Summary
Background
This study aimed to summarize a new technology for magnetic resonance imaging (MRI) of swallowing in the evaluation of esophageal function and gastroesophageal reflux disease (GERD) as well as for postoperative imaging after antireflux surgery.
Methods
A search was carried out in the Medline database to identify relevant publications.
Results
Magnetic resonance swallowing is a new, simple, nonionizing radiological method used to confirm the diagnosis of GERD or any motility disorder. The MR diagnosis of GERD was concordant with the pH-metry in 82% of patients. However, the main clinical indication is for evaluation of the cause of fundoplication failure in the postoperative patient who suffers new or recurrent symptoms. Magnetic resonance swallowing is the only method that enables a direct view of the wrap itself. In up to 93% of cases, the correct position of the fundoplication wrap could be determined; 67% of malpositions were assessed, as well as all cases of wrap.
Conclusion
Real-time MRI swallowing, as a noninvasive and nonionizing method, offers a new perspective for the combined anatomic and functional visualization of GERD, with the possibility of direct visualization of the surrounding structures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastroesophageal reflux disease (GERD) is one of the most common diseases in the modern world, with a prevalence of up to 20% [1]. The main cause is the dysfunction of the antireflux barrier, which is located at the gastroesophageal junction and includes many functional and anatomic components. In these times of more personalized therapy for GERD [2], the pathology of GERD is characterized by the functional impairment of the lower esophageal sphincter, the crural diaphragm, a widened angle of His, and poor esophageal motility [3].
Traditionally, the diagnosis of GERD has relied on pH-metric studies, manometry, endoscopy, and a barium swallow as an imaging method. Owing to their low diagnostic accuracy, barium esophagrams are currently no longer recommended in the diagnostic pathway. Because of various limitations, a contrast barium swallow cannot provide adequate anatomic information [4]. Repetitive assessment of esophageal motor function by barium esophagram is also restricted owing to ionizing radiation exposure [5].
With recent developments in magnetic resonance imaging (MRI) during the past few years, the imaging modality now offers excellent soft-tissue contrast in combination with increasing temporal resolution up to the subsecond level [6]. Therefore, dynamic imaging of swallowing has become a reality for the morphological and functional imaging of the esophagus and the gastroesophageal junction.
Moreover, MR swallowing is a completely noninvasive procedure without ionizing radiation, and, therefore, could also be applied in the near future in pediatric patients and pregnant women [7].
At present, MR swallowing has been successfully used in healthy volunteers [8,9,10] and patients with oropharyngeal disorders [11,12,13]. This new method was also reviewed in the assessment of motility disorders [14, 15], as well as in the diagnosis of GERD [4, 16,17,18,19]. Since 2014, it has been used to monitor treatment after antireflux surgery [20,21,22].
Methods
This is a narrative review of the clinical value of MR swallowing for the diagnosis of GERD and esophageal motility disorders, as well as for the assessment of failure after laparoscopic antireflux surgery (LARS).
A selective Medline search was conducted through PubMed with the terms “MRI”, “GERD”, “swallowing”, “gastroesophageal junction”, “esophageal motility”, and “fundoplication failure”.
Additional literature was supplemented from a reference list of leading articles.
Since there was no intention to perform a quantitative assessment of the available literature, no systematic review or meta-analysis was conducted. The imaging method described in this article has recently been developed, and, to date, most studies have dealt with a small patient population. Therefore, a meta-analysis would not be the appropriate method.
Results
Role of MRI in diagnosis of GERD
A consensus panel of GERD experts concluded, in 2012 [23], that the optimal diagnostic work-up for GERD should include pH monitoring, manometry, upper GI-endoscopy, and barium esophagram. The most important method in identifying GERD and the subsequent correct treatment is pH-impedance monitoring. Barium swallow examination is useful for testing the function of the esophagus, but, in diagnosing GERD, the sensitivity is only 34%, and thus, it is not recommended in the diagnostic work-up of GERD [24]. However, this method is required for the preoperative work-up before LARS, according to the American Esophageal Advisory Board [23], to determine whether there are structural abnormalities, such as hiatal hernia or paraesophageal hernia, as well as a short esophagus or other structural abnormalities in the oropharynx or esophagus. This aids in planning an adequate surgical therapy. However, a barium swallow cannot show surrounding structures and requires ionizing radiation exposure. Owing to the radiation exposure, the examination cannot be repeated at will.
Dynamic swallowing MRI has become a valuable tool in the assessment of morphological and functional imaging of the esophagus [4, 16, 18] in patients with typical GERD symptoms. Magnetic resonance swallowing is a noninvasive procedure without ionizing radiation and without the administration of intravenous contrast medium, and thus it could also be used for pediatric or pregnant patients.
Compared with a barium swallow, MR swallowing offers the possibility of multiplanar imaging in any desired plane, and thus the course of the esophagus and the gastroesophageal junction can be adjusted very precisely. The best coverage of the esophagus, which is the main challenge of this examination technique, can be reached with a combination of double-oblique sagittal and coronal views ([16]; Fig. 1). With these planes, reflux events can be studied quite well (Fig. 2), and the literature reports a good correlation between 83 and 91% with the DeMeester score in pH-metric studies [16, 18]. Other parameters, such as diaphragm-to-sphincter distance, sphincter length, as well as sphincter-transit-time, showed significant differences between healthy volunteers and symptomatic patients [18]. There was a small difference in the His angle between healthy volunteers with approximately 75° and reflux patients with about 100° [18]. However, this was not statistically significant, which is in contrast to the longstanding hypothesis that a smaller His angle forms a better antireflux barrier [25, 26]. The presence of a hiatal hernia has been shown to have a strong association with GERD [27, 28]. The detection and measurements of a hiatal hernia illustrates one of the main strengths of swallowing MRI. Exact measurements of the size of a hiatal hernia are possible in any desired plane [16]. The size of an axial hernia shows a strong correlation with the grade of the reflux [7]. About 60–80% of GERD patients suffer from a hiatal hernia, whereas only 3–7% of patients without hiatal hernia show signs of reflux esophagitis [27,28,29,30]. An increased hernia size is significantly correlated with total esophageal acid exposure, acid clearance, acid exposure time, and severity of esophagitis [31]. In MR swallowing examinations, the axial orientation is preferred for the detection and measurement of an axial hernia ([16]; Fig. 3).
This new method should not replace pH-metric studies, manometry, or endoscopy; rather, it should be seen as a complementary method in the diagnostic pathway for GERD. If the probe for pH measurements or manometry could not be placed for some reason, it could also serve as a helpful alternative method.
Dynamic MRI in motility disorders
Motility disorders of the esophagus are usually investigated with high-resolution manometry (HRM), a precise technique with measurements that are not assessable with other methods. While HRM even offers the capability to measure the intensity of a single peristaltic wave and the opening pressure of the lower esophageal sphincter (LES), a barium swallow or videofluoroscopy allows for a simultaneous view of the morphological changes and gastroesophageal function during swallowing events.
The combination of imaging and instrumental approaches was successfully introduced for the diagnosis and follow-up [32,33,34,35] of symptomatic patients. In the past few years, the field of radiology has moved from ionizing radiation studies toward radiation-free imaging modalities like MRI.
Today, MR swallowing studies for motility disorders represent an actual topic of research, but is still limited to a few working groups [14,15,16, 19].
As a noninvasive procedure, MR swallowing could, therefore, be used in young patients who suffer from noncardiac chest pain, globus without dysphagia, or unexplained cough as a first-choice investigation before HRM or a barium swallow [15]. This method allows for repeated examinations at short intervals thanks to the lack of ionizing radiation, as different oral contrast mediums are used, from a gadolinium-buttermilk mixture, yoghurt, pineapple juice, or the oral MR contrast agent Lumivision® (Bendergruppe, Baden-Baden, Germany). The good soft-tissue contrast of MR swallowing offers the possibility for multiplanar imaging of the surrounding structures, such as the mediastinum or diaphragm [15], which could be useful in detecting extra-esophageal findings that can clinically mimic achalasia or other motility disorders.
One limitation is that MR swallowing can be performed only in supine position in most MR machines. Thus, an upright swallow is not possible and can impair the examination in some subjects, especially in patients with achalasia (Fig. 4), in whom the bolus transport is possible only in an upright position [15]. Also, fine webs, Schatzki rings, or low-grade stenoses could be overlooked by an MR swallowing study owing to the limited spatial resolution, which is adequate for the assessment of esophageal function, but too low for precise evaluation of the esophageal wall [14].
Dynamic B‑FFE (Balanced Fast Field Echo) sequence in the sagittal view (a, b) as well as in coronal view (c) showing the delayed propagation of the bolus in the craniocaudal direction. The esophagus is dilated and the lower esophageal sphincter does not open sufficiently (arrow), which is the typical finding in achalasia. Numbers at the bottom indicate time in seconds after initiation of swallowing
Impact of swallowing MRI in failed LARS
Gastroesophageal reflux disease can be successfully treated by LARS with a high success rate of nearly 90% [36]. A small group of patients suffer from persistent, recurrent, or completely new symptoms after fundoplication. The clinical presentation includes dysphagia that does not resolve after the first 6 weeks, recurrent heartburn, regurgitation, or gas bloat syndrome [37]. Postsurgical failure and its definition have been extensively discussed in the literature [38]. It has been demonstrated that surgical success decreased with each reoperation. Thus, a detailed preoperative work-up is mandatory to identify the underlying cause.
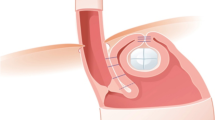
Most often, a multimodal diagnostic approach is chosen, which includes endoscopy, manometry, pH-metric studies, a barium swallow, or videocinematography. However, compared with fluoroscopy, where the wrap can be identified only indirectly, swallowing MRI is the first-choice radiological method, which offers a direct view of the wrap itself (Fig. 5). Thus, diagnostic reports should be structured and could be categorized according to five different failure patterns ([20]; Table 1). A significant problem includes intrathoracic migration of the wrap (Fig. 6). Therefore, it is not surprising that MRI achieves better results than endoscopy in diagnosing the location of the wrap or even its disruption [20]. Magnetic resonance imaging was able to confirm the correct position in 93% of patients and wrap disruption in all patients, when compared with the intraoperative results of the revision surgery [20]. In another study where endoscopy was used as a reference test, real-time MRI yielded an excellent sensitivity of 92%, with a specificity of only 17% [22]. The high number of “false-positive” diagnoses (i.e., low specificity) of MRI might indicate that in fact endoscopy missed a relevant portion of hernias [22]. In a combined test, where the reference test for the assessment of hernia was implemented either on endoscopy or real-time MRI, MRI showed a sensitivity of 94%, with a specificity of 100%, whereas endoscopy reached 71% sensitivity, with a specificity of 100% [22]. A recurrent hernia is one of the major causes of a second operation [38]. Thus, it is not surprising that 82.9% of the patient population in the revision surgery group had a recurrent hiatal hernia [21]. Swallowing MRI could detect 83% of cases, with five cases missed (17%), in a follow-up study with a total patient population of 79 [21]. Although these very few studies showed better results for MRI, endoscopy should not be replaced. Moreover, these two methods are complementary in diagnosing the postoperative wrap situation [21].
Normal postoperative appearance after Nissen fundoplication on magnetic resonance imaging. A ring-like “pseudotumor” (arrow) of the fundoplication, and a well-defined smooth defect in the fundus acquired in the axial plane (a). Additional coronal (b) and sagittal (c) views show the correct position of the wrap under the diaphragm
Intrathoracic migration of the wrap. T2-weighted HASTE (Half-Fourier-Acqired Single-shot Turbo spin Echo) sequences in the coronal and sagittal view (a, b) show that the entire wrap (arrow) lies above the esophageal hiatus in a patient with postprandial chest fullness. T2-weighted HASTE sequences in the axial view (c) demonstrate the integrity of the wrap (arrow)
The radiologist should be familiar with the terms “recurrent hernia” and “slipping.” In contrast to “slipping,” a recurrent hernia is diagnosed when gastric components are displaced above the hiatus level without regard to the status of the wrap [21]. “Slipping” means that the stomach slips proximally through an intact fundoplication wrap. Some authors use the term “slipped Nissen,” which means the transthoracic migration of the Nissen wrap [39]. Slipping is a difficult diagnosis to make with MRI, because the tube-like malformed part of the gastric fundus can easily be misinterpreted as distal to the esophagus [21].
Wrap disruption (Fig. 7) occurs in up to 12.8% of patients [40] who undergo revision surgery. Obviously, the correct diagnosis of wrap disruption requires a certain level of experience in interpreting swallowing MRI [21]. Sometimes, it can be difficult to distinguish between a complete and incomplete rupture of the wrap, but the decision for revision surgery in symptomatic patients remains the same. The knowledge of the correct fundoplication type before the MR examination is crucial. However, a “Toupet” fundoplication (270° circumference) can mimic a partial disruption of a Nissen fundoplication (360° complete circumference). Swallowing MRI has the major advantage of combining anatomical with functional information, enabling the assessment of esophageal dysmotility after fundoplication [20, 22]. A reduction of propulsive contractions and a bolus transit time more than 20 s are a strong indicator of esophageal dysmotility [20]. The diagnosis of re-reflux still remains a problem. Owing to the higher temporal resolution of the new sequences, the detection rate of reflux could be improved [22] in 86% of cases, but 15–30-min imaging time might be too short to achieve so as to compete with 24-h pH monitoring.
Conclusion
Swallowing MRI is a reliable, safe, and innovative diagnostic imaging modality with which to assess the anatomic and functional features of the gastroesophageal junction and the esophagus in one examination. It has the potential for a better understanding of GERD, as well as of motility disorders, and is a valuable complementary method in the diagnosis of failure patterns after LARS.
References
Dent J, El-Serag HB, Wallander MA, Johansson S. Epidemiology of gastro-oesophageal reflux disease. A systematic review. Gut. 2005;54:710–7.
Rieder E, Riegler M, Simic AP, Skrobic OM, Bonavina L, Gurski R, Paireder M, Castell DO, Schoppmann SF. Alternative therapies for GERD: a way to personalized antireflux surgery. Ann N Y Acad Sci. 2018;1434:360–9.
Koch OO, Antoniou SA. Advances in diagnosing GERD. Which examinations should be performed before interventional therapy ? Eur Surg. 2016;48:203–8.
Curcic J, Fox M, Kaufman E, Forras-Kaufman Z, et al. Gastroesophageal junction: structure and function as assessed by using MR imaging. Radiology. 2010;257:115–24.
Crawley MT, Savage P, Oakley F. Patient and operator dose during fluoroscopic examination of swallow mechanism. Br J Radiol. 2004;77:654–6.
Frahm J, Schätz S, Untenberger M, et al. On the temporal fidelity of nonlinear inverse reconstructions for real-time MRI—the motion challenge. Open Med Imaging J. 2014;8:1–7.
Kulinna-Cosentini C. The role of MRI in GERD. In: Memon MA, editor. Hiatal Hernia Surgery – An evidence Based Approach. Springer, Vol. 2018. 2018. pp. 17–27.
Kulinna-Cosentini C, Schima W, Cosentini EP. Dynamic MR imaging of the gastroesophageal junction in healthy volunteers during bolus passage. J Magn Reson Imaging. 2007;25:749–54.
Zhang S, Olthoff A, Frahm J. Real-time magnetic resonance imaging of normal swallowing. Magn Reson Imaging. 2012;35:1372–9.
Manabe T, Kawamitsu H, Higashino T, et al. Esophageal magnetic resonance fluoroscopy: optimization of the sequence. J Comput Assist Tomogr. 2004;28:697–703.
Hartl DM, Kolb F, Bretagne E, Marandas P, Sigal R. Cine magnetic resonance imaging with single-shot fast spin echo for evaluation of dysphagia and aspiration. Dysphagia. 2006;21:156–62.
Barkhausen J, Goyen M, von Winterfeld F, Lauenstein T, Arweiler-Harbeck D, Debatin JF. Visualization of swallowing using real-time TrueFISP MR fluoroscopy. Eur Rad. 2002;12:129–33.
Breyer T, Echternach M, Arndt S, et al. Dynamic magnetic resonance imaging of swallowing and laryngeal motion using parallel imaging at 3T. Magn Reson Imaging. 2009;27:48–54.
Panebianco V, Tomei E, Anzidei M, et al. Functional MRI in the evaluation of oesophageal motility: feasibility, MRI patterns of normality and preliminary experience in subjects with motility disorders. Radiol Med. 2006;111:881–9.
Panebianco V. Habib Fl, Toemi E. Initial Exp With Magn Reson Fluoroscopy Eval Oesophageal Motil Disord Comp With Manometry Barium Fluoroscopy Eur Rad. 2006;16:1926–33.
Kulinna-Cosentini C, Schima W, Lenglinger J, et al. Is there a role for dynamic swallowing MRI in the assessment of gastroesophageal reflux disease and oesophageal motility disorders? Eur. Rad, Vol. 22. 2012. pp. 364–70.
Manabe T, Kawamitsu H, Higashino T, et al. Observation of gastro-esophageal reflux by MRI: a feasibility study. Abdom Imaging. 2009;34:419–23.
Zhang S, Joseph AA, Gross L, Ghadimi M, Frahm J, Beham AW. Diagnosis of gastroesophageal reflux disease using real-time magnetic resonance imaging. Sci Rep. 2015;15(5):12112.
Seif Amir Hosseini A, Beham A, Uhlig J, et al. Intra-and interobserver variability in the diagnosis of GERD by real-time MRI. Eur J Radiol. 2018;104:14–9.
Kulinna-Cosentini C, Schima W, Ba-Ssalamah A, Cosentini EP. MRI patterns of Nissen fundoplication: normal appearance and mechanisms of failure. Eur Radiol. 2014;24:2137–45.
Arnoldner MA, Kristo I, Paireder M, Cosentini EP, Schima W, Weber M, Schoppmann SF, Kulinna-Cosentini C. Swallowing MRI—a reliable method fort he evaluation oft he postoperative gastroesophageal situs after Nissen fundoplication. Eur Rad 2018 (epub ahead of print). 2018. https://doi.org/10.1007/s00330-018-5779-2..
Seif Amir Hosseini A, Uhlig J, Streit U, Voit D, Uhlig A, Ellenrieder V, et al. Real-time MRI for the dynamic assessement of fundoplication failure in patients with gastroesophageal reflux disease. Eur Rad. 2019 (epub ahead of print). https://doi.org/10.1007/s00330-019-06025-x.
Jobe BA, Richter JE, Hoppo T, Peters JH, Bell R, Dengler WC, DeVault K, Fass R, Gyawali CP, Kahrilas PJ, Lacy BE, Pandolfino JE, Patti MG, Swanstrom LL, Kurian AA, Vela MF, Vaezi M, DeMeester TR. Preoperative diagnostic workup before antireflux surgery: an evidence and experienced-based consensus oft he Esophageal Diagnostic Advisory Panel. J Am Coll Surg. 2013;217:586–697.
Ott DJ. Gastroesophageal reflux disease. Radiol Clin North Am. 1994;32:1147–66.
Hill LD, et al. The gastroesophageal flap valve: in vitro and in vivo observations. Gastrointest Endosc. 1996;44:541–7.
Kahrilas PJ, et al. Esophageal peristaltic dysfunction in peptic esophagitis. Baillieres Clin Gastroenterol. 1986;91:897–904.
Van Herwaarden MA, Samsom M, Smout AJ. The role of hiatus hernia in gastro-oesophageal reflux disease. Eur J Gastroenterol Hepatol. 2004;16:831–5.
Wright RA, Hurwitz AL. Relationship of hiatal hernia to endoscopically proved reflux esophagitis. Dig Dis Sci. 1979;24:311–3.
Berstad A, Weberg R, Froyshov Larsen I, et al. Relationship of hiatus hernia to reflux esophagitis. Scand J Gastroenterol. 1986;21:55–8.
Petersen H, Johannessen T, Sandvik AK, et al. Relationship between endoscopic hiatus hernia and gastroesophageal reflux symptoms. Scand J Gastroenterol. 1991;26:921–6.
Jones MP, Sloan SS, Jovanovic B, et al. Impaired egress rather than increased access: an important independent predictor of erosive oesophagitis. Neurogastroenterol Motil. 2002;14:625–31.
Prabhakar A, Levine MS, Rubesin S, Laufer I, Katzka D. Relationship between diffuse esophageal spasm and lower esophageal sphincter dysfunction in barium studies and manometry in 14 patients. Ajr Am J Roentgenol. 2004;183:409–13.
Fuller L, Huprich JE, Theisen J, Hagen JA, Crookes PF, Demeester SR, Bremner CG, Demeester TR, Peters JH. Abnormal esophageal body function: radiographic-manometric correlation. Am Surg. 1999;65:911–91.
Schima W, Ryan JM, Harisinghani M, Schober E, Pokieser P, Denk DM, Stacher G. Radiographic detection of achalasia: diagnostic accuracy of videofluoroscopy. Clin Radiol. 1998;53:372–5.
Fiorentino E, Barbiera F, Grassi N, Buscemi G, Latteri S, Valenti A, Mastrosimone A. Digital videofluorography and esophageal achalasia: from diagnosis to follow-up. Chir Ital. 2005;57:59–64.
Bammer T, Hinder RA, Klaus A, Klingler PJ. Five- to eight-year outcome of the first laparoscopic Nissen fundoplications. J Gastrointest Surg. 2001;5:42–8.
Gopal DV, Chang EY, Kim CY, et al. EUS characteristics of Nissen fundoplication: normal appearance and mechanisms of failure. Gastrointest Endosc. 2006;63:35–44.
Smith CD, McClusky DA, Rajad MA, Lederman AB, Hunter JG. When fundoplication fails: redo? Ann Surg. 2005;241(6):861–71.
Puri R, Cline AM, DeArmond DT, Johnson SB. Transthoracic repair of slipped Nissen fundoplications: technique and results. Ann Thorac Surg. 2012;94:429–35.
Carlson MA, Frantzides CT. Complications and results of primary minimally invasive antireflux procedures: a review of 10,735 reported cases. J Am Coll Surg. 2001;193:428–39.
Funding
Open access funding provided by Medical University of Vienna.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
C. Kulinna-Cosentini, M.A. Arnoldner, I. Kristo, G. Jomrich, W. Schima, M. Riegler, S.F. Schoppmann, and E.P. Cosentini declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Kulinna-Cosentini, C., Arnoldner, M.A., Kristo, I. et al. Swallowing MRI for GERD—diagnosis and treatment monitoring. Eur Surg 51, 231–238 (2019). https://doi.org/10.1007/s10353-019-0601-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10353-019-0601-1