Summary
Functional dyspepsia (FD) and irritable bowel syndrome (IBS) are frequent disorders affecting quality of life. They often require long-term treatment. Abdominal symptoms of both disorders can overlap, making differential diagnosis and treatment challenging. The extracts of the herbal combination preparation STW 5 (Iberogast®) exert pharmacological effects in different gastrointestinal regions and can address symptoms of both FD and IBS. This review summarizes safety and efficacy data of 12 clinical trials using STW 5 in FD and IBS since 1990. Double-blind and randomized studies versus placebo or active control found statistically significant effects of STW 5 on patients’ symptoms with a comparable efficacy to a standard prokinetic. Non-interventional and retrospective studies confirmed these effects. Various studies evaluated the tolerability profile of STW 5: the incidence of adverse drug reactions was 0.04 %. The worldwide spontaneous reporting system confirmed this profile. STW 5 has a favorable tolerability which is relevant for long-term treatment.
Zusammenfassung
Funktionelle Dyspepsie (FD) und Reizdarmsyndrom (RDS) sind häufige Erkrankungen mit Einfluss auf die Lebensqualität, die evtl. eine Langzeitbehandlung erfordern. Ober- und Unterbauchsymptome können gemeinsam auftreten, was die Differenzialdiagnose und Therapie erschwert. Die Frischpflanzen- und Drogenauszüge im Kombinationspräparat STW 5 (Iberogast®) wirken auf verschiedene gastrointestinale Regionen und adressieren sowohl FD als auch RDS. Dieser Review beschreibt das Sicherheitsprofil von STW 5 und bezieht sich auf zwölf seit 1990 durchgeführte Studien bei diesen Indikationen. Doppelblinde und randomisierte Studien gegen Plazebo und/oder aktive Kontrolle fanden statistisch signifikante und klinisch relevante Effekte gegenüber Plazebo bzw. eine zu einem Standardprokinetikum vergleichbare Wirkung. Diese Effekte wurden in nicht-interventionellen bzw. retrospektiven Studien bestätigt. Klinische Studien unterschiedlichen Designs untersuchten das Verträglichkeitsprofil von STW 5. Die Inzidenz unerwünschter Arzneimittelwirkungen in diesen Studien betrug 0,04 %. Das weltweite Spontanmeldesystem bestätigte dieses Profil. STW 5 weist eine vorteilhafte Verträglichkeit auf, die für eine Langzeittherapie zu fordern ist.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Functional dyspepsia and irritable bowel syndrome (IBS) are the most frequent functional gastrointestinal disorders. In a Western population, their prevalence is estimated at approximately 10–20 % each [1]. Symptoms of both disorders may overlap, showing components of functional dyspepsia and of IBS at one time [2]. Functional gastrointestinal disorders are frequently perceived as psychological or psychosomatic disorders. They are the box to which patients are allocated, if organic disease is absent. In an attempt to find something “real”, patients may undergo unneeded diagnostic approaches [3].
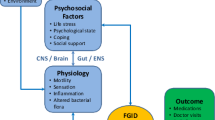
Since 1987, scientists and clinicians have worked on the understanding and classification of functional gastrointestinal disorders and have developed the Rome criteria, which are now applied for diagnosis [4]. The current Rome III diagnostic criteria classify functional gastrointestinal disorders into several categories according to the regional location of symptoms, the predominant symptoms, and the age. Functional gastrointestinal disorders are currently explained by a biopsychological model, which “allows for symptoms to be both physiologically multi-determined and modifiable by sociocultural and psychosocial influences”. In this model, psychosocial factors and physiology are interlinked through the “brain-gut axis”, with the elements influenced by genetic and environmental factors (Fig. 1) [3]. The Rome III diagnostic criteria for functional dyspepsia and IBS are shown in Tables 1 and 2 [5, 6].
Biopsychosocial conceptualization of functional gastrointestinal disorders [3]
The therapy for functional dyspepsia and IBS is multi-faceted and includes patient education, dietary recommendations, physical exercise, relaxation, and stress management. If such non-pharmacological measures are not successful, pharmacologic interventions like proton pump inhibitors, prokinetic drugs, antibiotics for the eradication of Helicobacter pylori, if positive, antidiarrhoics, dietary fibers, bulking agents, anticholinergic spasmolytics, tricyclic antidepressants, and herbal drugs are recommended [1, 2, 7, 8]. With the exception of herbal combination medicines, pharmacological drugs mostly treat only one symptom at a time and thus cannot address overlapping and variable complaints. In addition, side-effects with chronic treatment can be observed.
Functional gastrointestinal disorders are not life-threatening and in the strict sense of the word also not debilitating diseases. Accordingly, they are not desperate illnesses, which require desperate remedies. However, functional gastrointestinal disorders relevantly reduce the quality of life and often require chronic therapy [9]. Thus it is important not only to supply effective therapy to patients, but also to focus on the long-term tolerability.
The herbal combination preparation STW 5 (Iberogast®, Steigerwald Arzneimittelwerk GmbH, Darmstadt, Germany) has been comprehensively studied for the treatment of functional gastrointestinal disorders of the upper and lower abdomen. STW 5 contains alcoholic extracts of Iberis amara totalis recens, Angelicae radix, Cardui mariae fructus, Chelidonii herba, Liquiritiae radix, Matricariae flos, Melissae folium, Carvi fructus and Menthae piperitae folium and has been used for the therapy of functional gastrointestinal disorders for five decades. The extracts exert different proven pharmacological effects on different regions of the gastrointestinal tract and thus address the whole symptom complex of functional dyspepsia syndrome and IBS [10]. The tolerability of STW 5 was favorable both in clinical studies and in post-marketing use.
This review was performed to evaluate the available clinical data on STW 5 with a focus also on its tolerability profile.
Material and methods
We reviewed the following clinical studies and report information on study design, patient characteristics and efficacy and safety outcomes:
-
Six controlled and randomized studies with STW 5, five in functional dyspepsia and one in IBS [11–16]. The studies in functional dyspepsia have also been included in several meta-analyses [17–19].
-
Two post-marketing surveillance studies in various gastrointestinal diseases, including functional dyspepsia and IBS, and one retrospective cohort study in functional dyspepsia [20–22].
-
Two retrospective surveillance studies and one non-interventional study in children with gastrointestinal complaints, including functional dyspepsia and IBS [23–25].
Interventional studies had been reviewed by the appropriate ethics committee and were performed in accordance with the ethical standards laid down in the Declaration of Helsinki. All patients gave their informed consent prior to their inclusion in the studies. Eleven older studies with STW 5 performed before 1990, when the first European GCP guideline was adopted, were not taken into account.
Results
The characteristics of the studies are tabulated in Table 3. Overall, 413 patients were treated with STW 5 in randomized controlled studies, about 5,795 patients in prospective non-interventional studies, and more than 40,000 children in retrospective database surveillances.
Comparative clinical studies
Functional dyspepsia
Four clinical studies evaluated STW 5 versus placebo and one study tested the non-inferiority of STW 5 to the prokinetic drug cisapride. A panel of gastroenterological experts participated in planning the studies and developed the primary efficacy criterion, the gastrointestinal symptom score (GIS). This score is a summary score consisting of ten gastrointestinal symptoms, which are evaluated on five-point Likert scales. The GIS was validated and is sensitive, responsive, and specific for functional dyspepsia [26]. All studies had a similar design.
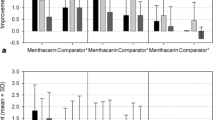
Von Arnim et al. [11, 27] performed a randomized, double-blind, placebo-controlled study, which included 315 patients with functional dyspepsia diagnosed according to the Rome II criteria. After a washout of 7 days, the patients were randomized to an 8 weeks treatment period with either STW 5 3 × 20 drops/day or placebo. Patients were followed up for 6 months. The primary endpoint was the change of the GIS over the treatment period. The GIS improved in both groups (see Fig. 2). After 4 and 8 weeks of treatment, the improvement was significantly higher in the STW 5 group compared to placebo and the difference between the two groups was clinically relevant. Investigators’ and patients’ global assessments confirmed the significant superiority of STW 5. On average, patients treated with STW 5 remained recurrence-free longer than patients who had received placebo. Though in the very good and good categories of tolerability assessments by patients, there was a slight imbalance in favor of placebo, the overall tolerability of STW 5 and placebo were comparable (see Fig. 3). No clinically relevant changes of laboratory or vital parameters and no serious adverse events occurred. The proportion of patients with documented adverse events was practically identical in both treatment groups. Five patients reported seven adverse events, in which a causal relationship to STW 5 could not be excluded (abdominal pain, pruritus, sore throat, alopecia, hypersensitivity, hypertension, gastrointestinal pain).
Gastrointestinal Symptom Profile of STW 5 (Iberogast®) in comparison to placebo in patients with functional dyspepsia [11]
Patients’ tolerability assessment of STW 5 (Iberogast®) in comparison to placebo in patients with functional dyspepsia. [11]
The other three studies in patients with functional dyspepsia comparing STW 5 to placebo showed basically similar results, i.e. a statistically significant and clinically relevant superiority of STW 5 versus placebo as determined by the GIS and an almost identical proportion of patients with adverse events in both treatment groups. Adverse events at least possibly related to STW 5 reported in these studies were esophagitis, bronchitis, diarrhea, nausea, stomatitis, and abdominal pain [12–14].
One of the studies examined gastric emptying as secondary parameter with the help of the 13C octanoic acid breath test for the assessment of the gastric half-emptying time [14]. The findings from this study suggested that the clinical effects of STW 5 were not simply mediated by accelerating gastric emptying, but by more complex processes, reflecting the multi-faceted pharmacological profile of the drug.
The studies of STW 5 versus placebo with patients suffering from functional dyspepsia were sufficiently comparable in design to be submitted to several meta-analyses [17–19]. These meta-analyses detected a significant efficacy of STW 5. The latest and largest included 637 patients and found a standardized mean difference of − 1.1, indicating a large and clinically relevant effect (p = 0.0005), although there was statistically significant heterogeneity between studies [19].
One clinical trial compared STW 5 to the prokinetic drug cisapride 3 × 20 mg/day [15]. To ensure double-blind conditions, this study used a double dummy design. One-hundred eighty-six patients with functional dyspepsia of the dysmotility type were enrolled and received STW 5, cisapride, or a research compound for 4 weeks after a 1-week washout phase. Patients were followed up for 6 months. Like in the placebo-controlled studies, the primary endpoint was the change of the GIS during the treatment period. During treatment, the GIS decreased significantly in both groups: from comparable baseline scores of 14.3–14.5 points, the GIS decreased to 2.3 points in the STW 5 group and to 3.5 in the cisapride group, showing a numerical superiority of STW 5 and a statistically confirmed non-inferiority to cisapride (see Fig. 4). Patients, who were symptom-free after treatment, were evaluated for recurrence during the 6-month follow-up period. There were no significant differences between the groups in this parameter. A further secondary endpoint, the efficacy assessments by investigators and patients, again showed comparable results for STW 5 and cisapride. The tolerability of STW 5 was assessed as being very good or good by more than 93 % of investigators and patients; in the cisapride group, this proportion was between 81 and 91 %. More patients reported adverse events in the cisapride group (33 %) compared to the STW 5 group (21 %). Two adverse events in the STW 5 group were classified as having a probable causal relation relationship to the study medication (abdominal cramps; dizziness and nausea), whereas in the cisapride group one such adverse event was reported (diarrhea).
Gastrointestinal Symptom Profile of STW 5 (Iberogast®) in comparison to cisapride in patients with functional dyspepsia [15]
IBS
One randomized, double-blind, and placebo-controlled clinical study analyzed the efficacy and safety of STW 5 in IBS [16]. As one primary efficacy endpoint, the study used an abdominal symptom score developed by a gastroenterological expert panel, which included eight IBS-specific symptoms evaluated on four-point Likert scale. Two-hundred and eight patients underwent a 1-week washout phase and were then randomized to STW 5, two research compounds, or placebo for 4 weeks. STW 5 reduced the IBS symptom score by 1.5 points more than placebo, a clinically relevant superiority that was also statistically significant (p < 0.0004). The co-primary endpoint abdominal pain, evaluated on four-point Likert scales for the four abdominal quadrants, showed comparable results and again a statistically significant superiority of STW 5 versus placebo. The tolerability as assessed by investigators was generally ‘very good’ or ‘good’ (STW 5: 98 %; placebo: 89 %), with no essential differences to patients’ assessments. There was one adverse drug reaction in the STW 5 group (constipation). No serious adverse events were reported and blood chemistry before and after treatment showed no clinically relevant variations.
Observational studies
Two non-interventional studies with a comparable design enrolled patients with functional dyspepsia (n = 2267) or IBS (n = 2548) [20, 21]. In both studies, patients received STW 5 for up to 4 weeks. In functional dyspepsia, 27 % of patients discontinued therapy after 1 week for freedom of symptoms. The GIS sum score decreased on average by 78 %. In IBS, the single symptoms of the abdominal sum score decreased by 65–80 % each. Approximately 80 % of physicians and patients assessed the effectiveness of STW 5 as very good or good. There were no adverse drug reactions to or interactions with STW 5 documented in these studies.
A retrospective cohort study analyzed 961 patients, who had received STW 5 or metoclopramide in the recommended dose for functional dyspepsia [22]. The primary endpoint of this analysis was the improvement of GIS, outcome parameters included the number of symptom-reef patients after therapy and the duration of inability to work. There were significantly more symptom-free patients after therapy with STW 5 compared to metoclopramide (72 versus 63 %; p < 0.05). Furthermore, the duration of inability to work was significantly shorter under STW 5 than under metoclopramide (median 1 day versus 3 days; p < 0.001). There were no adverse drug reactions to STW 5, but five patients receiving metoclopramide reported vertigo and dizziness. In line with these results, 90 % of physicians rated the tolerability of STW 5 as very good, compared to 71 % for metoclopramide.
Studies in children
Two retrospective database surveillance studies collected data on the use of STW 5 in children up to 12 years of age with gastrointestinal complaints including functional dyspepsia and irritable bowel disease. The studies documented 40,961 and 2,350 patients, respectively, and both studies used a four-point Likert scale for an assessment of efficacy [23, 24]. The physicians judged effectiveness to be very good or good in 88 and 96 % of patients, respectively. Both studies did not detect adverse drug reactions to or interactions with STW 5.
A more recent non-interventional study included 980 children (age 3–14 years) with functional gastrointestinal disorders preferably diagnosed by the Rome III criteria and eligible to treatment with STW 5 [25]. Patients were followed for approximately 1 week. The GIS, extended by four lower abdominal symptoms, served as primary endpoint for clinical effectiveness. Most patients were treated for IBS (43 %) or functional dyspepsia syndrome (26 %). The symptom score decreased by 76 % during the treatment period. The decrease in symptoms was similar for the different age groups, genders, and indications. Patients with a shorter duration of complaints had a lower score at study end (p < 0.0001). The global treatment effect was assessed as good or very good by 87–89 % of patients/parents and physicians. Physicians rated the global tolerability as very good or good for 95 % of the patients. Adverse events assessed as probably or possibly related to STW 5 were nausea, abdominal pain, increased gastrointestinal complaints, and vomiting. One patient experienced skin rash following the concomitant application of penicillin. These events were non-serious.
Safety overview
The safety profile of STW 5 was extensively evaluated in pre-clinical and in controlled and non-interventional or retrospective clinical studies. Pre-clinical evaluations included acute, subchronic, and chronic toxicity, with a specific focus on hepatotoxicity, reproductive toxicity, fertility, embryo- and fetotoxicity, mutagenicity, and cytotoxicity and showed no indications of safety signals relevant for human use [10, 28].
Table 4 shows the number of adverse events observed in the controlled and non-interventional or retrospective clinical studies reviewed above, in which the investigators considered a causal relationship to STW 5 as at least possible (i.e. the adverse events classified as adverse drug reactions). The incidence was 0.04 % and the adverse drug reactions documented were (in alphabetical order) abdominal cramps, abdominal pain, alopecia, bronchitis, constipation, diarrhea, dizziness, gastrointestinal complaints increased, gastrointestinal pain, hypersensitivity, hypertension, nausea, esophagitis, pruritus, skin rash, sore throat, stomatitis, and vomiting. No serious adverse drug reactions occurred and the studies also found no clinically relevant deviations of laboratory values. STW 5 was well tolerated in the populations examined, independent of concomitant diseases and without drug interactions.
In addition to the clinical or observational studies included in Table 4, the spontaneous reporting system in Germany and worldwide elicited a comparatively very small number of adverse events assessed as possessing a possible or probable causal relationship to STW 5 (n = 111), given that the exposed cohort is estimated at more than 25 million patients since the market launch of STW 5 over 50 years ago. The summary of product characteristics of STW 5 complements this information by specifying that hypersensitivity reactions may occur very rarely and may take the form of pruritus, dyspnea, or skin reactions in pre-disposed patients [28].
Discussion
On one hand, there is a widespread perception of herbal remedies as being only mild and moderately effective or not evidence based at all. On the other hand, some of the pharmacologically most active—and toxic—drugs are or have been derived from plants, among them aspirin, digitalis, colchicine, and the taxanes. Accordingly, in Europe herbal drugs have to meet regulatory requirements comparable to chemical or biopharmaceutical drugs. This means that they are required to undergo pre-clinical and clinical studies showing their safety and efficacy, before they are allowed to be sold or to remain on the markets. Under certain conditions, herbal medicines lacking a sufficiently scientific basis and evidence of efficacy may be marketed as traditionally used medicines [29]. It should furthermore be considered that efficacy is not identical to effectiveness. Roughly speaking, effectiveness describes an improvement of symptoms over time, whereas efficacy describes the course of symptoms in comparison to an active control or to a placebo group. Efficacy is thus effectiveness ‘cleaned’ of confounding effects like the natural course of the disease or placebo effects.
Treatment of functional gastrointestinal disorders remains a major challenge as pathomechanisms are complex. Thus, a multi-target approach is likely to be more successful than a drug directed to an individual target [30]. STW 5 as an herbal combination preparation with individually active components [31] has not only shown its effectiveness in improving symptoms over time in non-interventional studies as well as in retrospective database surveillances. It also passed the higher hurdle of proving its efficacy in studies versus placebo and cisapride as an active control. The efficacy observed was clinically relevant, as indicated by the effect size versus placebo as well as by an overall similar and even numerically superior effect when compared to cisapride.
The prokinetic cisapride was a standard for the treatment of functional (upper) gastrointestinal disorders before its withdrawal from most markets for safety reasons, namely, a prolongation of the QT time and resulting cardiac arrhythmia. Two other drugs of this class, domperidone and metoclopramide, have recently undergone labeling changes or are currently under review by the European Medicines Agency due to neurological and/or cardiovascular events [32, 33]. Other drugs for treating functional gastrointestinal disorders currently recommended in guidelines are proton pump inhibitors, antidiarrhoics, anticholinergic spasmolytics, tricyclic antidepressants, and herbal drugs [1, 2, 7, 8]. All possess specific tolerability issues which have to be evaluated and considered when selecting a suitable therapy for patients. Proton pump inhibitors tend to be over utilized. They have been associated with rebound acid hypersecretion, which may aggravate the symptoms of functional dyspepsia [34], whereas the compliance with spasmolytics like mebeverine, and also with tricyclic antidepressants as drugs of the last resort for functional gastrointestinal disorders, may be limited by their characteristic therapeutic profile [1, 2, 7, 8]. In addition to these safety issues, the drugs enumerated are rarely adequate for patients suffering from mixed complaints of the upper and lower gastrointestinal tract, as they address only one symptom at a time. The combination of drugs helpful for symptoms both of the upper and lower abdomen will generally further increase the side-effects the patient has to tolerate.
In several countries, STW 5 is approved as the only product both covering functional dyspepsia as well as IBS. It is furthermore included in the therapy guidelines for both upper and lower functional gastrointestinal disorders [7, 35]. STW 5 has been thoroughly evaluated for tolerability over approximately five decades. Pre-clinical and clinical studies have especially searched for signals of hepatotoxicity and abnormal liver function tests, as herbal drugs may possess hepatotoxic effects. They have shown that STW 5 has no such effects. For celandine herbs, very rare and reversible dose-dependent cases of hepatic side effects had been reported. However, the daily doses for which these hepatic side effects of celandine herbs have been described are approximately 100–200 times higher than those applied with STW 5. Furthermore, interventional and non-interventional clinical studies with STW 5 as well as database surveillances specifically assessed liver function as a safety parameter. These studies under controlled conditions found no hepatotoxicity cases associated temporally or causally with STW 5. The only adverse reactions considered attributable to STW 5 are hypersensitivity reactions as described in the summary of product characteristics. They have been observed very rarely in predisposed patients and may take the form of pruritus, dyspnea, or skin reactions. Thus, STW 5 shows the favorable tolerability profile important for a preparation used for functional gastrointestinal disorders, because the chronic nature of these diseases often requires long-term therapy.
Conflict of interest
The authors declare that there is no actual or potential conflict of interest in relation to this article.
References
Arzneimittelkommission der deutschen Ärzteschaft. Empfehlungen zur Therapie bei Funktioneller Dyspepsie und Reizdarmsyndrom. 2. Aufl. Arzneiverordn Prax. 2010;37:1–29.
Eckardt V. Funktionelle Magen-Darm-Erkrankungen. Selecta. 2009;1:6–11.
Drossmann DA. The functional gastrointestinal disorders and the Rome III process. Gastroenterology. 2006;130(5):1377–90.
Thompson WG. The road to Rome. Gastroenterology. 2006;130:1552–6.
Tack J, Talley NJ, Camilleri M, et al. Functional gastroduodenal disorders. Gastroenterology. 2006;130(5):1466–79.
Longstreth GF, Thompson WG, Chey WD, et al. Functional bowel disorders. Gastroenterology. 2006;130(5):1480–91.
Layer P, Andresen V, Pehl C, et al. S3-Leitlinie Reizdarmsyndrom: Definition, Pathophysiologie, Diagnostik und Therapie. Gemeinsame Leitlinie der Deutschen Gesellschaft für Verdauungs- und Stoffwechselkrankheiten (DGVS) und der Deutschen Gesellschaft für Neurogastroenterologie und Motilität (DGNM). AWMF-Registriernummer: 021/016,2011. Z Gastroenterol. 2011;49:237–93.
Teubner A, Jürgensen C, Stölzel U. Funktionelle Dyspepsie und Reizdarmsyndrom. Arzt Krankenh. 2007;6:169–74.
Chang L. Review article: epidemiology and quality of life in functional gastrointestinal disorders. Aliment Pharmacol Ther. 2004;20(Suppl 7):31–9.
Vinson B. Development of Iberogast: clinical evidence for multicomponent herbal mixtures. In: Cooper R, Kronenberg F, editors. Botanical medicine: from bench to bedside. New Rochelle: Mary Ann Liebert; 2009. pp. 167–89.
von Arnim U, Peitz U, Vinson B, et al. STW 5, a phytopharmacon for patients with functional dyspepsia: results of a multicenter, placebo-controlled double-blind study. Am J Gastroenterol. 2007;102:1268–75.
Buchert D. Wirksamkeit und Verträglichkeit von Iberogast bei Patienten mit gesicherter Non Ulcus Dyspepsie. Z Phytotherapie. 1994;15(1):45–6.
Madisch A, Melderis H, Mayr G, et al. Ein Phytotherapeutikum und seine modifizierte Rezeptur bei funktioneller Dyspepsie. Z Gastroenterologie. 2001;39:1–8.
Braden B, Caspary W, Börner N, et al. Clinical effects of STW 5 (Iberogast) are not based on acceleration of gastric emptying in patients with functional dyspepsia and gastroparesis. Neurogastroenterol Motil. 2009;21(6):632–8, e25.
Rösch W, Vinson B, Sassin I. A randomised clinical trial comparing the efficacy of a herbal preparation STW 5 with the prokinetic drug cisapride in patients with dysmotility type of functional dyspepsia. Z Gastroenterol. 2002;40(6):401–8.
Madisch A, Holtmann G, Plein K, et al. Treatment of irritable bowel syndrome with herbal preparations: results of a double-blind, randomized, placebo-controlled, multi-centre trial. Aliment Pharmacol Ther. 2004;19(3):271–9.
Gundermann K, Godehardt E, Ulbrich M. Efficacy of a herbal preparation in patients with functional dyspepsia: a meta-analysis of double-blind, randomized, clinical trials. Adv Ther. 2003;20(1):43–9.
Melzer J, Rösch W, Reichling J, et al. Meta-analysis: phytotherapy of functional dyspepsia with the herbal drug preparation STW 5 (Iberogast). Aliment Pharmacol Ther. 2004;20(11–12):1279–87.
Holtmann G, Nandurkar S, Talley NJ, et al. Herbal medicine for the treatment of functional dyspepsia: a systematic review of the literature and meta-analysis. Gastroenterology. 2007;132(4) Supp 2 (Abstract W1204).
Sassin I, Buchert D. Efficacy and tolerability of the herbal preparation Iberogast® in the therapy of functional dyspepsia. Phytomedicine. 2000;7(Suppl. II):91–2 (69P).
Klein-Galczinsky C, Sassin I. Anwendungsbeobachtung zur Wirksamkeit und Verträglichkeit von Iberogast® in der Therapie des Colon irritabile. Phytotherapie an der Schwelle zum neuen Jahrtausend 1999;125 (Abstract P25).
Raedsch R, Hanisch J, Bock P, et al. Wirksamkeit und Unbedenklichkeit des Phytopharmakons STW 5 versus Metoclopramid oral bei funktioneller Dyspepsie unter Praxisbedinungen. Z Gastroenterol. 2007;45(10):1041–8.
Gundermann KJ, Vinson B, Hänicke S. Die funktionelle Dyspepsie bei Kindern – eine retrospektive Studie mit einem Phytopharmakon. Päd. 2004;10:1–6.
Leichtle K. Experience reports of the application of Iberogast in children. Research report. Steigerwald: Arzneimittelwerk; 1999.
Vinson BR, Radke M. The herbal preparation STW 5 for the treatment of functional gastrointestinal diseases in children aged 3–14 years—a prospective non interventional study. Digestive disease week, Chicago, IL, USA. 8 May 2011 (Abstract #523).
Adam B, Liebregts T, Saadat-Gilani K, et al. Validation of the gastrointestinal symptom score for the assessment of symptoms in patients with functional dyspepsia. Aliment Pharmacol Ther. 2005;22(4):357–63.
von Arnim U, Vinson BR, Malfertheiner P, et al. Functional dyspepsia: are relapse rates influenced by active treatment. Gastroenterology. 2008;134 (Abstract W1085).
Summary of product characteristics (SmPC, Fachinformation) Iberogast®. STEIGERWALD Arzneimittelwerk GmbH. Oktober 2010.
Knöss W, Chinou I. Regulation of medicinal plants for public health—European community monographs on herbal substances. Planta Med. 2012;78:1311–6.
Wagner H. Multitarget therapy: the future of treatment for more than just functional dyspepsia. Phytomedicine. 2006;13(Suppl. 5):122–99.
Wegener T, Wagner H. The active components and the pharmacological multi-target principle of STW 5 (Iberogast). Phytomedicine. 2006;13(Suppl. 5):20–35.
Bundesinstitut für Arzneimittel und Medizinprodukte. Abwehr von Gefahren durch Arzneimittel, Stufe II. Metoclopramid-haltige Arzneimittel: Wirksamkeits- und Sicherheitsbedenken (hinsichtlich neurologischer und kardiovaskulärer Ereignisse). Bonn 2. Jan. 2012.
Arzneimittelkommission der deutschen Ärzteschaft. UAW-News International. Ventrikuläre Arrhythmien und plötzlicher Herztod im Zusammenhang mit Domperidon. Deutsches Ärzteblatt. 2012;109(35/36):A1779–80.
Heidelbaugh JJ, Kim AH, Chang R, et al. Overutilization of proton-pump inhibitors: what the clinician needs to know. Therap Adv Gastroenterol. 2012;5(4):219–32.
Malfertheiner P, Holtmann G, Peitz U, et al. Guidelines of the German society of metabolic and digestive diseases for the therapy of dyspepsia. Z Gastroenterol. 2001;39(11):937–56.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Ottillinger, B., Storr, M., Malfertheiner, P. et al. STW 5 (Iberogast®)—a safe and effective standard in the treatment of functional gastrointestinal disorders. Wien Med Wochenschr 163, 65–72 (2013). https://doi.org/10.1007/s10354-012-0169-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10354-012-0169-x