Abstract
Purpose
To evaluate the efficacy and safety of intravitreal aflibercept (IVT-AFL) versus IVT-AFL plus rescue photodynamic therapy (IVT-AFL + rPDT) in the subgroup of Japanese patients with polypoidal choroidal vasculopathy (PCV) enrolled in the PLANET study.
Study design
A 96-week, double-masked, sham-controlled phase-3b/4 randomized clinical trial conducted at multiple centers from May 2014 to August 2016.
Patients and methods
Patients with PCV (BCVA 73-24 ETDRS letters [20/40–20/320 Snellen]) received 3 initial monthly doses of IVT-AFL 2 mg. At week 12, the patients were randomly assigned 1:1 to IVT-AFL + sham PDT or IVT-AFL + rPDT. Patients not requiring rescue received IVT-AFL every 8 weeks; those requiring rescue received IVT-AFL monthly plus sham/active PDT. Following week 52, the treatment intervals could be extended > 8 weeks.
Results
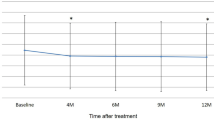
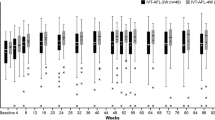
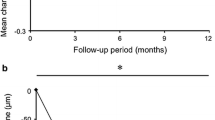
The baseline demographics for the 159 Japanese patients were balanced. At week 96, the mean BCVA change was + 9.7 (IVT-AFL) versus + 9.5 letters (IVT-AFL + rPDT) (least-squares mean difference of − 0.3; 95% CI, − 3.7 to 3.1); the mean central subfield thickness reduction was − 148.0 µm versus − 145.9 µm. Overall, 17.1% of the patients required rescue PDT. At week 96, 25.0% (IVT-AFL) and 37.9% (IVT-AFL + rPDT) of the patients had complete polyp regression; 84.1% (IVT-AFL) and 88.4% (IVT-AFL + rPDT) of the patients had no evidence of active polyps. The mean number of injections (weeks 52–96) were 4.6 (IVT-AFL) and 4.5 (IVT-AFL + rPDT). Overall, 36.0% (IVT-AFL) and 33.8% (IVT-AFL + rPDT) of the patients experienced ocular treatment-emergent adverse events.
Conclusion
IVT-AFL monotherapy was efficacious for the treatment of Japanese patients with PCV, and the addition of rescue PDT did not show additional benefits.



Similar content being viewed by others
References
Wong RL, Lai TY. Polypoidal choroidal vasculopathy: an update on therapeutic approaches. J Ophthalmic Vis Res. 2013;8:359–71.
Byeon SH, Lee SC, Oh HS, Kim SS, Koh HJ, Kwon OW. Incidence and clinical patterns of polypoidal choroidal vasculopathy in Korean patients. Jpn J Ophthalmol. 2008;52:57–62. https://doi.org/10.1007/s10384-007-0498-2.
Ladas ID, Rouvas AA, Moschos MM, Synodinos EE, Karagiannis DA, Koutsandrea CN. Polypoidal choroidal vasculopathy and exudative age-related macular degeneration in Greek population. Eye (Lond). 2004;18:455–9. https://doi.org/10.1038/sj.eye.6700706.
Lafaut BA, Leys AM, Snyers B, Rasquin F, De Laey JJ. Polypoidal choroidal vasculopathy in caucasians. Graefes Arch Clin Exp Ophthalmol. 2000;238:752–9.
Scassellati-Sforzolini B, Mariotti C, Bryan R, Yannuzzi LA, Giuliani M, Giovannini A. Polypoidal choroidal vasculopathy in Italy. Retina. 2001;21:121–5.
Lim LS, Mitchell P, Seddon JM, Holz FG, Wong TY. Age-related macular degeneration. Lancet. 2012;379:1728–38. https://doi.org/10.1016/s0140-6736(12)60282-7.
Schmidt-Erfurth U, Kaiser PK, Korobelnik JF, Brown DM, Chong V, Nguyen QD, et al. Intravitreal aflibercept injection for neovascular age-related macular degeneration: ninety-six-week results of the VIEW studies. Ophthalmology. 2014;121:193–201. https://doi.org/10.1016/j.ophtha.2013.08.011.
Brown DM, Michels M, Kaiser PK, Heier JS, Sy JP, Ianchulev T. Ranibizumab versus verteporfin photodynamic therapy for neovascular age-related macular degeneration: two-year results of the ANCHOR study. Ophthalmology. 2009;116(57–65):e5. https://doi.org/10.1016/j.ophtha.2008.10.018.
Holz FG, Tadayoni R, Beatty S, Berger A, Cereda MG, Cortez R, et al. Multi-country real-life experience of anti-vascular endothelial growth factor therapy for wet age-related macular degeneration. Br J Ophthalmol. 2015;99:220–6. https://doi.org/10.1136/bjophthalmol-2014-305327.
Eleftheriadou M, Vazquez-Alfageme C, Citu CM, Crosby-Nwaobi R, Sivaprasad S, Hykin P, et al. Long-term outcomes of aflibercept treatment for neovascular age-related macular degeneration in a clinical setting. Am J Ophthalmol. 2017;174:160–8. https://doi.org/10.1016/j.ajo.2016.09.038.
Cohen SY, Mimoun G, Oubraham H, Zourdani A, Malbrel C, Quere S, et al. Changes in visual acuity in patients with wet age-related macular degeneration treated with intravitreal ranibizumab in daily clinical practice: the LUMIERE study. Retina. 2013;33:474–81. https://doi.org/10.1097/IAE.0b013e31827b6324.
Souied EH, Oubraham H, Mimoun G, Cohen SY, Quere S, Derveloy A, et al. Changes in visual acuity in patients with wet age-related macular degeneration treated with intravitreal ranibizumab in daily clinical practice: the TWIN study. Retina. 2015;35:1743–9. https://doi.org/10.1097/IAE.0000000000000548.
Holz FG, Tadayoni R, Beatty S, Berger A, Cereda MG, Hykin P, et al. Key drivers of visual acuity gains in neovascular age-related macular degeneration in real life: findings from the AURA study. Br J Ophthalmol. 2016;100:1623–8. https://doi.org/10.1136/bjophthalmol-2015-308166.
Lotery A, Griner R, Ferreira A, Milnes F, Dugel P. Real-world visual acuity outcomes between ranibizumab and aflibercept in treatment of neovascular AMD in a large US data set. Eye (Lond). 2017;31:1697–706. https://doi.org/10.1038/eye.2017.143.
Eleftheriadou M, Gemenetzi M, Lukic M, Sivaprasad S, Hykin PG, Hamilton RD, et al. Three-year outcomes of aflibercept treatment for neovascular age-related macular degeneration: evidence from a clinical setting. Ophthalmol Ther. 2018. https://doi.org/10.1007/s40123-018-0139-5.
Almuhtaseb H, Johnston RL, Talks JS, Lotery AJ. Second-year visual acuity outcomes of nAMD patients treated with aflibercept: data analysis from the UK Aflibercept Users Group. Eye (Lond). 2017;31:1582–8. https://doi.org/10.1038/eye.2017.108.
Wong TY, Cheung CMG, Lai TYY, Chen SJ, Lee WK, Yoon YH, et al. Efficacy and safety of intravitreal aflibercept and ranibizumab in Asian patients with neovascular age-related macular degeneration: subgroup analyses from the View trials. Retina. 2017. https://doi.org/10.1097/iae.0000000000001986.
Cheung CMG, Lai TYY, Ruamviboonsuk P, Chen SJ, Chen Y, Freund KB, et al. Polypoidal choroidal vasculopathy: definition, pathogenesis, diagnosis, and management. Ophthalmology. 2018;125:708–24. https://doi.org/10.1016/j.ophtha.2017.11.019.
Hosokawa M, Shiraga F, Yamashita A, Shiragami C, Ono A, Shirakata Y, et al. Six-month results of intravitreal aflibercept injections for patients with polypoidal choroidal vasculopathy. Br J Ophthalmol. 2015;99:1087–91. https://doi.org/10.1136/bjophthalmol-2014-305275.
Cho HJ, Kim KM, Kim HS, Han JI, Kim CG, Lee TG, et al. Intravitreal aflibercept and ranibizumab injections for polypoidal choroidal vasculopathy. Am J Ophthalmol. 2016;165:1–6. https://doi.org/10.1016/j.ajo.2016.02.019.
Lee JE, Shin JP, Kim HW, Chang W, Kim YC, Lee SJ, et al. Efficacy of fixed-dosing aflibercept for treating polypoidal choroidal vasculopathy: 1-year results of the VAULT study. Graefes Arch Clin Exp Ophthalmol. 2017;255:493–502. https://doi.org/10.1007/s00417-016-3489-5.
Ijiri S, Sugiyama K. Short-term efficacy of intravitreal aflibercept for patients with treatment-naive polypoidal choroidal vasculopathy. Graefes Arch Clin Exp Ophthalmol. 2015;253:351–7. https://doi.org/10.1007/s00417-014-2707-2.
Inoue M, Arakawa A, Yamane S, Kadonosono K. Short-term efficacy of intravitreal aflibercept in treatment-naive patients with polypoidal choroidal vasculopathy. Retina. 2014;34:2178–84. https://doi.org/10.1097/iae.0000000000000229.
Arakawa A, Inoue M, Sato S, Yamane S, Kadonosono K. Efficacy of intravitreal aflibercept injections for Japanese patients with polypoidal choroidal vasculopathy. Clin Ophthalmol. 2017;11:797–802. https://doi.org/10.2147/opth.S129164.
Koh A, Lee WK, Chen LJ, Chen SJ, Hashad Y, Kim H, et al. EVEREST study: efficacy and safety of verteporfin photodynamic therapy in combination with ranibizumab or alone versus ranibizumab monotherapy in patients with symptomatic macular polypoidal choroidal vasculopathy. Retina. 2012;32:1453–64. https://doi.org/10.1097/IAE.0b013e31824f91e8.
Yamamoto A, Okada AA, Kano M, Koizumi H, Saito M, Maruko I, et al. One-year results of intravitreal aflibercept for polypoidal choroidal vasculopathy. Ophthalmology. 2015;122:1866–72. https://doi.org/10.1016/j.ophtha.2015.05.024.
Wong CW, Cheung CM, Mathur R, Li X, Chan CM, Yeo I, et al. Three-year results of polypoidal choroidal vasculopathy treated with photodynamic therapy: retrospective study and systematic review. Retina. 2015;35:1577–93. https://doi.org/10.1097/iae.0000000000000499.
Lee WK, Iida T, Ogura Y, Chen SJ, Wong TY, Mitchell P, et al. Efficacy and safety of intravitreal aflibercept for polypoidal choroidal vasculopathy in the PLANET study: a randomized clinical trial. JAMA Ophthalmol. 2018;136:786–93. https://doi.org/10.1001/jamaophthalmol.2018.1804.
Koh A, Lai TYY, Takahashi K, Wong TY, Chen LJ, Ruamviboonsuk P, et al. Efficacy and safety of ranibizumab with or without verteporfin photodynamic therapy for polypoidal choroidal vasculopathy: a randomized clinical trial. JAMA Ophthalmol. 2017;135:1206–13. https://doi.org/10.1001/jamaophthalmol.2017.4030.
Wong TY, Ogura Y, Lee WK, Iida T, Chen SJ, Mitchell P, et al. Efficacy and safety of intravitreal aflibercept for polypoidal choroidal vasculopathy: 2-year results of the PLANET study. Am J Ophthalmol. 2019. https://doi.org/10.1016/j.ajo.2019.02.027.
Acknowledgements
The authors thank all the patients and investigators who contributed to this study. Medical writing and editorial support for the preparation of this manuscript (under the guidance of the authors) were provided by Mia Cahill (ApotheCom, UK). Mia Cahill has no conflict of interest to declare. The authors thank Zhongqi Zhang, PhD, for providing statistical support.
Funding
Funding was provided by Bayer Consumer Care AG, Basel, Switzerland for the overall study and for medical writing and editorial assistance for this article.
Author information
Authors and Affiliations
Contributions
All authors take full responsibility for the content and scientific accuracy of the manuscript and the online supplementary materials. YO conception and design of the study, drafting of the manuscript, and administrative supervision. TI conception and design of the study, acquisition of data, critical review of intellectual content, and administrative supervision. WKL conception and design of the study, acquisition of data, critical review of intellectual content, and administrative supervision. CMGC analysis and interpretation of the data, critical review of intellectual content, and administrative supervision. PM acquisition of data, analysis and interpretation of the data, critical review of intellectual content, and administrative supervision. SL conception and design of the study, analysis and interpretation of the data, drafting of the manuscript, critical review of intellectual content, obtaining funding, and administrative supervision. TS analysis and interpretation of the data, critical review of intellectual content, and administrative supervision. TI conception and design of the study, acquisition of data, critical review of intellectual content, and administrative supervision.
Corresponding author
Ethics declarations
Conflicts of interest
Y. Ogura, Grant (Novartis, Santen, Boehringer Ingelheim), Consultant fee (Novartis, Santen, Senju, HOYA, Alcon Japan, Kowa, Jansen, Boehringer Ingelheim, Astellas), Lecture fee (Santen, Nikon, Wakamoto, Otsuka) and personal fees from Bayer in relation to the conduct of the PLANET study; T. Iida, Consultant fee, Advisory board member (Bayer, Novartis, Alcon Pharma, Chugai, Kowa); W. K. Lee, Consultant fee (Novartis, Bayer, Roche, Boehringer Ingelheim, Santen); C. M. G. Cheung, Consultant fee (Bayer, Novartis, Roche, Boehringer Ingelheim, Allergan), Lecture fee (Topcon, Zeiss), Grant (Bayer, Novartis, Topcon, Boehringer Ingelheim, Zeiss), Nonfinancial support (Bayer, Novartis, Roche, Topcon, Boehringer Ingelheim); P. Mitchell, Consultant fee, Advisory board member (Bayer, Novartis, Allergan, Mylan); S. Leal, Employee, Stockholder (Bayer Consumer Care AG, Pharmaceuticals, Basel, Switzerland), Patent WO2018/229034 pending); T. Schmelter, Employee, Stockholder (Bayer AG, Berlin, Germany); T. Ishibashi, Consultant fee, Advisory board member (Bayer, Novartis, Alcon Pharma, HOYA, Kowa, Bausch + Lomb).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Corresponding Author: Yuichiro Ogura
Supplementary Information
Below is the link to the electronic supplementary material.
About this article
Cite this article
Ogura, Y., Iida, T., Lee, W.K. et al. Efficacy and safety of intravitreal aflibercept for polypoidal choroidal vasculopathy: 96-week outcomes in the Japanese subgroup of the PLANET study. Jpn J Ophthalmol 65, 344–353 (2021). https://doi.org/10.1007/s10384-020-00805-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10384-020-00805-5