable [43], in our review, attendance for dietary and psychological care was commendable, exercise attendance was 56–76%, with inadequate and acceptable programs. Notably, participation rates were acceptable (76%) [14, 29] for low- to moderate-intensity supervised exercise included in multimodal prehabilitation, with low dropout rates due to health-related or other reasons (0–7.7%), suggesting better feasibility. As patients with esophageal or gastroesophageal junction cancer experience multiple medical problems during NAT, multimodal interventions [14, 29] may be beneficial to promote adherence to prehabilitation. Previous studies [44,45,46] theoretically indicated the importance of combining exercise therapy with nutritional and psychological care, supporting our hypothesis. Additionally, multilevel intensity exercise [47] and intensive monitoring may be needed for patients with disincentive factors such as baseline fitness weakness or poor motivation [48]. As for prehabilitation programs involving unsupervised or high-intensity exercise, adherence tends to be insufficient; thus, innovations for enhancing feasibility are warranted.
This review examined the safety of various evidence-based prehabilitation programs. Prehabilitation during NAT may be safe, showing no significant adverse events related to prehabilitation, irrespective of its frequency, intensity, time, or type. Previous reviews found exercise therapy during chemotherapy, including NAT, is generally safe for patients with cancers other than esophageal or gastroesophageal junction cancer [16, 17, 49]. Our review supports this, suggesting that prehabilitation during NAT for patients with esophageal or gastroesophageal junction cancer may also be safe.
Quality of evidence
The quality of the studies included in this review ranged from low to high; therefore, the results must be interpreted with caution. Specifically, we found that both RCTs and non-RCTs have a high risk of bias in the measurement of outcomes. This issue needs to be addressed in future clinical trials. Three RCTs were conducted, all of which had small sample sizes, raising concerns regarding imprecision. To determine the effectiveness and feasibility of prehabilitation during the NAT period, a meta-analysis should be conducted using clinical trials with larger sample sizes.
Limitations
This systematic review had several limitations. First, there was heterogeneity in the NAT regimens. Second, there is insufficient RCTs regarding its effect on clinical outcomes. Future studies may reveal additional benefits for postoperative clinical outcomes, considering the potential effectiveness of prehabilitation during NAT. Third, we could not examine what composition would be best for multimodal prehabilitation during NAT. In recent years, multimodal supportive care for patients with advanced cancer are becoming the standard, and additional studies are needed to determine optimal combinations of multiple components. Fourth, the small number of included studies made it difficult to accurately assess reporting bias using funnel plot. Fifth, the sample sizes of the two RCTs are small, the intervention methods vary, and the results differ from the results of the sensitivity analysis, thus the robustness of the results is weak. Finally, due to the small sample size, we have not conducted a subgroup analysis to examine the impact of age on the effectiveness of prehabilitation. Considering the negative impact of postoperative complications on survival in older patients undergoing intensive chemotherapy [50], there is a critical need for prehabilitation during NAT to benefit older patients with advanced esophageal cancer. Further clinical studies are needed to examine the impact of age on the effect of prehabilitation during NAT.
Conclusion
This review demonstrates that prehabilitation during NAT may safely maintain physical fitness, improve tolerance to NAT, and reduce postoperative complications in patients with esophageal or gastroesophageal junction cancer. However, although the potential usefulness of prehabilitation was demonstrated, only two RCTs were eligible for inclusion, which suggested that the high-quality evidence was currently lacking. In the future, the benefit of prehabilitation during NAT and the mechanisms through which prehabilitation improves clinical outcomes should be examined in RCTs with sufficient sample sizes.
References
Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49.
Allum WH, Stenning SP, Bancewicz J, et al. Long-term results of a randomized trial of surgery with or without preoperative chemotherapy in esophageal cancer. J Clin Oncol. 2009;27:5062–7.
Kitagawa Y, Ishihara R, Ishikawa H, et al. Esophageal cancer practice guidelines 2022 edited by the Japan esophageal society: part 2. Esophagus. 2023;20:373–89.
Sjoquist KM, Burmeister BH, Smithers BM, et al. Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: an updated meta-analysis. Lancet Oncol. 2011;12:681–92.
Reijneveld EAE, Bor P, Dronkers JJ, et al. Impact of curative treatment on the physical fitness of patients with esophageal cancer: a systematic review and meta-analysis. Eur J Surg Oncol. 2022;48:391–402.
Wang P, Wang S, Li X, et al. Skeletal muscle wasting during neoadjuvant therapy as a prognosticator in patients with esophageal and esophagogastric junction cancer: a systematic review and meta-analysis. Int J Surg. 2022;97: 106206.
Sivakumar J, Sivakumar H, Read M, et al. The role of cardiopulmonary exercise testing as a risk assessment tool in patients undergoing oesophagectomy: a systematic review and meta-analysis. Ann Surg Oncol. 2020;27:3783–96.
Fang P, Zhou J, Xiao X, et al. The prognostic value of sarcopenia in oesophageal cancer: a systematic review and meta-analysis. J Cachexia Sarcopenia Muscle. 2023;14:3–16.
Bor P, Kingma BF, Kerst A, et al. Decrease of physical fitness during neoadjuvant chemoradiotherapy predicts the risk of pneumonia after esophagectomy. Dis Esophagus. 2021;34:doab008.
Harada T, Tsuji T, Yanagisawa T, et al. Skeletal muscle mass recovery after oesophagectomy and neoadjuvant chemotherapy in oesophageal cancer: retrospective cohort study. BMJ Support Palliat Care. 2023. https://doi.org/10.1136/spcare-2023-004245.
Harada T, Tsuji T, Ueno J, et al. Prognostic impact of the loss of skeletal muscle mass during neoadjuvant chemotherapy on older patients with esophageal cancer. Ann Surg Oncol. 2022;29:8131–9.
Tukanova KH, Chidambaram S, Guidozzi N, et al. Physiotherapy regimens in esophagectomy and gastrectomy: a systematic review and meta-analysis. Ann Surg Oncol. 2022;29:3148–67.
Xu YJ, Cheng JC, Lee JM, et al. A walk-and-eat intervention improves outcomes for patients with esophageal cancer undergoing neoadjuvant chemoradiotherapy. Oncologist. 2015;20:1216–22.
Allen SK, Brown V, White D, et al. Multimodal prehabilitation during neoadjuvant therapy prior to esophagogastric cancer resection: effect on cardiopulmonary exercise test performance, muscle mass and quality of life-a pilot randomized clinical trial. Ann Surg Oncol. 2022;29:1839–50.
Zylstra J, Whyte GP, Beckmann K, et al. Exercise prehabilitation during neoadjuvant chemotherapy may enhance tumour regression in oesophageal cancer: results from a prospective non-randomised trial. Br J Sports Med. 2022;56:402–9.
Furmaniak AC, Menig M, Markes MH. Exercise for women receiving adjuvant therapy for breast cancer. Cochrane Database Syst Rev. 2016;9:CD005001.
Latrille M, Buchs NC, Ris F, et al. Physical activity programmes for patients undergoing neo-adjuvant chemoradiotherapy for rectal cancer: a systematic review and meta-analysis. Medicine (Baltimore). 2021;100: e27754.
Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372: n71.
Ouzzani M, Hammady H, Fedorowicz Z, et al. Rayyan-a web and mobile app for systematic reviews. Syst Rev. 2016;5:210.
Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366: l4898.
Wells GA, Shea B, O´Connell D, et al. The Newcastle‐Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta‐analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm Accessed 20 June 2023.
National Institutes of Health, National Heart, Lung and Blood Institute Quality assessment tool for before-after (pre-post) studies with no control group. https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools Accessed 20 June 2023.
Guyatt G, Oxman AD, Akl EA, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64:383–94.
Higgins JPT, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors. 2nd ed: Cochrane 2022; 2022: https://training.cochrane.org/handbook/current Accessed 20 June 2023.
Loughney L, Tully R, Bolger JC, et al. The effect of a pre- and post- operative exercise programme versus standard care on physical fitness of patients with oesophageal and gastric cancer undergoing neoadjuvant treatment prior to surgery (The PERIOP-OG Trial): a randomised controlled trial. Br J Surg. 2021;108:S9.
Christensen JF, Simonsen C, Banck-Petersen A, et al. Safety and feasibility of preoperative exercise training during neoadjuvant treatment before surgery for adenocarcinoma of the gastro-oesophageal junction. BJS Open. 2018;3:74–84.
Knight W, Zylstra J, White G, et al. Structured prehabilitation reduces physical deconditioning and improves emotional and physical well-being during neo-adjuvant chemotherapy prior to surgery for oesophageal cancer. Br J Surg. 2022;109:S5.
Halliday LJ, Boshier PR, Doganay E, et al. The effects of prehabilitation on body composition in patients undergoing multimodal therapy for esophageal cancer. Dis Esophagus. 2023;36:doac046.
Ikeda T, Noma K, Maeda N, et al. Effectiveness of early exercise on reducing skeletal muscle loss during preoperative neoadjuvant chemotherapy for esophageal cancer. Surg Today. 2022;52:1143–52.
Halliday LJ, Doganay E, Wynter-Blyth V, et al. Adherence to pre-operative exercise and the response to prehabilitation in oesophageal cancer patients. J Gastrointest Surg. 2021;25:890–9.
Kenneth. Feasibility of multimodal prehabilitation to enhance preoperative functional capacity of esophageal cancer patients during concurrent neoadjuvant chemotherapies. 2021. https://escholarship.mcgill.ca/concern/theses/3t945w63r Accessed 20 June 2023.
Chmelo J, Phillips AW, Greystoke A, et al. A feasibility trial of prehabilitation before oesophagogastric cancer surgery using a multi-component home-based exercise programme: the ChemoFit study. Pilot Feasibility Stud. 2022;8:173.
Yang K, Oh D, Noh JM, et al. Feasibility of an interactive health coaching mobile app to prevent malnutrition and muscle loss in esophageal cancer patients receiving neoadjuvant concurrent chemoradiotherapy: prospective pilot study. J Med Internet Res. 2021;23: e28695.
Carla M, Inês RC, Catarina C, et al. Effects of exercise training on cancer patients undergoing neoadjuvant treatment: a systematic review. J Sci Med Sport. 2023;26:586–592.
Lockhart AC, Reed CE, Decker PA, et al. Phase II study of neoadjuvant therapy with docetaxel, cisplatin, panitumumab, and radiation therapy followed by surgery in patients with locally advanced adenocarcinoma of the distal esophagus (ACOSOG Z4051). Ann Oncol. 2014;25:1039–44.
Pinto E, Cavallin F, Scarpa M. Psychological support of esophageal cancer patient. J Thorac Dis. 2019;11:S654–62.
Ethun CG, Bilen MA, Jani AB, et al. Frailty and cancer: implications for oncology surgery, medical oncology, and radiation oncology. CA Cancer J Clin. 2017;67:362–77.
Williams GR, Dunne RF, Giri S, et al. Sarcopenia in the older adult with cancer. J Clin Oncol. 2021;39:2068–78.
Gérard S, Bréchemier D, Lefort A, et al. Body composition and anti-neoplastic treatment in adult and older subjects - a systematic review. J Nutr Health Aging. 2016;20:878–88.
Papadopoulos E, Helal AA, Jin R, et al. The impact of pre-treatment muscle strength and physical performance on treatment modification in older adults with cancer following comprehensive geriatric assessment. Age Ageing. 2022;51:afac152.
Harada T, Tsuji T, Ueno J, et al. Association of sarcopenia with relative dose intensity of neoadjuvant chemotherapy in older patients with locally advanced esophageal cancer: a retrospective cohort study. J Geriatr Oncol. 2023;14: 101580.
West MA, Loughney L, Lythgoe D, et al. Effect of prehabilitation on objectively measured physical fitness after neoadjuvant treatment in preoperative rectal cancer patients: a blinded interventional pilot study. Br J Anaesth. 2015;114:244–51.
Hawley-Hague H, Horne M, Skelton DA, et al. Review of how we should define (and measure) adherence in studies examining older adults’ participation in exercise classes. BMJ Open. 2016;6: e011560.
Gillis C, Fenton TR, Gramlich L, et al. Malnutrition modifies the response to multimodal prehabilitation: a pooled analysis of prehabilitation trials. Appl Physiol Nutr Metab. 2022;47:141–50.
Huddy JR, Huddy FMS, Markar SR, et al. Nutritional optimization during neoadjuvant therapy prior to surgical resection of esophageal cancer-a narrative review. Dis Esophagus. 2018;31:1–11.
Tsimopoulou I, Pasquali S, Howard R, et al. Psychological prehabilitation before cancer surgery: a systematic review. Ann Surg Oncol. 2015;22:4117–23.
Naito T, Mitsunaga S, Miura S, et al. Feasibility of early multimodal interventions for elderly patients with advanced pancreatic and non-small-cell lung cancer. J Cachexia Sarcopenia Muscle. 2019;10:73–83.
Ormel HL, van der Schoot GGF, et al. Predictors of adherence to exercise interventions during and after cancer treatment: a systematic review. Psychooncology. 2018;27:713–24.
Cave J, Paschalis A, Huang CY, et al. A systematic review of the safety and efficacy of aerobic exercise during cytotoxic chemotherapy treatment. Support Care Cancer. 2018;26:3337–51.
Matsuda S, Kitagawa Y, Okui J, et al. Old age and intense chemotherapy exacerbate negative prognostic impact of postoperative complication on survival in patients with esophageal cancer who received neoadjuvant therapy: a nationwide study from 85 Japanese esophageal centers. Esophagus. 2023;20:445–54.
Acknowledgements
We would like to thank Editage (www.editage.com) for English language editing.
Funding
Open Access funding provided by Okayama University. This study did not receive any specific grants from funding agencies in the public, commercial, or non-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical statement
All studies included in this systematic review involve human subjects and have received approval from the ethics review boards of the respective institutions.
Conflict of interest
All authors declare that they have no conflict of interest.
Informed consent
Consent has been obtained from all participants.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ikeda, T., Toyama, S., Harada, T. et al. Effectiveness of prehabilitation during neoadjuvant therapy for patients with esophageal or gastroesophageal junction cancer: a systematic review. Esophagus 21, 283–297 (2024). https://doi.org/10.1007/s10388-024-01049-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10388-024-01049-9