Summary
Background
Tissue Engineering is a multidimensional approach that aims to produce a structure similar to target tissue. Main targets in laryngeal tissue engineering (TE) are chronic vocal fold scars and vocal fold atrophy which occurs in elderly patients. The main obstacle for restoration is the highly complex architecture of the human vocal fold. Deterioration of its micro-architecture leads to altered vibration characteristics that have deleterious effects on voice quality, causing hoarseness (dysphonia). The aim of this study is to give an actual clinical and experimental overview of this field as well as to further elucidate promising new findings and trends.
Methods
We performed a PubMed survey on articles relevant to this field.
Results
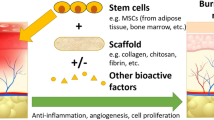
Fifty relevant articles were included that reflect the latest advances with growth factors, cell therapy and scaffold engineering, as well the first applications in humans. Cell therapy, using somatic or stem cells, remains highly promising; however, there is a lack of applications in humans due to safety concerns. Different types of scaffolds have been investigated and atelocollagen sheets were already implanted in human vocal folds. Many trials used a combined approach of cell therapy, scaffolds, and different growth factors.
Conclusions
Experiments and trials in laryngeal tissue engineering have emerged over the last decades and clinical use is near. Within this review, we discuss the latest developments to encourage further research on this fascinating topic. Similar to other fields, a close cooperation between different professions such as physicians, bioengineers, as well as industrial partners is required to succeed in laryngeal TE.


Similar content being viewed by others
References
Ling C, Yamashita M, Waselchuk EA, Raasch JL, Bless DM, Welham NV. Alteration in cellular morphology, density and distribution in rat vocal fold mucosa following injury. Wound Repair Regen. 2010;18:89–97.
Ruben RJ. Redefining the survival of the fittest: communication disorders in the 21st century. Laryngoscope. 2000;110:241–5.
Hirano M. Morphological structure of the vocal cord as a vibrator and its variations. Folia Phoniatr (Basel). 1974;26:89–94.
Gray SD, Titze IR, Alipour F, Hammond TH. Biomechanical and histologic observations of vocal fold fibrous proteins. Ann Otol Rhinol Laryngol. 2000;109:77–85.
Hansen JK, Thibeault SL. Current understanding and review of the literature: vocal fold scarring. J Voice. 2006;20:110–20.
Suehiro A, Hirano S, Kishimoto Y, Rousseau B, Nakamura T, Ito J. Treatment of acute vocal fold scar with local injection of basic fibroblast growth factor: a canine study. Acta Otolaryngol. 2010;130:844–50.
Suehiro A, Hirano S, Kishimoto Y, Tateya I, Rousseau B, Ito J. Effects of basic fibroblast growth factor on rat vocal fold fibroblasts. Ann Otol Rhinol Laryngol. 2010;119:690–6.
Kishimoto Y, Hirano S, Suehiro A, et al. Effect of exogenous hepatocyte growth factor on vocal fold fibroblasts. Ann Otol Rhinol Laryngol. 2009;118:606–11.
Kishimoto Y, Hirano S, Kitani Y, et al. Chronic vocal fold scar restoration with hepatocyte growth factor hydrogel. Laryngoscope. 2010;120:108–13.
Ohno T, French LC, Hirano S, Ossoff RH, Rousseau B. Effect of hepatocyte growth factor on gene expression of extracellular matrix during wound healing of the injured rat vocal fold. Ann Otol Rhinol Laryngol. 2008;117:696–702.
Hirano S, Kishimoto Y, Suehiro A, Kanemaru S, Ito J. Regeneration of aged vocal fold: first human case treated with fibroblast growth factor. Laryngoscope. 2008;118:2254–9.
Branski RC, Barbieri SS, Weksler BB, et al. Effects of transforming growth factor-beta1 on human vocal fold fibroblasts. Ann Otol Rhinol Laryngol. 2009;118:218–26.
Hirano S, Nagai H, Tateya I, Tateya T, Ford CN, Bless DM. Regeneration of aged vocal folds with basic fibroblast growth factor in a rat model: a preliminary report. Ann Otol Rhinol Laryngol. 2005;114:304–8.
Hirano S. Current treatment of vocal fold scarring. Curr Opin Otolaryngol Head Neck Surg. 2005;13:143–7.
Rousseau B, Sohn J, Montequin DW, Tateya I, Bless DM. Functional outcomes of reduced hyaluronan in acute vocal fold scar. Ann Otol Rhinol Laryngol. 2004;113:767–76.
Matsumoto K, Nakamura T. Hepatocyte growth factor (HGF) as a tissue organizer for organogenesis and regeneration. Biochem Biophys Res Commun. 1997;239:639–44.
Hirano S, Bless D, Heisey D, Ford C. Roles of hepatocyte growth factor and transforming growth factor beta1 in production of extracellular matrix by canine vocal fold fibroblasts. Laryngoscope. 2003;113:144–8.
Sheen-Chen SM, Liu YW, Eng HL, Chou FF. Serum levels of hepatocyte growth factor in patients with breast cancer. Cancer Epidemiol Biomarkers Prev. 2005;14:715–7.
Gugatschka M, Kojima T, Ohno S, Kanemaru S, Hirano S. Recruitment patterns of side population cells during wound healing in rat vocal folds. Laryngoscope. 2011;121:1662–7.
Chhetri DK, Berke GS. Injection of cultured autologous fibroblasts for human vocal fold scars. Laryngoscope. 2011;121:785–92.
Kanemaru S, Nakamura T, Omori K, et al. Regeneration of the vocal fold using autologous mesenchymal stem cells. Ann Otol Rhinol Laryngol. 2003;112:915–20.
Xu W, Hu R, Fan E, Han D. Adipose-derived mesenchymal stem cells in collagen-hyaluronic acid gel composite scaffolds for vocal fold regeneration. Ann Otol Rhinol Laryngol. 2011;120:123–30.
Kumai Y, Kobler JB, Herrera VL, Zeitels SM. Perspectives on adipose-derived stem/stromal cells as potential treatment for scarred vocal folds: opportunity and challenges. Curr Stem Cell Res Ther. 2010;5:175–81.
Park H, Karajanagi S, Wolak K, et al. Three-dimensional hydrogel model using adipose-derived stem cells for vocal fold augmentation. Tissue Eng Part A. 2010;16:535–43.
Zhang F, Sprecher AJ, Wei C, Jiang JJ. Implantation of gelatin sponge combined with injection of autologous fat for sulcus vocalis. Otolaryngol Head Neck Surg. 2010;143:198–203.
Luo Y, Kobler JB, Heaton JT, Jia X, Zeitels SM, Langer R. Injectable hyaluronic acid-dextran hydrogels and effects of implantation in ferret vocal fold. J Biomed Mater Res B Appl Biomater. 2010;93:386–93.
Long JL. Tissue engineering for treatment of vocal fold scar. Curr Opin Otolaryngol Head Neck Surg. 2010;40(5):521–5.
Prestwich GD, Shu XZ, Liu Y, et al. Injectable synthetic extracellular matrices for tissue engineering and repair. Adv Exp Med Biol. 2006;585:125–33.
Prestwich GD. Hyaluronic acid-based clinical biomaterials derived for cell and molecule delivery in regenerative medicine. J Control Release. 2011;155:193–9.
Bartlett RS, Thibeault SL, Prestwich GD. Therapeutic potential of gel-based injectables for vocal fold regeneration. Biomed Mater. 2012;7:024103–6041/7/2/024103. Epub 2012 Mar 29.
Kishimoto Y, Welham NV, Hirano S. Implantation of atelocollagen sheet for vocal fold scar. Curr Opin Otolaryngol Head Neck Surg. 2010;18(6):507–11.
Ohno S, Hirano S, Tateya I, et al. Atelocollagen sponge as a stem cell implantation scaffold for the treatment of scarred vocal folds. Ann Otol Rhinol Laryngol. 2009;118:805–10.
Teramachi M, Nakamura T, Yamamoto Y, Kiyotani T, Takimoto Y, Shimizu Y. Porous-type tracheal prosthesis sealed with collagen sponge. Ann Thorac Surg. 1997;64:965–9.
Nakamura T, Teramachi M, Sekine T, et al. Artificial trachea and long term follow-up in carinal reconstruction in dogs. Int J Artif Organs. 2000;23:718–24.
Omori K, Nakamura T, Kanemaru S, et al. Cricoid regeneration using in situ tissue engineering in canine larynx for the treatment of subglottic stenosis. Ann Otol Rhinol Laryngol. 2004;113:623–7.
Omori K, Tada Y, Suzuki T, et al. Clinical application of in situ tissue engineering using a scaffolding technique for reconstruction of the larynx and trachea. Ann Otol Rhinol Laryngol. 2008;117:673–8.
Okano W, Nomoto Y, Wada I, et al. Bioengineered trachea with fibroblasts in a rabbit model. Ann Otol Rhinol Laryngol. 2009;118:796–804.
Nomoto Y, Kobayashi K, Tada Y, Wada I, Nakamura T, Omori K. Effect of fibroblasts on epithelial regeneration on the surface of a bioengineered trachea. Ann Otol Rhinol Laryngol. 2008;117:59–64.
Nomoto Y, Okano W, Imaizumi M, Tani A, Nomoto M, Omori K. Bioengineered prosthesis with allogenic heterotopic fibroblasts for cricoid regeneration. Laryngoscope. 2012;122:805–9.
Igai H, Chang SS, Gotoh M, et al. Widespread and early tracheal cartilage regeneration by synchronous slow release of b-FGF and BMP-2. ASAIO J. 2009;55:266–70.
Yamashita M, Kanemaru S, Hirano S, et al. Glottal reconstruction with a tissue engineering technique using polypropylene mesh: a canine experiment. Ann Otol Rhinol Laryngol. 2010;119:110–7.
Hou N, Cui P, Luo J, Ma R, Zhu L. Tissue-engineered larynx using perfusion-decellularized technique and mesenchymal stem cells in a rabbit model. Acta Otolaryngol. 2011;131:645–52.
Macchiarini P, Jungebluth P, Go T, et al. Clinical transplantation of a tissue-engineered airway. Lancet. 2008;372:2023–30.
Laurance J. British boy receives trachea transplant built with his own stem cells. BMJ. 2010;340:c1633.
Baiguera S, Jungebluth P, Burns A, et al. Tissue engineered human tracheas for in vivo implantation. Biomaterials. 2010;31:8931–8.
Jungebluth P, Alici E, Baiguera S, et al. Tracheobronchial transplantation with a stem-cell-seeded bioartificial nanocomposite: a proof-of-concept study. Lancet. 2011;378(9808):1997–2004.
Fuchs JR, Terada S, Ochoa ER, Vacanti JP, Fauza DO. Fetal tissue engineering: in utero tracheal augmentation in an ovine model. J Pediatr Surg. 2002;37:1000–6; discussion 1000–6.
Kunisaki SM, Freedman DA, Fauza DO. Fetal tracheal reconstruction with cartilaginous grafts engineered from mesenchymal amniocytes. J Pediatr Surg. 2006;41:675–82; discussion 675–82.
Elliott MJ, De Coppi P, Speggiorin S, et al. Stem-cell-based, tissue engineered tracheal replacement in a child: a 2-year follow-up study. Lancet. 2012;380:994–1000.
Baiguera S, Gonfiotti A, Jaus M, et al. Development of bioengineered human larynx. Biomaterials. 2011;32:4433–42.
Conflict of interest
The authors declare that there is no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gugatschka, M., Graupp, M. & Friedrich, G. Applications of tissue engineering in modern laryngology . Eur Surg 45, 136–141 (2013). https://doi.org/10.1007/s10353-013-0214-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10353-013-0214-z