Abstract
Purpose
The temporal and spatial characteristics of cone degeneration in the Royal College of Surgeons (RCS) rat were studied to provide information for treatment strategies of retinitis pigmentosa.
Methods
Nonpigmented dystrophic RCS rats (RCS) and pigmented nondystrophic RCS rats (controls) were used. Cone processes were visualized with peanut agglutinin (PNA).
Results
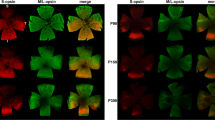
Cone development appears to have been completed by postnatal day 21 (P21) in both the RCS and control rats. Signs of cone degeneration were obvious by P30, with shorter outer segments (OSs) and enlarged inner segments (ISs). At that time, 81.7% of the cones retained stained ISs. The rate of IS density decline was slower in the peripheral, nasal, and superior retina, and only 43.6% of the cones with ISs were present at P45. By P60, PNA-labeled cone ISs were distorted and restricted to the peripheral retina, and by P90, few cone pedicles were detected.
Conclusions
Our findings indicate that therapeutic strategies aimed at rescuing cones in the degenerating retina should be applied before P21 and no later than P45 while substantial numbers of cones retain their ISs. Either the middle or peripheral regions of the nasal and superior retina are the best locations for transplantation strategies.
Similar content being viewed by others
References
Marc RE, Jones BW, Watt CB, Strettoi E. Neural remodeling in retinal degeneration. Prog Retin Eye Res 2003;22:607–655.
Lund RD, Kwan AS, Keegan DJ, Sauve Y, Coffey PJ, Lawrence JM. Cell transplantation as a treatment for retinal disease. Prog Retin Eye Res 2001;20:415–449.
Aramant RB, Seiler MJ. Progress in retinal sheet transplantation. Prog Retin Eye Res 2004;23:475–494.
Pinilla I, Cuenca N, Sauve Y, Wang S, Lund RD. Preservation of outer retina and its synaptic connectivity following subretinal injections of human RPE cells in the Royal College of Surgeons rat. Exp Eye Res 2007;85:381–392.
Wojciechowski AB, Englund U, Lundberg C, Wictorin K, Warfvinge K. Subretinal transplantation of brain-derived precursor cells to young RCS rats promotes photoreceptor cell survival. Exp Eye Res 2002;75:23–37.
Rosenfeld PJ, Cowley GS, McGee TL, Sandberg MA, Berson EL, Dryja TP. A null mutation in the rhodopsin gene causes rod photoreceptor dysfunction and autosomal recessive retinitis pigmentosa. Nat Genet 1992;1:209–213.
McLaughlin ME, Sandberg MA, Berson EL, Dryja TP. Recessive mutations in the gene encoding the beta-subunit of rod phosphodiesterase in patients with retinitis pigmentosa. Nat Genet 1993;4:130–134.
Girman SV, Wang S, Lund RD. Time course of deterioration of rod and cone function in RCS rat and the effects of subretinal cell grafting: a light- and dark-adaptation study. Vision Res 2005;45:343–354.
LaVail MM. Legacy of the RCS rat: impact of a seminal study on retinal cell biology and retinal degenerative diseases. Prog Brain Res 2001;131:617–627.
Huang XY, Yin ZQ, Tan XL. Characteristics of retinal stem cells from rat optic cup at embryonic day 12.5 (tailbud stage). Cell Tissue Res 2008;333:381–393.
Liu DN, Yin ZQ, Wu N, Wang YH, Chen LF. Rat bone marrow stromal cells express retinal phenotypic markers following different induction protocols. Ophthalmic Res 2009;41:186–193.
LaVail MM, Sidman M, Rausin R, Sidman RL. Discrimination of light intensity by rats with inherited retinal degeneration: a behavioral and cytological study. Vision Res 1974;14:693–702.
Cicerone CM, Green DG, Fisher LJ. Cone inputs to ganglion cells in hereditary retinal degeneration. Science 1979;203:1113–1115.
Cotter JR, Noell WK. Ultrastructure of remnant photoreceptors in advanced hereditary retinal degeneration. Invest Ophthalmol Vis Sci 1984;25:1366–1375.
Sauve Y, Pinilla I, Lund RD. Partial preservation of rod and cone ERG function following subretinal injection of ARPE-19 cells in RCS rats. Vision Res 2006;46:1459–1472.
Li L, Turner JE. Optimal conditions for long-term photoreceptor cell rescue in RCS rats: the necessity for healthy RPE transplants. Exp Eye Res 1991;52:669–679.
Krishnamoorthy V, Jain V, Cherukuri P, Baloni S, Dhingra NK. Intravitreal injection of fluorochrome-conjugated peanut agglutinin results in specific and reversible labeling of mammalian cones in vivo. Invest Ophthalmol Vis Sci 2008;49:2643–2650.
Li A, Lane WS, Johnson LV, Chader GJ, Tombran-Tink J. Neuronspecific enolase: a neuronal survival factor in the retinal extracellular matrix? J Neurosci 1995;15:385–393.
Johnson LV, Hageman GS. Structural and compositional analyses of isolated cone matrix sheaths. Invest Ophthalmol Vis Sci 1991;32:1951–1957.
LaVail MM, Sidman RL, Gerhardt CO. Congenic strains of RCS rats with inherited retinal dystrophy. J Hered 1975;66:242–244.
Dowling JE, Sidman RL. Inherited retinal dystrophy in the rat. J Cell Biol 1962;14:73–109.
Rapaport DH, Wong LL, Wood ED, Yasumura D, LaVail MM. Timing and topography of cell genesis in the rat retina. J Comp Neurol 2004;474:304–324.
Blanks JC, Bok D. An autoradiographic analysis of postnatal cell proliferation in the normal and degenerative mouse retina. J Comp Neurol 1977;174:317–327.
Rich KA, Zhan Y, Blanks JC. Migration and synaptogenesis of cone photoreceptors in the developing mouse retina. J Comp Neurol 1997;388:47–63.
Rubin GR, Kraft TW. Flicker assessment of rod and cone function in a model of retinal degeneration. Doc Ophthalmol 2007;115:165–172.
Lin B, Masland RH, Strettoi E. Remodeling of cone photoreceptor cells after rod degeneration in rd mice. Exp Eye Res 2009;88:589–599.
Nguyen-Legros J, Hicks D. Renewal of photoreceptor outer segments and their phagocytosis by the retinal pigment epithelium. Int Rev Cytol 2000;196:245–313.
Winkler BS. An hypothesis to account for the renewal of outer segments in rod and cone photoreceptor cells: renewal as a surrogate antioxidant. Invest Ophthalmol Vis Sci 2008;49:3259–3261.
Sakai T, Calderone JB, Lewis GP, Linberg KA, Fisher SK, Jacobs GH. Cone photoreceptor recovery after experimental detachment and reattachment: an immunocytochemical, morphological, and electrophysiological study. Invest Ophthalmol Vis Sci 2003;44: 416–425.
Mohand-Said S, Hicks D, Dreyfus H, Sahel JA. Selective transplantation of rods delays cone loss in a retinitis pigmentosa model. Arch Ophthalmol 2000;118:807–811.
Leveillard T, Mohand-Said S, Lorentz O, et al. Identification and characterization of rod-derived cone viability factor. Nat Genet 2004;36:755–759.
Mohand-Said S, Hicks D, Simonutti M, et al. Photoreceptor transplants increase host cone survival in the retinal degeneration (rd) mouse. Ophthalmic Res 1997;29:290–297.
Komeima K, Rogers BS, Campochiaro PA. Antioxidants slow photoreceptor cell death in mouse models of retinitis pigmentosa. J Cell Physiol 2007;213:809–815.
Pinilla I, Lund RD, Sauve Y. Cone function studied with flicker electroretinogram during progressive retinal degeneration in RCS rats. Exp Eye Res 2005;80:51–59.
Milam AH, Li ZY, Fariss RN. Histopathology of the human retina in retinitis pigmentosa. Prog Retin Eye Res 1998;17:175–205.
Fariss RN, Li ZY, Milam AH. Abnormalities in rod photoreceptors, amacrine cells, and horizontal cells in human retinas with retinitis pigmentosa. Am J Ophthalmol 2000;129:215–223.
Li ZY, Jacobson SG, Milam AH. Autosomal dominant retinitis pigmentosa caused by the threonine-17-methionine rhodopsin mutation: retinal histopathology and immunocytochemistry. Exp Eye Res 1994;58:397–408.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Huang, Y.M., Yin, Z.Q., Liu, K. et al. Temporal and spatial characteristics of cone degeneration in RCS rats. Jpn J Ophthalmol 55, 155–162 (2011). https://doi.org/10.1007/s10384-010-0908-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10384-010-0908-8