Summary
Background
The beneficial outcomes of hepatectomy in patients with colorectal metastases have encouraged the attempts of repeated hepatectomy in patients with recurrent disease. Although studies have provided encouraging results regarding perioperative outcomes and survival rates following repeated hepatectomy, it remains unclear whether the reported outcomes reflect the therapeutic results of redo hepatectomy or rather reflect the effect of selection bias. The aim of this study was to investigate differences among patients who underwent single and repeated hepatectomy and to hereby identify prognostic factors that contribute to the premises of repeated resection.
Methods
Patients who underwent hepatectomy due to colorectal metastases were listed in a retrospective database. Study participants were divided into a single partial hepatectomy group, a multiple partial hepatectomies group, and into subgroups of two or more than two hepatectomies.
Results
A total of 338 patients with 439 partial liver resections were included in the analysis. The overall survival rate after 1, 3, and 5 years was 89%, 56%, and 36%, respectively. The survival benefit in patients who underwent multiple partial liver resections versus those with a single partial resection was 10%, 16%, and 4% after 1, 3, and 5 years, respectively. Repeated hepatectomy was not associated with increased rates of surgical and non-surgical complications.
Conclusion
Beneficial outcomes have been found in terms of median overall survival and perioperative morbidity in patients with recurrence of colorectal hepatic metastases after partial and tissue-sparing repeated liver resections.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
-
Repeated hepatectomy with complete resection of recurrent colorectal hepatic metastases should be considered the treatment of choice.
-
Tissue-saving redo hepatectomies with preservation of crucial anatomic structures are recommended.
-
Repeated hepatectomy is associated with beneficial outcomes in median overall survival and perioperative morbidity in patients with recurrence of colorectal hepatic metastases.
Introduction
Colorectal cancer represents the third most common cause of malignancy and the third leading cause of cancer-related mortality worldwide. It is estimated that up to 25% of patients have colorectal liver metastases at the time of diagnosis, whereas 30–50% of patients develop metastases during the course of their cancer disease [1, 2]. Compared to other therapeutic approaches such as locoregional ablation treatments, surgical resection is associated with the highest 5‑year survival rate and is considered the gold standard in treatment of liver cancer [3, 4].
Hepatic or extrahepatic recurrence is reported in up to 80% of patients after hepatectomy [5, 6]. The beneficial outcomes of hepatectomy in patients with colorectal metastases have encouraged attempts of repeated hepatectomy in patients with recurrent liver disease. Repeated hepatectomy has been associated with acceptable rates of postoperative morbidity and mortality; however, recent studies have reported conflicting results regarding the impact of repeated hepatectomy on long-term survival [5, 7,8,9]. Although some studies have demonstrated a survival benefit following redo hepatectomy compared to single resection [5, 8, 10, 11], others have yielded comparable or less favorable long-term results [7, 12, 13].
It remains unclear whether the documented beneficial outcomes truly reflect the therapeutic impact of repeated resection or rather simply reflect the effect of selection bias. Furthermore, although attempts have been made to provide selection criteria for repeated hepatectomy, factors that predict the recurrence rate after hepatectomy are not clearly defined [5, 11].
The aim of this study is to investigate differences among patients who underwent single and repeated hepatic resections and to hereby identify prognostic factors that contribute to the premises of repeated hepatectomy. We investigated differences in the course of the first partial hepatectomy which potentially prejudice the possibility of repeated resection. The key question is whether redo hepatectomy for recurrent disease after successful index surgery improves overall survival.
Materials and methods
Within a period of 14 years, a total of 338 patients with colorectal liver metastases underwent 439 partial liver resections in curative intent due to one or more metastases and were included in the analysis. The study was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki.
All cases were previously presented and discussed in an interdisciplinary tumor board. The primary tumor was resected either prior to the liver resection or synchronously. According to current guidelines and the initial tumor stage, radiation therapy and/or chemotherapy was performed.
Anatomical partial liver resections according to Couinaud liver segmentation, atypical partial liver resection, or a combination of both procedures was conducted. If necessary, the Pringle-maneuver technique was used to prevent blood loss. “Major partial hepatectomy” was defined as resection of three or more liver segments. All patients underwent an intraoperative ultrasound examination to verify the location and size of metastases and to detect potential previously unknown metastases. Perihepatic lymphadenectomy was only performed in case of lymph node enlargement, while cholecystectomy was conducted as standard. Parenchyma dissection was performed using an ultrasound dissection device (CUSA; Tyco Healthcare, Mansfield, MA, USA).
All patients were transferred to the intensive care unit postoperatively and were monitored for at least 1 day. Patients received red cell concentrates only in case of hemoglobin concentration less than 6 g/dl or less than 8 g/dl in patients with existing cardiovascular-related diseases.
All patients were registered in an Access database (Microsoft Access, Microsoft Office Professional Plus 2010; Microsoft Cooperation, Redmond, Washington, United States). Demographical and medical history data including stage and location of the primary tumor, initial tumor therapy, extent of partial liver resection, and course of inpatient treatment were listed. From January 2017 to December 2021, a retrospective analysis and completion of follow-up data were conducted. Institutional approval was obtained for the conduction of this retrospective study.
Study participants were divided into two groups. Patients with only one partial hepatectomy were assigned to the group “single partial hepatectomy” (sPhx); those with two or more partial liver resections were assigned to the group “multiple partial hepatectomies” (mPhx). The mPhx group was subdivided into the following subgroups: patients who underwent two (2Phx) or more than two (> 2Phx) partial liver resections. The different groups were compared with regard to the above-mentioned data.
The collected data were analyzed using Microsoft Excel (Microsoft Excel, Microsoft Office Professional Plus 2010, Microsoft Cooperation) and SPSS 23.0® (IBM Corp., Armonk, NY, United States). Categorical values are given as absolute values and percentages. Continuous values are given as the mean value with standard deviation or median and 95% confidence range.
The comparison of categorical values was performed using chi-square test and Fisher’s exact test. Continuous values were compared using Mann–Whitney U test. Survival time was defined as the time after the first partial liver resection until the date of death. Overall survival was assessed using the method of Kaplan–Meier. Survival rates were compared between groups using the log-rank test. The influence of each variable on overall survival was investigated using a Cox regression model. Statistical significance was interpreted considering a p-value < 0.05.
Results
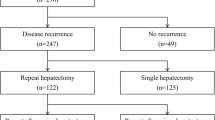
A total of 338 patients underwent 439 partial liver resections due to colorectal metastases. Single partial hepatectomy was performed in 259 patients (group sPhx), while 79 patients underwent multiple partial liver resections (group mPhx). Of the latter patients, 56 underwent two partial liver resections (group 2Phx), whereas 23 patients underwent more than two partial liver resections (group > 2Phx). In the > 2Phx group, three, four, and five hepatectomies were performed in 16, 7, and 1 patient, respectively.
Demographic data and data concerning the primary tumor are shown in Table 1. The mean age at the time of index hepatectomy in the mPhx group was 61.2 ± 1.2 years compared to 64.5 ± 0.6 years in the sPhx group (p = 0.018). In addition, the age of the patients with > 2Phx was significantly lower compared to the 2Phx group (> 2Phx vs. 2Phx: 58.4 ± 2.1 vs. 62.4 ± 1.4 years, p = 0.03).
With regard to gender distribution, body mass index, rate of rectal cancer, presence of hepatitis B or C, T4 stage of the primary tumor, and adjuvant chemotherapy or radiation therapy, there were no significant differences between the groups. Patients’ ASA (American Society of Anesthesiologists) score was higher in the sPhx group compared to mPhx (sPhx vs. mPhx: 2.4 ± 0.0 vs. 2.2 ± 0.1, p = 0.011). Tumor stage UICC IV at diagnosis was more frequent in the mPhx group (sPhx vs. mPhx: 43.2% vs. 59.5%, p = 0.022). There was no significant difference comparing these variables between the groups 2Phx and > 2Phx.
Characteristics of liver metastases at the time of index hepatectomy, type of first liver resection, and the macroscopic illustration of the tumors are demonstrated in Table 2 and Fig. 1. Although the interval between resection of the primary tumor and the first partial liver resection differed nonsignificantly, it is remarkable that patients out of the sPhx group underwent surgery on average 4 months later than patients out of the mPhx group. The time between surgery for primary cancer and the first Phx was on average 6.5 months earlier in the group with > 2Phx compared to the 2Phx group.
Synchronous liver metastases were diagnosed in 54.5% and 41.7% of patients in the mPhx and sPhx group, respectively (p = 0.053). Comparison of the two subgroups showed no significant difference in the incidence of synchronous liver metastases (> 2Phx vs. 2Phx: 60.9% vs. 51.8%, p = 0.620). The number of metastases and the presence of bilobular liver metastases differed nonsignificantly between the groups. Patients of the sPhx group underwent a segmentectomy significantly more frequently (sPhx vs. mPhx: 19.7% vs. 8.9%; p = 0.026), whereas atypical multiple partial liver resection was significantly more common in the mPhx group (sPhx vs. mPhx: 25.5% vs. 40.5%, p = 0.016).
Operating time was similar between the groups. The average amount of blood loss, the maximum diameter of tumor lesion, and the average weight of the resected liver tissue were not significantly different between the four groups.
Perioperative and long-term outcomes were comparable between the groups (Table 3). Specifically, patients in the sPhx and mPhx group spent 2 days on average in the intensive care unit and were hospitalized for 12 days. Moreover, comparison of the postoperative complications showed no significant differences between the groups (sPhx vs. mPhx: 26.3% vs. 24.1%, p = 0.770; 2Phx vs. > 2Phx: 17.9% vs. 39.1%, p = 0.079). Surgical or interventional revision was necessary in 22 cases (8.5%) in the sPhx group and in 5 cases (6.3%) in the mPhx group. Patients out of the 2Phx group required surgical revision in 5 cases (8.9%), whereas there was no necessity for revision in the > 2Phx group. Six patients (2.3%) in the sPhx group and none of the other groups died within 30 days after surgery.
Fig. 2 shows the Kaplan–Meier curves for overall survival and recurrence-free survival for the sPhx and mPhx groups. Median survival and median recurrence-free survival was 25.1 (25.5–38.6) and 10.0 (9.1–13.6) months in the sPhx group, respectively. Patients in the mPhx group survived longer; however, the level of significance was not reached (mPhx vs. sPhx: 44.7 vs. 25.1 months, p = 0.072). The median recurrence-free survival in the mPhx group was 13.5 (9.6–16.4) months and statistically comparable to the sPhx group. Patients out of the > 2Phx group showed the longest overall survival (66.6 [49.3–76.1] months) and the shortest recurrence-free survival (9.0 [7.2–11.0] months). Overall and recurrence-free survival were 40.3 (38.9–55.4) and 12.0 (11.4–19.4) months in the 2Phx group, respectively (Table 3).
Factors influencing survival are shown in Table 4. Patients out of the sPhx group survived significantly longer in case of primary rectal cancer as indicated by uni- and multivariate analysis. In addition, surgical complications had a statistically significant impact on survival. Metachronous appearance of liver metastases seems to “positively” influence survival, since it was statistically associated with increased survival in univariate analysis (p = 0.04) but not in multivariate analysis (p = 0.056).
With respect to the patients with mPhx, factors such as age > 70 years at the time of index hepatectomy and overall or non-surgical complications had an impact on survival in the univariate analysis. Using multivariate analysis, no factor was observed to predict survival in a statistically significant manner.
Discussion
Despite improvements in the treatment of colorectal liver metastases in recent decades, recurrence can be observed in up to 80% of cases after hepatectomy [5, 6, 14]. In 40% of patients, recurrence is isolated in the liver [15]. In case of potentially resectable disease, it is broadly accepted that repeated hepatectomy can be offered as a treatment option [16, 17]. Using multiple redo resections of liver-limited tumor recurrences, survival rates may match those of recurrence-free patients [18]. In recent studies, prolonged overall survival rates were reported in the repeated hepatectomy group compared to the sPhx group [5, 8, 10, 11, 17, 19]. This is explained by the fact that within the mostly retrospective studies, some of the patients with recurrences underwent a repeated resection that improved their prognosis, whereas patients with unresectable recurrences were assigned to the sPhx group. In some studies, long-term survival of patients undergoing repeated hepatectomy was compared with the entire cohort of patients who underwent index hepatectomy, including those patients with repeated resection for hepatic recurrence [5]. In other studies, the redo hepatectomy group was compared with patients who only underwent the index hepatectomy and did not undergo repeated resections [8, 10, 11, 19]. Furthermore, the favorable outcomes in terms of survival rates after salvage resection of recurrent disease could be attributed to the extreme selection bias. We conducted this retrospective study to evaluate survival rates following repeated hepatectomy for recurrent hepatic metastases and to identify prognostic factors that are associated with a repeated resection pattern of treatment.
In our analysis, median overall survival and recurrence-free survival were comparable in the sPhx and mPhx groups. Studies that evaluated survival rates after repeated hepatectomy for colorectal liver metastases are shown in Table 5; [10, 20,21,22]. A retrospective study with 488 patients reported 5‑year overall survival rates of 48.4% and 26.2% in repeated and index resection groups, respectively. In accordance with those results, Kulik et al. reported in a retrospective study with a total of 1026 patients that repeated hepatectomy is associated with better median survival compared to single resection [8]. However, it was mentioned that no difference was found in median survival after the index operation and the last hepatectomy.
Yamazaki et al. included patients with similar tumor stage, number of lesions, and tumor size. They concluded that median overall survival was similar between the groups with one, two, and three hepatic resections [9]. On the contrary, Matsuoka et al. reported a significantly longer median survival time after single resection compared to multiple hepatectomies, with 83.2 and 35.3–42.9 months, respectively [7].
A meta-analysis reported similar rates of overall survival between patients who underwent only single resection and those who underwent redo hepatectomy [23]. However, it was mentioned that when the analysis included only high-quality studies or studies with more than 500 patients, a significant benefit in overall survival was detected in favor of repeated hepatectomy.
The question regarding prognostic factors that are associated with resectable recurrences is highly interesting. The following factors are suggested to be prognostically favorable after redo liver resection in the majority of studies: R0 resection, a long recurrence-free interval between the first and second liver resection, unilobular metastases, singular metastases, maximum diameter < 5 cm, no extrahepatic metastases, and a non-advanced UICC stage of primary tumor [8, 9, 15, 18, 23, 24]. The R0 resection rate is influenced by the surgeon’s experience and skills, while the other prognostic factors, such as long recurrence-free survival, unilobular recurrence, singular and small metastases, and the absence of extrahepatic recurrences, are at least in part tumor specific and indicative of a low tendency to metastasize and/or slower growth rate, which are associated with a better outcome.
The aim of this study was to identify specific prognostic factors that allow repeated partial liver resections. Patient-specific data in Table 1 show that neither gender nor body weight exerts an influence on the probability of repeated hepatectomy. On the other hand, there are significant differences concerning the age, ASA score, and UICC stage at first resection. Younger and healthier patients as well as patients with advanced UICC stage IV more frequently undergo redo hepatectomy. Furthermore, there is a trend towards multiple liver resections in patients with primary rectal cancer. In contrast, patients with primary T4 stage cancer at diagnosis undergo redo resections less often.
In general, the treatment strategy should be determined in an interdisciplinary tumor board. The decision for a more radical procedure with repeated resection in case of isolated hepatic recurrences seems to be made more easily in young and healthy patients. Elderly patients and patients with comorbidities seem to be treated more often less invasively, with chemotherapy, local radiation therapy, or ablation. In this study, the mean age of patients who underwent single or multiple partial liver resections was 64.5 and 61.2 years, respectively. Life expectancy varies between 75.8 and 81.2 years for German men and between 81.8 and 85.7 years for German women [25], whereas a 75-year-old man has a mortality risk of approximately 40% (Federal Statistical Office, Germany). In an Australian study with 29 patients older than 75 years, Gandy et al. showed a 5-year survival rate of 58% after resection of colorectal liver metastases [26]. In 1997, Fong et al. concluded that colorectal liver metastases are resectable in elderly patients over 65 years [27]. In contrast, recent studies classify elderly patients from the age of 70 years onward [28, 29].
Repeated liver resection should be taken into consideration in case of high ASA score or the presence of comorbidities. There is a lack of evidence concerning the influence of comorbidities such as severe cardiovascular and pulmonary diseases, diabetes, or renal insufficiency on postoperative outcomes following repeated liver resection. Available data suggest that redo hepatectomy in obese patients and diabetics is associated with increased perioperative mortality and morbidity. Nevertheless, good long-time outcomes can also be achieved in this group of patients [30].
Patients who underwent multiple liver resections showed a shorter interval between primary cancer treatment and the first Phx. More synchronous liver metastases occurred in these patients. There was no difference between the mPhx and the sPhx group concerning the occurrence of multiple and bilobular liver metastases. This demonstrates that early metastasis leads tendentially more often to repeated liver resections in contrast to bilobular and multiple metastases. However, the presence of bilobular or multiple hepatic lesions at first Phx is not an exclusion criterion for repeated liver resections. Patients with > 2Phx experienced the highest median survival with 66.6 months, although they had the shortest recurrence-free survival.
In our study, the rate of adjuvant chemotherapy or radiation of the primary tumor was similar between the groups. Lee et al. reported similar rates of adjuvant chemotherapy in patients treated with sPhx and those with mPhx [22]. In a retrospective study with 488 participants, neoadjuvant treatment was administrated in 43% of the patients in the index hepatectomy group and in 51% of the patients with a second hepatectomy [5]. Similarly, 41% and 48% of the patients received adjuvant treatment in each group. Neither treatment was associated with any difference in the survival rates of the patients compared to those who did not receive chemotherapy. According to the guidelines of the Japanese Society of Hepato-Biliary-Pancreatic Surgery, adjuvant chemotherapy is weakly recommended in patients with resectable colorectal metastases [31]. The ESMO guidelines recommend neoadjuvant and adjuvant chemotherapy in patients with an unfavorable prognosis, marginal resectable tumors, or synchronous onset of the metastases. In patients with favorable oncological and surgical criteria, there is no strong evidence to support the use of chemotherapy [32].
According to our study, repeated hepatectomy with complete resection of metastases should be considered as a treatment option in patients with recurrent disease. Tissue-saving redo hepatectomies should be preferred whenever possible, achieving voluminous anatomical resections and R0 surgical margins with maximum preservation of crucial anatomic structures. Repeated hepatectomy is associated with beneficial outcomes in terms of median overall survival and perioperative morbidity in patients with recurrence of colorectal hepatic metastases.
References
Favoriti P, Carbone G, Greco M, et al. Worldwide burden of colorectal cancer: a review. Updates Surg. 2016;68(1):7–11.
Meijerink MR, Puijk RS, van Tilborg A, et al. Radiofrequency and microwave ablation compared to systemic chemotherapy and to partial hepatectomy in the treatment of colorectal liver metastases: a systematic review and meta-analysis. Cardiovasc Intervent Radiol. 2018;41(8):1189–204.
European Association for the Study of the Liver. Electronic address eee, European Association for the Study of the L. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182–236.
Vogel A, Cervantes A, Chau I, et al. Hepatocellular carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29(Suppl 4):iv238–iv55.
Neal CP, Nana GR, Jones M, et al. Repeat hepatectomy is independently associated with favorable long-term outcome in patients with colorectal liver metastases. Cancer Med. 2017;6(2):331–8.
Park J, Lee SD, Han SS, et al. Repeat hepatectomy for recurred colorectal liver metastasis: is it justified? Ann Surg Treat Res. 2019;97(1):7–14.
Matsuoka H, Morise Z, Tanaka C, et al. Repeat hepatectomy with systemic chemotherapy might improve survival of recurrent liver metastasis from colorectal cancer—a retrospective observational study. World J Surg Oncol. 2019;17(1):33.
Kulik U, Bektas H, Klempnauer J, Lehner F. Repeat liver resection for colorectal metastases. Br J Surg. 2013;100(7):926–32.
Yamazaki S, Takayama T, Okada S, et al. Good candidates for a third liver resection of colorectal metastasis. World J Surg. 2013;37(4):847–53.
Shaw IM, Rees M, Welsh FK, et al. Repeat hepatic resection for recurrent colorectal liver metastases is associated with favourable long-term survival. Br J Surg. 2006;93(4):457–64.
Wicherts DA, de Haas RJ, Salloum C, et al. Repeat hepatectomy for recurrent colorectal metastases. Br J Surg. 2013;100(6):808–18.
Jonsson K, Grondahl G, Salo M, et al. Repeated liver resection for colorectal liver metastases: a comparison with primary liver resections concerning perioperative and long-term outcome. Gastroenterol Res Pract. 2012;2012:568214.
Rolff HC, Calatayud D, Larsen PN, Wettergren A. Good results after repeated resection for colorectal liver metastases. Dan Med J. 2012;59(2):A4373.
Hallet J, Cunha SA, Adam R, et al. Factors influencing recurrence following initial hepatectomy for colorectal liver metastases. Br J Surg. 2016;103(10):1366–76.
Battula N, Tsapralis D, Mayer D, et al. Repeat liver resection for recurrent colorectal metastases: a single-centre, 13-year experience. HPB. 2014;16(2):157–63.
Lam VW, Pang T, Laurence JM, et al. A systematic review of repeat hepatectomy for recurrent colorectal liver metastases. J Gastrointest Surg. 2013;17(7):1312–21.
Lillemoe HA, Kawaguchi Y, Passot G, et al. Surgical resection for recurrence after two-stage Hepatectomy for colorectal liver metastases is feasible, is safe, and improves survival. J Gastrointest Surg. 2019;23(1):84–92.
Rahbari NN, D’Angelica MI. Surgical salvage of recurrence after resection of colorectal liver metastases: incidence and outcomes. Hepat Oncol. 2017;4(1):25–33
Nanji S, Tsang ME, Wei X, Booth CM. Outcomes after repeat hepatic resection for recurrent metastatic colorectal cancer: a population-based study. Am J Surg. 2016;213(6):1053–9.
Adam R, Pascal G, Azoulay D, et al. Liver resection for colorectal metastases: the third hepatectomy. Ann Surg. 2003;238(6):871–83. discussion 83–4.
de Jong MC, Mayo SC, Pulitano C, et al. Repeat curative intent liver surgery is safe and effective for recurrent colorectal liver metastasis: results from an international multi-institutional analysis. J Gastrointest Surg. 2009;13(12):2141–51.
Lee H, Choi SH, Cho YB, et al. Repeat hepatic resection in patients with colorectal liver metastases. World J Gastroenterol. 2015;21(7):2124–30.
Luo LX, Yu ZY, Huang JW, Wu H. Selecting patients for a second hepatectomy for colorectal metastases: an systemic review and meta-analysis. Eur J Surg Oncol. 2014;40(9):1036–48.
Andreou A, Brouquet A, Abdalla EK, et al. Repeat hepatectomy for recurrent colorectal liver metastases is associated with a high survival rate. HPB. 2011;13(11):774–82.
Rau R, Schmertmann CP. District-level life expectancy in Germany. Dtsch Arztebl Int. 2020;117(29–30):493–9.
Gandy RC, Stavrakis T, Haghighi KS. Short- and long-term outcomes of elderly patients undergoing liver resection for colorectal liver metastasis. ANZ J Surg. 2018;88(3):103–107.
Fong Y, Brennan MF, Cohen AM, et al. Liver resection in the elderly. Br J Surg. 1997;84(10):1386–90.
Shirabe K, Kajiyama K, Harimoto N, et al. Early outcome following hepatic resection in patients older than 80 years of age. World J Surg. 2009;33(9):1927–32.
Schiergens TS, Lindenthaler A, Thomas MN, et al. Time-dependent impact of age and comorbidities on long-term overall survival after liver resection. Liver Int. 2016;36(9):1340–50.
Langella S, Russolillo N, Forchino F, et al. Impact of obesity on postoperative outcome of hepatic resection for colorectal metastases. Surgery. 2015;158(6):1521–9.
Yamamoto M, Yoshida M, Furuse J, et al. Clinical practice guidelines for the management of liver metastases from extrahepatic primary cancers 2021. J Hepatobiliary Pancreat Sci. 2021;28(1):1–25.
Van Cutsem E, Cervantes A, Adam R, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol. 2016;27(8):1386–422.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
M. von Heesen and J. Schuld conceived of the presented idea and developed the study protocol. S. Holländer, A.E. Spiliotis, and A. Merscher conducted the retrospective analysis and verified the analytical methods. P.R. Scherber, D. Igna, G. Gäbelein, and M. Glanemann supervised the findings of this work. All authors discussed the results and contributed to the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
M. von Heesen, J. Schuld, S. Holländer, A.E. Spiliotis, A. Merscher, P.R. Scherber, D. Igna, G. Gäbelein, and M. Glanemann declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The authors M. von Heesen and J. Schuld contributed equally to the manuscript.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
von Heesen, M., Schuld, J., Holländer, S. et al. Repeated hepatic resection for colorectal liver metastases: is this concept safe and feasible?. Eur Surg 54, 317–325 (2022). https://doi.org/10.1007/s10353-022-00783-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10353-022-00783-7