Abstract
Background
Ferroptosis suppressor protein 1 and glutathione peroxidase 4 have been identified as key molecules in two independent pathways associated with ferroptosis inhibition. This study investigated the prognostic significance and clinical associations of FSP1 and GPX4 expression in esophageal squamous cell carcinoma (ESCC) and assessed the therapeutic potential of regulating these molecules in ESCC cells.
Methods
Immunohistochemical analysis was performed on surgical specimens of 97 patients with ESCC for FSP1 and GPX4 expression. To identify the change in ESCC cell viability, FSP1 and GPX4 inhibitors were administered to three cell lines. In addition, ferroptosis as the cause of reduced cell viability by FSP1 and GPX4 inhibition was confirmed.
Results
Prognosis was significantly worse for patients in the group positive for both FSP1 and GPX4 compared with the other groups (p < 0.001). In multivariate analysis, positivity for both FSP1 and GPX4 was an independent poor prognostic factor (p = 0.002). The combination of FSP1 and GPX4 inhibitors induced cell death more potently than each inhibitor did alone. Furthermore, the ferroptosis inhibitor markedly canceled this cell death.
Conclusions
Overexpression of FSP1 and GPX4 is a poor prognostic factor for patients with ESCC. Simultaneous suppression of both FSP1 and GPX4 caused potent cell death, which was markedly abrogated by ferroptosis inhibitors. These findings indicate that simultaneous regulation of FSP1 and GPX4 may be a new therapeutic target in ESCC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Esophageal cancer is the sixth most common cancer globally, with more than 480,000 new cases annually [1]. Moreover, this cancer is the fifth leading cause of cancer-related mortality, with 400,000 deaths annually [1]. In addition, the 5-year survival rate after esophagectomy is only 55.6% [2]. Thus, esophageal cancer persists as one of the malignant tumors with a poor prognosis [2].
Inducing cancer cell death is an important strategy in chemotherapy. Chemotherapeutic drugs for esophageal cancer include 5-fluorouracil (5-FU), platinum, and taxane [3], all of which induce apoptosis [4]. Esophageal squamous cell carcinoma (ESCC) has an extremely high mutation rate of apoptosis-inducing p53 (> 90%) [5] and is known to be resistant to chemotherapy [6].
In 2012, researchers discovered ferroptosis, defined as cell death by iron-dependent lipid peroxidation reaction [7]. Research further showed that ferroptosis is a different mechanism of cell death than that which occurs from apoptosis and necrosis, suggesting that ferroptosis is able to induce cell death even in apoptosis-resistant cancer cells [7]. Glutathione peroxidase 4 (GPX4) was reported in 2014 as a factor that inhibits ferroptosis [8]. Since then, several reports have implicated GPX4 in cancer prognosis [9, 10]. Subsequently, in 2019, ferroptosis suppressor protein 1 (FSP1) was reported as a new ferroptosis inhibitor [11, 12], previously known as apoptosis-inducing factor mitochondrion-associated 2 [13]. Both GPX4 and FSP1 negatively regulate ferroptosis by inhibiting lipid peroxidation reactions through different pathways [8, 11, 12].
We previously reported that high expression of GPX4 correlated with worse prognosis in ESCC [14]. The present study aimed to determine the prognostic significance of FSP1 and its association with GPX4 in patients with ESCC. We used the same cohort and performed additional experiments with FSP1, which is associated with another ferroptosis inhibitory pathway. Furthermore, we also examined the therapeutic potential for ferroptosis inhibition with FSP1 and GPX4 in patients with ESCC.
Materials and methods
Patients and pathologic specimens
Study participants were 97 patients who underwent radical esophagectomy for ESCC at Tottori University Hospital, Yonago, Japan, from January 2009 to December 2017. Stored paraffin-embedded specimens were obtained for immunohistochemical analysis. Patients with multiple primary cancers were excluded. Pathological diagnosis was confirmed according to the Japanese Classification of Esophageal Cancer [15]. The patients with clinical T1N0 underwent surgery without preoperative treatment. Patients with ≥ T2 or with lymph node metastasis (cStage ≥ 2) received neoadjuvant chemotherapy (NAC), followed by esophagectomy. In principle, patients treated with NAC underwent surgery 5–7 weeks after NAC completion avoid the influence of NAC. As standard chemotherapeutic drugs, 5-FU and cisplatin were used for all eligible patients except those with impaired renal function, who were instead treated with 5-FU and nedaplatin. The standard surgical procedure was subtotal esophagectomy by a right thoracic approach with three-field lymphadenectomy and reconstruction using a gastric tube. The tumor samples were fixed in 10% neutral buffered formalin solution and embedded in paraffin.
Immunohistochemical analysis
Immunohistochemistry was performed according to the standard protocols, which are further described in the Online Resource.
The expression of FSP1 was defined as < 20% negative and ≥ 20% positive in terms of the tumor-stained area. Immunolabeling was evaluated by three investigators (W.M., Y.S., and Y.U.); consensus was reached in all cases. To evaluate GPX4 expression, our previous staining results were used as follows [14]: “Expression of GPX4 and HMOX1 in the tumor was assessed on a 4-point scale based on the percentage of tumor cells with positive staining (immunohistochemistry) score: 0 [< 10%], 1 [10–50%], 2 [50–90%], 3 [> 90%]).” An immunohistochemistry score of ≥ 2 was considered a positive expression. Immunolabeling was evaluated by three investigators (W.M., Y.S., and Y.U.); consensus was reached in all cases.
Cell lines and cell culture
Three human ESCC cell lines, including KYSE30, KYSE510, and KYSE520, were grown in Roswell Park Memorial Institute Medium supplemented with 10% fetal bovine serum. All cell lines were maintained under 37 °C in atmospheric air supplemented with 5% carbon dioxide and passaged at a ratio of 1:3–1:10 every 2–3 days. Cell line and reagent details are described in the Online Resource. Clinical information on these cell lines is summarized in Table S1 in the Online Resource.
Western blotting analysis
In this analysis, the KYSE30, KYSE510, and KYSE520 cells were seeded at 2 × 105 cells in four 6-cm dishes and incubated overnight. After 24 h, the cells were lysed and protein extracted. Detailed descriptions of the subsequent steps are included in the Online Resource.
Cell proliferation assay after treatment with GPX4 or FSP1 inhibitors
ESCC cell lines were treated with iFSP1—an FSP1 inhibitor—and (1S, 3R)-RSL3 (RSL3; a GPX4 inhibitor) alone or in combination. Cell proliferation was assessed using the Cell Counting Kit-8 (CCK8) according to the manufacturer’s protocol, which is further detailed in the Online Resource.
Cell death inhibition assay
The procedure and elapsed time from cell seeding, addition of drugs, and cell survival evaluation are the same as the cell proliferation assay. The added drug included a combination of iFSP1 and RSL3 plus liproxstatin-1 (Lipro-1, a ferroptosis inhibitor) or Z-VAD-FMK Caspase Inhibitor VI (Z-VAD, an apoptosis inhibitor) or Necrostatin-1 (Necro-1, a necrosis inhibitor), respectively. Detailed descriptions of the subsequent steps are included in the Online Resource.
Statistical analysis
For overall survival and relapse-free survival, survival curves were estimated by Kaplan–Meier analysis; differences in survival curves were compared using the log-rank test. Cox proportional hazards models were used for univariate and multivariate analyses. All quantitative values are presented as the median. For FSP1 and GPX4, association between expression and patient's characteristics was examined using the chi-squared test and Fisher exact test for categorical variables. All p values < 0.05 were considered statistically significant. Statistical analyses were performed using GraphPad Prism (GraphPad Software, La Jolla, CA, USA) and SPSS version 25.0 (IBM, Armonk, NY, USA).
Results
FSP1 and GPX4 expression associated with ESCC prognosis
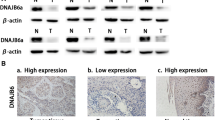
Expression of FSP1 and GPX4 in resected specimens from patients with ESCC was evaluated by immunohistochemistry and classified as positive or negative based on the percentage of positively tumor-stained area (Fig. 1).
Representative immunohistochemical stains for FSP1 and GPX4 in patients with ESCC. Magnification × 100; scale bar, 100 μm. a High-expressing FSP1 ESCC. The FSP1 protein is localized in cytoplasmic regions. b Low-expressing FSP1 ESCC (FSP1-negative case). c High-expressing GPX4 ESCC. The GPX4 protein is localized in cytoplasmic regions. d Low-expressing GPX4 ESCC (GPX4-negative case). ESCC esophageal squamous cell carcinoma; FSP1 ferroptosis suppressor protein 1; GPX4 glutathione peroxidase 4
Of the 97 patients, 20 (20.6%) were positive for FSP1 and 40 (41.2%) for GPX4. The FSP1-positive group had a significantly higher invasion depth (p = 0.001) and disease stage (p = 0.029) than the FSP1-negative group. The GPX4-positive group had significantly higher invasion depth (p = 0.014), lymph node metastasis (p = 0.001), lymphatic involvement (p = 0.019), vascular involvement (p = 0.011), and disease stage (p = 0.002) than the GPX4-negative group (Table 1).
Next, we examined the correlation between FSP1 and GPX4 expression and prognosis of patients with ESCC. The FSP1-positive group had significantly worse overall survival and relapse-free survival than the negative group (Fig. 2a, b). Similar results were obtained for the GPX4-positive and -negative groups (Fig. 2c, d).
Kaplan–Meier survival curves categorized by FSP1 and GPX4 immunoreactivity in ESCC. a, b Survival based on FSP1 expression. c, d Survival based on GPX4 expression. e, f Survival based on simultaneous expression of FSP1 and GPX4. ESCC esophageal squamous cell carcinoma, FSP1 ferroptosis suppressor protein 1, GPX4 glutathione peroxidase 4
In addition, we compared the prognosis between three classified groups based on the combined expression patterns of FSP1 and GPX4. The breakdown of FSP1 and GPX4 positivity/negativity is shown below. Both FSP1- and GPX4-positive; n = 14, FSP1-positive and GPX4-negative; n = 6, FSP1-negative and GPX4-positive; n = 26, Both FSP1- and GPX4-negative; n = 51. Groups that were positive for only one of FSP1 or GPX4 were considered together in one group. The group with both FSP1- and GPX4-positive expression had a significantly worst prognosis (p < 0.001) (Fig. 2e, f). In contrast, the group with both FSP1- and GPX4-negative results showed the best prognosis compared with the other groups.
We also examined whether the combined FSP1 and GPX4 expression pattern was associated with NAC in patients with ≥ T2 or lymph node metastasis (Table S2). The prognostic comparison of the patients with ≥ T2 or lymph node metastasis among the same three groups categorized according to the FSP1 and GPX4 expression revealed a pattern that was similar to that observed in the entire patient population (Fig. S1). In multivariate analysis, positivity for both FSP1 and GPX4 was an independent poor prognostic factor (p = 0.002) (Table 2). For FSP1 and GPX4 expression, both positive groups, which had a particularly poor prognosis, were used as one of the covariates in the multivariate analysis.
Degree of cell death depends on FSP1 or GPX4 expression level
We used three human ESCC-derived cell lines to examine whether FSP1 and GPX4 inhibition induce cell death in ESCC cells. The intensities of FSP1 and GPX4 expression assessed by Western blotting varied between the three cell lines, with negative FSP1 expression in KYSE30 and low expression in KYSE510 and KYSE520. All three cell lines expressed GPX4, as follows: moderate expression in KYSE30, high in KYSE510, and low in KYSE520 (Fig. 3).
To examine the correlations between the differences in FSP1 and GPX4 expression and the degree of ferroptosis induced by inhibiting FSP1 and GPX4, we administered the FSP1 inhibitor iFSP1, the GPX4 inhibitor RSL3, and the ferroptosis inhibitor Lipro-1 to each cell line and evaluated cell viability. Administration of iFSP1 alone had no effect on cell death in KYSE30, which showed negative FSP1 expression (Fig. 4a). In KYSE510 and KYSE520, both of which expressed FSP1, cell viability tended to decrease in KYSE510 (Fig. 4b) and significantly reduced in KYSE520 (Fig. 4c). Administration of RSL3 (GPX4 inhibitor) alone caused significant cell death in all cell lines (Fig. 4a–c). The iFSP1 and RSL3 combination induced cell death more potently in KYSE510 and KYSE520 than with their administration alone (Fig. 4b, c). In all dosing patterns, cell death caused by iFSP1 and RSL3 was canceled by the addition of Lipro-1 (ferroptosis inhibitor) (Fig. 4a–c).
iFSP1 and RSL3 administered alone or in combination with examination of the effect adding Lipro-1 on the decrease in cell viability and the inhibition of the decrease in cell viability. *p < 0.05; **p < 0.01; ***p < 0.001. a KYSE30, b KYSE510, c KYSE520. FSP1 ferroptosis suppressor protein 1, FSP1 inhibitor iFSP1, GPX4 glutathione peroxidase 4, GPX4 inhibitor RSL3, Liproxstatin-1 Lipro-1, NS not significant
FSP1 and GPX4 inhibitor-induced cell death caused by ferroptosis
Finally, we tested whether cell death caused by the combination of iFSP1 and RSL3 could be counteracted by other cell death inhibitors. In all cell lines, cell death was markedly inhibited by the combination of Lipro-1, but administration of Z-VAD and Necro-1 did not cancel the decrease in cell viability (Fig. 5). These results indicated that cell death induced by iFSP1 and RSL3 was due to ferroptosis and not apoptosis or necrosis.
Examination of the different effects of the combination of iFSP1 and RSL3 plus Lipro-1, Z-VAD, and Necro-1 on the three cell lines in preventing the reduction of cell viability. *p < 0.05; **p < 0.01; ***p < 0.001. a KYSE30, b KYSE510, c KYSE520. FSP1 ferroptosis suppressor protein 1, FSP1 inhibitor iFSP1, GPX4 glutathione peroxidase 4, GPX4 inhibitor RSL3, Liproxstatin-1 Lipro-1, Necrostatin-1 Necro-1, Z-VAD-FMK Caspase Inhibitor VI Z-VAD, NS not significant
Discussion
This study showed that expression of FSP1 and GPX4, identified as inhibitors of ferroptosis, correlated with prognosis for patients with ESCC. Furthermore, regulation of these factors markedly induced ferroptosis in ESCC cell lines. To the best of our knowledge, this report is the first to show a correlation between the combination of FSP1 and GPX4 and prognosis in ESCC.
Immunostaining of ESCC and analysis of clinical data indicated that high FSP1 and GPX4 expression was a significantly poor prognostic factor. Both FSP1 and GPX4 are capable of suppressing iron-dependent lipid peroxidation reactions [11, 12]. Oxidative stress is known to induce cell death [16], and the present results are consistent with the evidence. However, previous literature reported better prognosis in lung squamous cell carcinoma when both FSP1 and GPX4 were highly expressed compared with other expression patterns, although no difference in prognosis was observed when FSP1 or GPX4 were analyzed alone [17].
The expression pattern of GPX4 also differs depending on the cancer type. For example, GPX4 expression is reported to be upregulated in hepatocellular carcinoma and colorectal cancer [18, 19], and high GPX4 expression is associated with poor prognosis in gastric cancer [9] and lung adenocarcinoma [10]. Conversely, GPX4 expression is weaker in pancreatic cancer than in nontumor areas [20]. The reason for the conflicting expression patterns in different types of cancer is unclear, but reactive oxygen species affect cancer development in seemingly contradictory ways—promoting tumorigenesis or causing cell death, depending on the concentration [21]. Furthermore, different thresholds of reactive oxygen species concentration at which cell death occurs may be related to different levels of FSP1 or GPX4 expression in different cancers.
Ferroptosis is a mechanism of cell death that differs from apoptosis, and it is proven to have the potential to induce cell death even in apoptosis-resistant tumors [7]. Chemotherapy for esophageal cancer primarily uses apoptosis-inducing agents [3, 4]. Inhibition of GPX4 is reported to induce ferroptosis in persister tumor cells [22], the source of drug-resistant tumor cells. Therefore, cancer therapy may be improved if a novel therapy for the induction of ferroptosis is developed. In the aforementioned report showing the relationship between lung squamous cell carcinoma prognosis and FSP1 and GPX4 expression, the combination of iFSP1 and RSL3-induced marked ferroptosis, and the same was true when FSP1 and GPX4 were knocked out by CRISPR–Cas9 [17]. Furthermore, in an in vivo study, 5-aminolevulinic acid, a natural amino acid, suppressed GPX4 and resulted in tumor shrinkage [14]. Considered with the current study results, simultaneous FSP1 and GPX4 regulation may represent a new target for cancer therapy via induction of ferroptosis. Although these results were obtained only in in vitro experiments, further validation with animal experiments is needed because FSP1 and GPX4 inhibitors were simply used instead of genome editing technology, and administration in vivo is also technically simple.
The preclinical development of both iFSP1 and RSL3 has not been well reported [23]. Several reports focusing on GPX4 suppression in animals showed that mice lacking GPX4 in a nerve-specific manner developed neurodegeneration [24, 25] or died [26]. However, all studies were conducted after genome editing and cannot be directly applied iFSP1 and RSL3 administration to adult mice for whom organ development was already completed. Future studies must confirm the tumor suppression effect and any adverse events in animal experiments.
This study had several limitations. First, it was a single-center study, and the number of samples used was small. Second, the study did not examine the effects of iFSP1 and RSL3 on normal cells. Third, this study contained only a cellular experiment and not an in vivo evaluation.
Conclusion
Overexpression of FSP1 and GPX4, especially in cases of simultaneous overexpression, is a significant poor prognostic factor in ESCC tumors. In ESCC cell lines, simultaneous suppression of both FSP1 and GPX4 caused potent cell death, which was markedly abrogated by ferroptosis inhibitors. These results indicate that simultaneous regulation of FSP1 and GPX4 may be a new target for therapy in patients with ESCC.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90.
Tachimori Y, Ozawa S, Numasaki H, et al. Comprehensive registry of esophageal cancer in Japan, 2012. Esophagus. 2019;16:221–45.
Yamamoto S, Kato K. Immuno-oncology for esophageal cancer. Future Oncol. 2020;16:2673–81.
Su XY, Yin HT, Li SY, et al. Intervention effects of nedaplatin and cisplatin on proliferation and apoptosis of human tumour cells in vitro. Asian Pac J Cancer Prev. 2012;13:4531–6.
Kobayashi T, Makino T, Yamashita K, et al. APR-246 induces apoptosis and enhances chemo-sensitivity via activation of ROS and TAp73-Noxa signal in oesophageal squamous cell cancer with TP53 missense mutation. Br J Cancer. 2021;125:1523–32.
Yamasaki M, Miyata H, Fujiwara Y, et al. p53 genotype predicts response to chemotherapy in patients with squamous cell carcinoma of the esophagus. Ann Surg Oncol. 2010;17:634–42.
Dixon SJ, Lemberg KM, Lamprecht MR, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060–72.
Yang WS, SriRamaratnam R, Welsch ME, et al. Regulation of ferroptotic cancer cell death by GPX4. Cell. 2014;156:317–31.
Wang Y, Zheng L, Shang W, et al. Wnt/beta-catenin signaling confers ferroptosis resistance by targeting GPX4 in gastric cancer. Cell Death Differ. 2022;29(11):2190–202.
Liu K, Jin M, Xiao L, et al. Distinct prognostic values of mRNA expression of glutathione peroxidases in non-small cell lung cancer. Cancer Manag Res. 2018;10:2997–3005.
Doll S, Freitas FP, Shah R, et al. FSP1 is a glutathione-independent ferroptosis suppressor. Nature. 2019;575:693–8.
Bersuker K, Hendricks JM, Li Z, et al. The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature. 2019;575:688–92.
Horikoshi N, Cong J, Kley N, et al. Isolation of differentially expressed cDNAs from p53-dependent apoptotic cells: activation of the human homologue of the Drosophila peroxidasin gene. Biochem Biophys Res Commun. 1999;261:864–9.
Shishido Y, Amisaki M, Matsumi Y, et al. Antitumor effect of 5-aminolevulinic acid through ferroptosis in esophageal squamous cell carcinoma. Ann Surg Oncol. 2021;28:3996–4006.
Japanese Classification of Esophageal Cancer. 11th edition: part I. Esophagus. 2017;14:1–36.
Sekine Y, Hatanaka R, Watanabe T, et al. The Kelch repeat protein KLHDC10 regulates oxidative stress-induced ASK1 activation by suppressing PP5. Mol Cell. 2012;48:692–704.
Asakawa A, Kawade G, Kurata M, et al. Stratification of lung squamous cell carcinoma based on ferroptosis regulators: potential for new therapeutic strategies involving ferroptosis induction. Lung Cancer. 2022;165:82–90.
Guerriero E, Capone F, Accardo M, et al. GPX4 and GPX7 over-expression in human hepatocellular carcinoma tissues. Eur J Histochem. 2015;59:2540.
Yagublu V, Arthur JR, Babayeva SN, et al. Expression of selenium-containing proteins in human colon carcinoma tissue. Anticancer Res. 2011;31:2693–8.
Liu J, Du J, Zhang Y, et al. Suppression of the malignant phenotype in pancreatic cancer by overexpression of phospholipid hydroperoxide glutathione peroxidase. Hum Gene Ther. 2006;17:105–16.
Hayes JD, Dinkova-Kostova AT, Tew KD. Oxidative stress in cancer. Cancer Cell. 2020;38:167–97.
Hangauer MJ, Viswanathan VS, Ryan MJ, et al. Drug-tolerant persister cancer cells are vulnerable to GPX4 inhibition. Nature. 2017;551:247–50.
Conrad M, Lorenz SM, Proneth B. Targeting ferroptosis: new hope for as-yet-incurable diseases. Trends Mol Med. 2021;27:113–22.
Wirth EK, Conrad M, Winterer J, et al. Neuronal selenoprotein expression is required for interneuron development and prevents seizures and neurodegeneration. FASEB J. 2010;24:844–52.
Seiler A, Schneider M, Förster H, et al. Glutathione peroxidase 4 senses and translates oxidative stress into 12/15-lipoxygenase dependent- and AIF-mediated cell death. Cell Metab. 2008;8:237–48.
Yoo SE, Chen L, Na R, et al. Gpx4 ablation in adult mice results in a lethal phenotype accompanied by neuronal loss in brain. Free Radic Biol Med. 2012;52:1820–7.
Funding
None.
Author information
Authors and Affiliations
Contributions
WM, YS, and YF established the study design and analytical concept. WM, YS, YM, and YU perfromed material preparation, data collection, and analysis. WM wrote the first draft of the manuscript, and all authors commented on the previous versions of the manuscript. All the authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical statement
The Institutional Review Board of our institution approved this study (19A084). All procedures were performed according to the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later versions. Informed consent or substitute for it was obtained from all patients for being included in the study.
Conflict of interest
Author Miyauchi, Author Shishido, Author Matsumi, Author Matsunaga, Author Makinoya, Author Shimizu, Author Miyatani, Author Sakamoto, Author Umekita, Author Hasegawa, and Author Fujiwara declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Miyauchi, W., Shishido, Y., Matsumi, Y. et al. Simultaneous regulation of ferroptosis suppressor protein 1 and glutathione peroxidase 4 as a new therapeutic strategy of ferroptosis for esophageal squamous cell carcinoma. Esophagus 20, 492–501 (2023). https://doi.org/10.1007/s10388-022-00982-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10388-022-00982-x