Abstract
Background
Poor oral health is an independent risk factor for upper-aerodigestive tract cancers, including esophageal cancer. Several studies have investigated short-term outcomes after esophagectomy and the impact of periodontal disease, but few have examined the impact of periodontal disease on long-term outcomes. The purpose of this study was to investigate the rate of periodontitis among esophagectomy patients and the prognostic value of periodontitis and its effect on prognosis after esophagectomy.
Methods
A total of 508 patients who underwent esophagectomy received oral health care from a dentist before cancer treatment at Akita University Hospital between January 2009 and December 2021. We assessed the presence and severity of the patients’ periodontitis and divided them into no-periodontitis, mild periodontitis, severe periodontitis and edentulous jaw groups. We then assessed 10-year overall survival (OS) and disease-specific survival (DSS) and determined whether periodontitis was an independent prognostic factor affecting OS and DSS.
Results
We found that 101 (19.9%) patients had no periodontitis, 207 (40.8%) had mild periodontitis, 176 (34.6%) had severe periodontitis requiring tooth extraction, and 24 (4.7%) had edentulous jaw. Both OS and DSS were significantly poorer in the periodontitis than no-periodontitis group (p < 0.001). In detail, the edentulous jaw group had the poorest prognosis (p < 0.001). Multivariate analysis showed that periodontitis was an independent risk factor affecting OS and DSS.
Conclusion
Esophageal cancer patients had a high prevalence of periodontitis. Moreover, the presence of periodontitis and severity of periodontitis are independent risk factors contributing to a poorer prognosis after esophagectomy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Esophageal squamous cell carcinoma (ESCC) is the most common histological type of esophageal cancer in Africa, Central South America, and Asia [1, 2]. Known risk factors for ESCC include habitual cigarette smoking, heavy alcohol consumption, hereditary inactive acetaldehyde dehydrogenase 2 (ALDH2) and a poor diet lacking green and yellow vegetables [3, 4]. More recently, it was reported that poor oral health is also an independent risk factor for upper-aerodigestive tract cancers, including ESCC [5, 6].
Poor oral health usually involves periodontitis, which reportedly correlates with a variety of systemic diseases, including diabetes, heart disease, atherosclerosis, stroke, Alzheimer’s disease, immature birth and various cancers [7,8,9,10]. We previously reported that approximately 70% of our ESCC patients have periodontal disease and about half of those patients required one or more dental extractions before treatment of their cancer [11]. In that regard, tooth loss was recently shown to be associated with a poorer prognosis in ESCC patients [12, 13].
Thanks to advances in multidisciplinary treatments, the prognosis of esophageal cancer patients has improved in recent years [14, 15]. One important treatment is esophagectomy. However, this is a highly invasive gastrointestinal surgery with a high incidence of complications [15]. Among these complications, postoperative pneumonia is commonly observed, and there have been reports suggesting that appropriate oral care can reduce its incidence [11, 16]. Perioperative oral care is therefore considered essential for safe postoperative management after esophagectomy.
In Japan, perioperative management of the oral health of patients with malignant tumors was first covered by health insurance in 2012. Nonetheless, since 2009 we have been routinely providing oral health assessment and oral care by dentists to ESCC patients prior to the start of cancer treatment. By 2021, a total of 508 patients underwent surgery after preoperative oral health evaluation at our hospital. Up to now there have been scattered reports on the relationship between periodontal disease and short-term outcomes after esophagectomy, but there have been few on the effect of periodontal disease on long-term outcomes. In the present study, therefore, we investigated the presence and severity of periodontal disease and its impact on 10-year overall survival (OS) and disease-specific survival (DSS) after esophagectomy for ESCC. We also investigated whether the presence of periodontal disease is the independent prognostic factor affecting 10-year OS.
Materials and methods
Patients
This study was approved by the Ethics Committee of Akita University School of Medicine (#2959, March 2023), and all experiments were performed in accordance with the Helsinki Declaration. All study participants provided informed written consent. Between January 2009 and December 2021, 563 ESCC patients received esophagectomy at Akita University Hospital. Among them, 508 patients received oral health assessment and care by a dentist before cancer treatment. For all these patients, tumor staging was based on the International Union Against Cancer Tumor-Node-Metastasis (TMN) Classification of Malignant Tumors (seventh edition) [17]. Diagnostic evaluation prior to treatment included esophagography, endoscopy, computed tomography (CT) and 18F-fluorodeoxyglucose positron-emission tomography CT. The patients’ preoperative nutritional status and respiratory function were analyzed based on clinical examination data, including %VC, FEV1.0% and albumin. Surgical invasion was assessed based on operation type, operation time and operation bleeding.
Dental management
Dental examinations were performed by a dentist at the time of the initial visit to our hospital. In each patient, periodontitis was classified into 1 of 3 categories: no periodontitis, mild periodontitis or severe periodontitis. Severe periodontitis was accompanied by the need for dental extraction, the criteria for which was (i) Miller’s tooth mobility classification class 3: > 1 mm horizontal + vertical mobility, (ii) > 4 mm periodontal pocket with bleeding or purulent discharge, (iii) severe caries of the teeth with periodontitis, and (iv) teeth with a periapical abscess or cyst [18]. An edentulous jaw was considered to have lost teeth already and was included as periodontal disease. For slight periodontitis, removal of plaque and dental calculus was performed. For dental caries without criteria for tooth extraction, removal of infected tissue and tooth crown restoration were performed. The dentist also explained the importance of oral care and how to perform self-care to all patients.
Treatment strategy
For all patients, staging and treatment strategies were defined at a conference attended by radiologists, physicians and surgeons. In general, patients with locally advanced or node-positive tumors underwent neoadjuvant chemoradiotherapy (NACRT). Briefly, the radiotherapy consisted of 41.4 Gy in 23 fractions. The chemotherapy consisted of a protracted infusion of 5-fluorouracil (800 mg/m2/day) on days 1–5 combined with cisplatin or nedaplatin (80 mg/m2/day) on day 1. This chemotherapy protocol was repeated twice with 3- to 4-weeks interval in between [19, 20]. Our standard operative procedure was transthoracic or thoracoscopic esophagectomy with three-field lymphadenectomy of the mediastinal (involving the periesophageal region and areas around the trachea and bilateral main bronchus), abdominal (involving the perigastric region and areas around the celiac axis) and cervical (involving the bilateral periesophageal region and supraclavicular region) lymph nodes. Reconstruction commonly involved inserting a gastric tube or pedicled colon [21]. A jejunostomy feeding tube was used for patients who needed reconstruction with a pedicled colon graft or were in poor general condition.
Statistical analyses
To test for statistical differences between the periodontitis and no-periodontitis groups, the Wilcoxon test was used for continuous variables and the chi-square and Fisher’s exact tests were used for categorical variables. Outcomes included DSS and OS, which were respectively calculated as the time from the date of esophagectomy to death from esophageal cancer or from any cause. Patients known to be alive or lost to follow-up on the date of last contact were censored in OS. Patients who passed away with other than esophageal cancer were also censored in DSS. Patients The Kaplan–Meier method was used to construct OS and DSS curves, which were compared using the log-rank test. To investigate the impact of periodontitis and to identify independent prognostic factors affecting DSS and OS, we applied a Cox proportional hazard model to calculate the hazard ratios (HRs) and 95% confidence intervals (CIs). After entering all potential predictors for DSS and OS, we ran backward selection based on type III score chi‐square test statistics to identify the most suitable models. We then checked whether the selected variables satisfied the proportional hazard assumption. To minimize the influence of a short observation period, we run sensitivity analyses by eliminating those who were followed 6 months or shorter.
All statistical analyses were performed using JMP Pro15.0 (SAS Institute, Cary, NC, USA), and two-sided p values < 0.05 were considered statistically significant.
Results
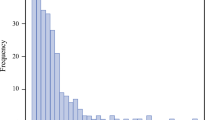
In the 508 cases, the histological types were SCC in 460 patients (90.6%), adenocarcinoma in 36 patients (7.1%) and other cancer types in 12 patients (2.3%). Based on dental examinations, the oral health profile of the 508 study participants was as follows: 101 (19.9%) had no periodontitis, 207 (40.8%) had mild periodontitis, 176 (34.6%) had severe periodontitis requiring tooth extractions, and 24 (4.7%) had edentulous jaws. The numbers of teeth extracted from patients with severe periodontitis are shown in Fig. 1. While most of these patients required extraction of 1 or 2 teeth, some required extraction of as many as 22 teeth.
The clinicopathological features of the no-periodontitis and periodontitis groups are summarized in Table 1. There were no significant differences with respect to sex, age at surgery, Brinkman index, alcohol consumption, tumor location, histologic type, cN, clinical stage (UICC7), pN, pathological stage (UICC7), albumin, %VC, FEV1.0%, operation type, operation time or operation bleeding. On the other hand, the periodontitis group had significantly higher smoking habitation (p = 0.030), higher cT (p = 0.033), higher neoadjuvant therapy rate (p = 0.004), and higher pT (p = 0.026).
Kaplan–Meier curves comparing survival in the periodontitis and no-periodontitis groups showed that both OS (Fig. 2A) and DSS (Fig. 2B) were significantly higher in the no-periodontitis group (p < 0.001). When patients in the periodontitis group were subdivided based on the severity of their periodontitis (Table 2), Kaplan–Meier curves showed OS to be significantly higher in the no-periodontitis than in either the mild or severe periodontitis subgroup (p < 0.001) (Fig. 3A). No significant difference was detected between the mild and severe periodontal subgroups. Notably, the edentulous jaw group had the poorest prognosis. Comparison of DSS between the no-periodontitis and three periodontitis subgroups yielded similar results (Fig. 3B).
Univariate analyses showed that periodontitis (periodontitis vs no-periodontitis), sex (male vs female), age at surgery (65 over vs under 65), tumor invasion (cT; T3-4 vs T1-2), lymph node metastasis (cN; N + vs N0), clinical stage (UICC’7; over 3A vs under 2B), tumor invasion (pT; T3-4 vs T1-2), lymph node metastasis (N + vs N0), pathological stage (UICC7; over 3A vs under 2B), %VC (under 80% vs other) and operation type (transthoracic vs thoracoscopic) were all significant prognostic factors significantly affecting OS. Among these factors, multivariate analysis showed that periodontitis, sex, age at surgery, tumor invasion (cT), pathological stage (UICC7), and %VC were independent risk factors affecting 10-year OS. The HR for the effect of periodontitis on OS was 1.525 (Table 3) and on DSS was 2.040 (Table 4). Moreover, the severity of periodontitis was also independent risk factors affecting OS (Table 5). The HR for the effect of the mild periodontitis, severe periodontitis and edentulous jaw group on OS were 1.424, 1.592 and 2.083, respectively.
Sensitivity analyses by eliminating those who were followed 6 months or shorter confirmed significance of periodontitis associated with both of OS and DSS (Supplementary Table 1,2).
Discussion
The present study shows that more than 80% of our patients who underwent esophagectomy for esophageal cancer had periodontitis and that 35% of those patients required tooth extraction. We also showed that periodontitis, irrespective of severity, was an independent risk factor reducing OS and that edentulous jaw patients had the poorest prognoses.
We did not detect a difference in OS between patients with severe and mild periodontitis, but edentulous jaw patients had the poorest prognosis. We suggest that the oral environment is improved by tooth extraction and that professional oral care by a dentist was effective. On the other hand, patients with edentulous jaws have already lost many teeth and are the most severely affected by periodontitis. Consistent with our findings, an earlier study reported that patients who required more than 13 tooth extractions had the poorest prognosis [13]. However, the median age of this group was 5 years older than the other groups (median age: 67, 65, 65 and 70, respectively).
Several studies have reported that periodontal disease increases the risk not only for esophageal cancer but also for a variety of other cancers, including gastric, colorectal, pancreatic, lung, kidney and hematologic cancers [22,23,24,25]. It also appears to be closely associated with such life-threatening diseases as heart disease, atherosclerosis, stroke and aspiration pneumonia [8, 9, 26]. Notably, we detected a significant difference in DSS between the periodontitis and no-periodontitis groups, suggesting that periodontitis plays a key role specifically in the development, progression and recurrence of esophageal cancer.
The molecular mechanisms underlying the poorer prognosis among ESCC patients with periodontitis remain unclear. However, it is known that periodontitis is most often caused by the gram-negative bacteria Porphyromonas gingivalis, Tannerella forsythia and Treponema denticola, which together are categorized as the “red complex.” For example, P. gingivalis is detected in about 80% of patients with chronic periodontitis [27]. These gram-negative bacteria release lipopolysaccharides (LPS), which are associated with the progression and metastasis of ESCC cells [28, 29]. LPS is recognized by Toll-like receptor 4 (TLR4), high expression of which was previously shown to be an independent factor contributing to a poorer prognosis after esophagectomy [30, 31]. Accordingly, periodontitis may contribute to tumor progression and development by stimulating TLR4, which in turn leads to a poorer OS and DSS.
On the other hand, we recently reported that high expression of TLR6, which recognizes peptidoglycan (PGN) released from gram-positive bacteria in cancer tissue, is predictive of a significantly better prognosis after esophagectomy [32]. So-called “beneficial bacteria” in the gut bacterial flora, such as members of the genus Lactobacillus as well as Bacillus subtilis and several butyrate-producing strains are all gram-positive bacteria [33,34,35]. More importantly, normal inhabitants in oral flora and categorized “early colonizer”, including Streptococcus mitis, Streptococcus oralis and Streptococcus salivarius are all gram-positive bacteria [36]. These gram-positive strains release PGN, which we previously showed to stimulate TLR6 expressed on the surface of ESCC lines and to suppress the proliferation of these cells [32]. This suggests that a predominance of “beneficial” gram-positive bacteria in the oral environment is an important factor in improving prognosis after esophagectomy.
In our previous study, microorganisms most frequently encountered in sputum cultures from the patients with postoperative pneumonia after esophagectomy were MRSA (Methicillin-resistant Staphylococcus aureus), MRSE (Methicillin-resistant Staphylococcus epidermidis) and candida albicans [11]. However, periodontal gram-negative bacteria were anaerobic and categorized as oral bacteria, consequently not detected in our study methods. Another previous study showed that anaerobic oral bacteria were more frequently detected in older patients with aspiration pneumonia than previously believed [37]. Therefore, there is a possibility that periodontal gram-negative bacteria cause postoperative pneumonia in patients with loss of swallowing function after esophagectomy.
Accumulating evidences have shown that postoperative complications are correlated with poorer survival after esophagectomy [38, 39]. Among those evidences, Yuda M, et al. reported that the presence of allopatric bacteria in the oropharynx was an independent risk factor for postoperative pneumonia and correlated with significantly poorer survival [39]. Though the severity of periodontal disease of patients was not mentioned in that study, this result suggests that patients with poor oral condition are predictive of both the occurrence of postoperative pneumonia and poor prognosis after esophagectomy. Taken together, we can speculate that postoperative complications are correlated with poorer survival after esophagectomy, but those patients have poor oral conditions at the bottom.
Based on this idea and previous our findings, we started a specified clinical trial; Improvement of oral environment with toothpaste and mouse wash containing Lactobacillus and the incident rate of pneumonia after esophagectomy for esophageal cancer patients (LacPEC study, jRCTs021230010) in June 2023. Those toothpaste and mouse wash containing Lactobacillus, “beneficial” gram-positive bacteria, are already commercially available in Japan. It was already proved that this Lactobacillus significantly reduced periodontal gram-negative bacteria and candida albicans but not affected normal inhabitants in oral flora, including Streptococcus mitis, Streptococcus oralis. The results of this specified clinical trial may prove the benefit of the predominance of “beneficial” gram-positive bacteria in the oral environment before esophagectomy for preventing postoperative complications, and consequently for better survival.
This study has several limitations. First, there was selection bias due to this being a retrospective, single-center study. Second, the presence or absence and degree of periodontitis were determined by the dentist but could not be expressed in an objective measure. Third, only one evaluation of oral health was performed before the start of treatment, and there were no follow-up data on postoperative oral health. Fourth, there was no preoperative analysis of the oral microbiota in these patients. It would have been ideal if we could have tracked the degree to which periodontitis was improved by the dentist's intervention, the degree to which the patients were able to perform oral care by themselves after discharge from the hospital and how the oral microbiota changed with oral care. In the future, it will be important to establish a system to evaluate changes in the oral environment of patients after esophagectomy and its impact on patient outcome. Fifth, there were 289 censored cases although among these, there were 53 who were alive or did not have a recurrence that should be discriminated from the usual “drop-out”. After subtracting 53 from 289, there were 236 drop-out which consisted 46% of the total numbers.
In summary, patients who underwent esophagectomy had a high prevalence of periodontitis, and about half of the periodontally ill patients had severe periodontitis requiring tooth extraction. Moreover, the presence of periodontitis and severity of periodontitis are independent risk factors contributing to a poorer prognosis after esophagectomy.
Abbreviations
- ESCC:
-
Esophageal squamous cell carcinoma
- ALDH2:
-
Acetaldehyde dehydrogenase 2
- LPS:
-
Lipopolysaccharide
- TLR4:
-
Toll-like receptor 4
- TLR6:
-
Toll-like receptor 6
References
Rustgi AK, El-Serag HB. Esophageal carcinoma. N Engl J Med. 2014;371:2499–509.
Arnold M, Soerjomataram I, Ferlay J, et al. Global incidence of oesophageal cancer by histological subtype in 2012. Gut. 2015;64:381–7.
Morita M, Kumashiro R, Kubo N, et al. Alcohol drinking, cigarette smoking, and the development of squamous cell carcinoma of the esophagus: epidemiology, clinical findings, and prevention. Int J Clin Oncol. 2010;15(2):126–34.
Uhlenhopp DJ, Then EO, Sunkara T, et al. Epidemiology of esophageal cancer: update in global trends, etiology and risk factors. Clin J Gastroenterol. 2020;13(6):1010–21.
Ahrens W, Pohlabeln H, Foraita R, et al. Oral health, dental care and mouthwash associated with upper aerodigestive tract cancer risk in Europe: the ARCAGE study. Oral Oncol. 2014;50(6):616–25.
Baba Y, Iwatsuki M, Yoshida N, et al. Review of the gut microbiome and esophageal cancer: pathogenesis and potential clinical implications. Ann Gastroenterol Surg. 2017;1(2):99–104.
Pihlstrom BL, Michalowicz BS, Johnson NW. Periodontal diseases. Lancet. 2005;366(9499):1809–20.
Awano S, Ansai T, Takata Y, et al. Oral health and mortality risk from pneumonia in the elderly. J Dent Res. 2008;87:334–9.
Abnet CC, Qiao YL, Dawsey SM, et al. Tooth loss is associated with increased risk of total death and death from upper gastrointestinal cancer, heart disease, and stroke in a Chinese population-based cohort. Int J Epidemiol. 2005;34(2):467–74.
Holmlund A, Holm G, Lind L. Number of teeth as a predictor of cardiovascular mortality in a cohort of 7,674 subjects followed for 12 years. J Periodontol. 2010;81(6):870–6.
Sato Y, Motoyama S, Takano H, et al. Esophageal cancer patients have a high incidence of severe periodontitis and preoperative dental care reduces the likelihood of severe pneumonia after esophagectomy. Dig Surg. 2016;33(6):495–502.
Miura S, Nakamura T, Hasegawa T, et al. Tooth loss predicts long-term prognosis of esophageal cancer after esophagectomy. Ann Surg Oncol. 2020;27(3):683–90.
Watanabe T, Sohda M, Kim M, et al. Preoperative evaluation of oral hygiene may predict the overall survival of patients with esophageal cancer. Esophagus. 2023;20:99–108.
Saeki H, Sohda M, Sakai M, et al. Role of surgery in multidisciplinary treatment strategies for locally advanced esophageal squamous cell carcinoma. Ann Gastroenterol Surg. 2020;4:490–7.
Watanabe M, Okamura A, Toihata T, et al. Recent progress in perioperative management of patients undergoing esophagectomy for esophageal cancer. Esophagus. 2018;15:160–4.
Akutsu Y, Matsubara H, Shuto K, et al. Pre-operative dental brushing can reduce the risk of postoperative pneumonia in esophageal cancer patients. Surgery. 2010;147:497–502.
Sobin L, Gospodarowicz M, Wittekind C. International union against cancer. In: TNM classification of malignant tumors. 7th ed. Chichester: Wiley-Blackwell; 2009.
Miller PD Jr. A classification of marginal tissue recession. Int J Periodontics Restor Dent. 1985;5:8–13.
Sato Y, Motoyama S, Wada Y, et al. Neoadjuvant chemoradiotherapy followed by esophagectomy with three-field lymph node dissection for thoracic esophageal squamous cell carcinoma patients with clinical stage III and with supraclavicular lymph node metastasis. Cancers (Basel). 2021;13(5):983.
Sato Y, Motoyama S, Maruyama K, et al. A second malignancy is the major cause of death among thoracic squamous cell esophageal cancer patients negative for lymph node involvement. J Am Coll Surg. 2005;201:188–93.
Sato Y, Motoyama S, Nanjo H, et al. CXCL10 expression status is prognostic in patients with advanced thoracic esophageal squamous cell carcinoma. Ann Surg Oncol. 2016;23:936–42.
Arora M, Weuve J, Fall K, et al. An exploration of shared genetic risk factors between periodontal disease and cancers: a prospective co-twin study. Am J Epidemiol. 2010;171(2):253–9.
Michaud DS, Lu J, Peacock-Villada AY, et al. Periodontal disease assessed using clinical dental measurements and cancer risk in the ARIC study. J Natl Cancer Inst. 2018;110(8):843–54.
Michaud DS, Liu Y, Meyer M, et al. Periodontal disease, tooth loss, and cancer risk in male health professionals: a prospective cohort study. Lancet Oncol. 2008;9(6):550–8.
Shakeri R, Malekzadeh R, Etemadi A, et al. Association of tooth loss and oral hygiene with risk of gastric adenocarcinoma. Cancer Prev Res (Phila). 2013;6(5):477–82.
Komiya K, Ishii H, Kadota J. Healthcare-associated pneumonia and aspiration pneumonia. Aging Dis. 2014;6(1):27–37.
Xu W, Zhou W, Wang H, et al. Roles of Porphyromonas gingivalis and its virulence factors in periodontitis. Adv Protein Chem Struct Biol. 2020;120:45–84.
Lau MC, Ng KY, Wong TL, et al. FSTL1 promotes metastasis and chemoresistance in esophageal squamous cell carcinoma through NFκB-BMP signaling cross-talk. Cancer Res. 2017;77(21):5886–99.
Zu Y, Ping W, Deng T, et al. Lipopolysaccharide-induced toll-like receptor 4 signaling in esophageal squamous cell carcinoma promotes tumor proliferation and regulates inflammatory cytokines expression. Dis Esophagus. 2017;30(2):1–8.
Chen MF, Lu MS, Hsieh CC, et al. Porphyromonas gingivalis promotes tumor progression in esophageal squamous cell carcinoma. Cell Oncol (Dordr). 2021;44(2):373–84.
Sato Y, Motoyama S, Wakita A, et al. High TLR4 expression predicts a poor prognosis after esophagectomy for advanced thoracic esophageal squamous cell carcinoma. Esophagus. 2020;17:408–16.
Sato Y, Wakita A, Maeda E, et al. High TLR6 expression status predicts a more favorable prognosis after esophagectomy for locally advanced thoracic esophageal squamous cell carcinoma. Curr Oncol. 2023;30:4724–35.
Yue Y, Wang S, Shi J, et al. Effects of Lactobacillus acidophilus KLDS1.0901 on proliferation and apoptosis of colon cancer cells. Front Microbiol. 2022;12: 788040.
Botta C, Spyridopoulou K, Bertolino M, et al. Lactiplantibacillus plantarum inhibits colon cancer cell proliferation as function of its butyrogenic capability. Biomed Pharmacother. 2022;149: 112755.
Zhang L, Yi H. Potential antitumor and anti-inflammatory activities of an extracellular polymeric substance (EPS) from Bacillus subtilis isolated from a housefly. Sci Rep. 2022;12:1383.
Butler CA, Adams GG, Blum J, et al. Breastmilk influences development and composition of the oral microbiome. J Oral Microbiol. 2022;14:2096287.
Yamasaki K, Kawanami T, Yatera K, et al. Significance of anaerobes and oral bacteria in community-acquired pneumonia. PLoS ONE. 2013;8: e63103.
Horinouchi T, Yoshida N, Toihata T, et al. Postoperative respiratory morbidity can adversely affect prognosis in thoracoscopic esophagectomy for esophageal cancer: a retrospective study. Surg Endosc. 2023;37:2104–11.
Yuda M, Yamashita K, Okamura A, et al. Influence of preoperative oropharyngeal microflora on the occurrence of postoperative pneumonia and survival in patients undergoing esophagectomy for esophageal cancer. Ann Surg. 2020;272(6):1035–43.
Funding
Open Access funding provided by Akita University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical statement
This study was approved by the Ethics Committee of Akita University School of Medicine (#2959, March 2023), and all experiments were performed in accordance with the Helsinki Declaration.
Conflict of interest
All authors state that they have no conflict of interest to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nozaki, S., Sato, Y., Takano, H. et al. Pretreatment periodontitis is predictive of a poorer prognosis after esophagectomy for esophageal cancer. Esophagus 21, 120–130 (2024). https://doi.org/10.1007/s10388-024-01045-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10388-024-01045-z