Abstract
Crustose coralline algae (CCA) are important ecosystem engineers and carbonate producers today and in the geological past. While there is an increasing number of publications on CCA every year, it is evident that there are many misunderstandings and inconsistencies in the assignment of CCA to taxonomic and functional groups. This is partly because CCA are treated by biologists, ecologists and palaeontologists as well as covered by studies published in journals ranging from geo- to biosciences, so that there is often a mixture of terminology used and differing scientific focus. In this review, a comprehensive overview is given on what is known about CCA, their functional traits and their roles in environments from the present and the past. In this context, some bridges are built between the commonly different viewpoints of ecologists and palaeontologists, including suggesting a common and straightforward terminology, highlighting and partially merging different taxonomic viewpoints as well as summarizing the most important functional traits of CCA. Ideally, future studies should seek to quantitatively analyse potential implications for CCA and their associated organisms under ongoing global change.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Crustose coralline algae (CCA) are a phylogenetic group of heavily calcifying macroalgae that occur globally in recent environments, ranging from the tropics to polar latitudes (Teichert et al. 2012; van der Heijden and Kamenos 2015; López Correa et al. 2023). Having a great bathymetric range, CCA can be found on shallow reef crests (Dean et al. 2015), in tidal pools (Nalin et al. 2008, see also Fig. 1) but also down to 268 m water depth in the Bahamas, to date the deepest known plant life on Earth (Littler et al. 1985). CCA are characterized by their crustose growth mode and are grouped in the coralline red algae, consolidated in the subclass Corallinophycidae Le Gall and Saunders 2007. The Corallinophycidae share commonalities on the cellular level. Their pit plugs feature two cap layers at cytoplasmic faces; they have two-celled carpogonial branches and their tetrasporangia are zonate or cruciate in division. Most importantly from the geoscience perspective, their thallus features calcification in the form of calcite. CCA are a marine group, with so far only one record of a freshwater species (Ragazzola et al. 2020). As a result of their calcified cell walls, CCA have a very good fossil record (Aguirre et al. 2000). However, the timing of their origination is still disputed, ranging from the Palaeozoic (Teichert et al. 2019) to the Mesozoic (Peña et al. 2020).
In recent environments, CCA play a major role in the ecosystem functioning of benthic communities. In the form of rhodoliths (one type of free-living CCA), they act as ecosystem engineers by providing three-dimensional habitat (Teichert 2014, see also Fig. 2), and in modern coral reefs, they commonly act as the cement that holds these reefs together (Littler and Littler 2013; Teichert et al. 2020a; Cornwall et al. 2023 and references therein). Diversity analyses in marine habitats increasingly incorporate CCA. In view of the difficulties involved in taxonomic descriptions solely based on morphological characters, molecular methods are more and more applied in this context (Rindi et al. 2019; Auer and Piller 2020; Twist et al. 2020). CCA are threatened by climate change, especially by ocean acidification (Cornwall et al. 2022), coupled with warming temperatures (Cornwall et al. 2019).
In this review, the most important facts about CCA from an interdisciplinary point of view are highlighted and there is a focus on the advances in science that have been made since the last comprehensive treatments on various focal points (Steneck 1986; McCoy and Kamenos 2015; Sissini et al. 2022). An overview is provided on the two main phenotypes of CCA (attached and free-living CCA) in recent and past environments, the environmental factors that their development depends on, as well as on their ecological and sedimentological functions. The importance in analysing functional traits within the group of CCA is emphasized, in addition to taxonomic studies, when it comes to an assessment of the role CCA play in today’s ecosystems as well as in those of the geological past.
Taxonomic and phylogenetic aspects
Despite the obvious importance of CCA for biodiversity and carbonate production, several aspects regarding their geological history, their phylogenetics and taxonomy, as well as their ecological significance are still insufficiently known. CCA are researched by phycologists, ecologists, as well as palaeontologists and covered in journals from geo- and biosciences, so there is often a mixture of the terminology used and the scientific focus applied. The complicated taxonomy of this group makes quantitative studies on CCA diversity difficult, especially when it comes to the investigation of fossil CCA specimens. These need to be assigned taxonomically solely by morphological characters, while for the recent counterparts, molecular data can be used additionally. At present, there are five orders placed within the subclass Corallinophycidae, comprising 952 species plus another 5 species of incertae sedis. The orders are listed in Table 1 and their phylogenetic relationship is highlighted in Fig. 3. These data are according to AlgaeBase, which to date is the most complete and maintained database on marine algae publicly available (Guiry and Guiry 2023).
Phylogenetic tree based on Bayesian analysis of concatenated sequences (psbA, rbcL, COI-5P) highlighting the phylogenetic relationship of the five CCA orders currently known, according to Jeong et al. (2021) but pruned to order level. Order level boxes contain the presently accepted families with species numbers in brackets, according to AlgaeBase (Guiry and Guiry 2023). Values above branches denote Bayesian posterior probabilities (BPP) > 0.75/maximum likelihood bootstrap values (BP, %) > 50. BPP values < 0.75 and BP values < 50% are indicated by hyphens (–). BPP values of 1.00 and BP values of 100% are indicated by asterisks (*). Scale indicates substitutions per site, note that lengths of fading branches do not correspond to substitutions per site
Unfortunately, there is not such a repository for the fossil counterparts of CCA, as AlgaeBase does indeed include published occurrences of fossil CCA, but lacks the function of a distinct stratigraphical and palaeogeographical assignment. Although the Palaeobiology Database includes these and further functionalities for quantitative analyses focusing on fossil material, (https://paleobiodb.org), it is currently outdated in terms of CCA taxonomy.
While the taxonomy of (fossil and recent) CCA is still an issue, studies focusing on functional traits of CCA repeatedly highlight their ecological importance with respect to their particular phenotype (Steneck 1986; Hinojosa-Arango and Riosmena-Rodriguez 2004; Gagnon et al. 2012; Teichert 2014; Teichert et al. 2020a). Functional traits have been defined as those that affect organismal performance, that is, survival, development (increase in complexity/differentiation), growth (increase in size/mass) and reproduction (Violle et al. 2007). The development and ontogeny of CCA depends on biotic and abiotic environmental factors, like feeding pressure (Steneck 1986), carbonate saturation (Ragazzola et al. 2016), light conditions (Williams et al. 2018a) and water energy (Bosence 1983c). Depending on the environment, the same species can develop different thallus morphologies, ranging from extremely thin crusts over reef-like encrustations to free-living rhodoliths, thereby exhibiting an extreme phenotypic plasticity (Fusco and Minelli 2010). Nevertheless, sequencing data reveal that some so-called highly plastic species are in fact multiple species and it has been shown that there is also considerable genetic diversity masked by cryptic morphological differences (Nelson et al. 2015; Rindi et al. 2019). Therefore, there are both plastic morphologies and convergent morphologies in CCA.
A note on cryptic diversity in CCA
Strictly, two or more species are cryptic if they are, or have been, classified as a single nominal species because they are at least superficially morphologically indistinguishable (Bickford et al. 2007). CCA as a group are notoriously difficult to identify based on morphological characters and consequently they are often lumped together into vague categories like, as an extreme case, just “CCA” when it comes to the description of ecosystems and their share in local biodiversity (Hind et al. 2019). As cryptic species can be involved in both, attached and free-living CCA, the actual biodiversity is commonly underestimated and the high phenotypic plasticity of the group as a response to varying environmental parameters complicates this even further. Nevertheless, recognizing cryptic diversity among CCA is gaining momentum as a consequence of DNA sequencing analyses. DNA barcoding techniques allow for the accurate identification of species and have demonstrated that some of the most common taxa under the morphological species concept in fact represent several to more than a dozen distinct genetic species (Gabrielson et al. 2018; Hind et al. 2019). Of course, this is a significant issue when analysing fossil CCA communities with regard to taxonomic diversity, as this has to rely completely on morphological characters which also need to be preserved during the fossilization process.
Concerning this matter, Auer and Piller (2020) recently applied nanoscale morphotaxonomy to investigate the taxonomic value of nanometre-scale ultrastructures within calcified CCA thalli by comparing these independent morphological data to existing molecular phylogenies. This idea has been triggered by studies on several organism groups showing that the morphology of skeletal ultrastructures is strictly biologically controlled and thus not easily perturbated by environmental conditions such as changing pH levels (see Auer and Piller (2020) and references therein). Morphological evidence suggests that calcification in the primary cell walls follows the initiation of calcification in the middle lamella (de Carvalho et al. 2022; McCoy et al. 2023) and the results of the study indicate that there are distinct morphotypes of primary crystallites which can serve as taxonomically viable parameters: the genotype of CCA is expressed within the nanoscale ultrastructure (Auer and Piller 2020), regardless of phenotypic plasticity. On a higher taxonomic level, independent morphological analysis of nanoscale ultrastructures shows a clear correspondence with the phylogenetic clades/subfamilies defined by Rösler et al. (2016). As the authors point out, their study only represents a first step and additional analyses are necessary, especially to document the interclade (order level) morphological variability in CCA. Despite the early state of research on cryptic CCA and especially their fossil counterparts, the application of calcification traits for cryptic species identification represents a promising pathway for refined diversity estimations in past and present environments.
In this context, another study (Smith et al. 2012) explored the correlation between the skeletal mineralogy of CCA and their molecular phylogeny, mainly employing patterns of the magnesium/calcium ratio and testing these for a phylogenetic signal by calculating Blomberg’s K (Blomberg et al. 2003). Despite the significant mineralogical differences between families, variation and overlap in these patterns prevented the use of the CCA carbonate mineralogy as a taxonomic character.
Geniculate coralline red algae
Morphologically, the coralline red algae (Corallinophycidae) are divided into two main groups, the non-geniculate (non-articulated or encrusting) and the geniculate (articulated) forms. However, the taxonomic value traditionally attributed to the genicula to separate non-geniculate from geniculate taxa is no longer accepted. The latter are thought to be derived from the former, and later reversals to the ancestral state have also occurred (Peña et al. 2020). The non-geniculate thalli calcify completely and therefore have the highest preservation potential. The geniculate forms have uncalcified filaments (genicula) between their calcified segments (intergenicula) and grow upright or pendulously branched (Fig. 4). The genicula decay quickly after death, making geniculate CCA prone to rapid disarticulation, which results in a rather poor fossil record of this group. A literature search performed in the ISI Web of Knowledge in June 2023 reveals only two references to fossil geniculate coralline red algae. One of the difficulties in this context is that just based on the morphology of thallus parts of these algae, it cannot be concluded whether these are geniculate or not, as long as the actual genicula are not preserved in the fossil specimens. This is rarely the case, as the genicula do not calcify. Therefore, it has been suggested that the placing of fossil specimens into geniculate genera without appropriate evidence must be avoided by grouping all potentially intergeniculate fragments under the informal group “Geniculate sensu lato” (Bassi et al. 2000). Indeed, the intergenicula can be preserved in the fossil record (Mude and Kundal 2010) but due to their scarcity, they are of minor relevance regarding the fossil record of CCA, at least at the present state of knowledge.
Geniculate coralline red algae. a Thallus of Corallina officinalis from a tidal pool on the Isle of Skye, Scotland, illustrating the branched structure of geniculate forms. b Micro-CT reconstruction of a branch from the same specimen; calcified intergenicula have a higher density and appear in light gray (note the growth increments resembling tree rings); uncalcified genicula have a lower density and appear in dark gray
Non-geniculate coralline red algae
The crustose forms of the corallines have a high phenotypic plasticity. “Phenotypic plasticity is the ability of individual genotypes to produce different phenotypes when exposed to different environmental conditions” (Pigliucci et al. 2006). This includes the possibility to modify developmental trajectories in response to specific environmental triggers and the ability of an individual organism to change its phenotypic state in response to environmental variations (Fusco and Minelli 2010). A great variety of CCA that share significance for present environments and the fossil record can develop as two main groups. On the one hand these are CCA firmly attached to a fixed substrate and on the other hand, these are the free-living forms rhodoliths and CCA-coated grains (Fig. 5).
Comparison between attached and free-living CCA. a Thick crusts of Clathromorphum compactum firmly attached to the bedrock in 15 m water depth. b Thin crusts of Boreolithothamnion glaciale colonizing glaciogenic cobbles in 11 m water depths; such cobbles can be moved when exposed to wave action or browsing organisms. c Rhodolith bed mainly formed by B. glaciale in 40 m water depth as a classic example for free-living CCA. d Rhodoliths mainly formed by B. glaciale and a glaciogenic boulder in the lower right corner of image densely colonized by thin crusts of the same species. The large boulder can be regarded as a fixed or loose substrate, depending on water energy. This highlights that the transitions between attached and free-living CCA can be seamless. Images recorded in Mosselbukta, northern coast of Spitsbergen, Norway, courtesy of JAGO-team Geomar, Kiel
Attached CCA
Attached, also called epigenous, CCA are non-geniculate coralline red algae that commonly grow prostrate on hard substrates and as epibionts on other plants and animals (Steneck 1986). Their morphological characteristics have been comprehensively described and classified by Woelkerling (1988) and Woelkerling et al. (1993). Within the attached CCA, the thallus form can be divided arbitrarily into four broad categories: unconsolidated, taeniform, encrusting and protuberant.
Regarding the first group, the thallus consists largely of unconsolidated or anastomosing filaments spreading over the substrate whereas taeniform thalli are composed almost entirely of flattened, ramified, ribbon-like branches. These two categories are expressed rather seldomly in species compared to the encrusting and protuberant forms.
All encrusting thalli are pseudoparenchymatous, i.e. the thallus is composed of numerous coherent filaments which commonly, but not always are organized into a thallus of consistent form, resulting in recognizable dorsal and ventral surfaces. With few exceptions, encrusting thalli do not produce distinct holdfasts. The thallus can be anchored to the substratum only by localized cell adhesions, by enveloping part of the host axis or by being firmly attached, conforming to the contours of the substrate. When colonizing a substrate, encrusting CCA thalli grow, among several varieties, mostly in a monomerous or dimerous pattern (Woelkerling 1988). In the monomerous construction pattern, there is a single system of repeatedly branched filaments in which some derivatives of each filament contribute to a core, which runs more or less parallel to the thallus surface whereas other derivatives curve outwards and form a more peripheral region. In the dimerous construction pattern, two groups of filaments are produced successively, and these are usually oriented more or less at right angles to one another. These crusts can be fully adherent to the substrate or have non-adherent margins, a condition called leafy. The dorsal surface of those thalli can be smooth or feature more or less perpendicularly arising protuberances, which may be branched or not. Within the dimerous species, all these alternatives may appear within the same specimen.
The crust thicknesses can vary greatly in attached CCA, from about 50 µm to well over 10,000 µm; crusts are defined arbitrarily as “thin” if their thickness is less or equals 500 µm; otherwise they are considered “thick” (Steneck 1986). All these states (crust thickness, thallus construction, etc.) are rarely constrained phylogenetically, so most genera have species in several morphological categories. However, several genera show a predominance of certain morphological states, as, e.g. most species in the genus Lithothamnion are branched; most species in the genus Mesophyllum are thin and leafy and in the genus Clathromorphum, most species are thick and all species are unbranched, not developing protuberances (Steneck 1986; Guiry and Guiry 2023). Whereas the lateral growth of CCA is theoretically indeterminate, vertical growth (i.e. crust thickness) is usually limited and happens at a fractional amount of lateral growth speed. The vertical growth rate has been shown to vary mainly with temperature (positively) and with species, while species biogeography is linked to isotherms (Lüning 1990), whereas an increase in nutrient availability can decrease CCA growth (Russell et al. 2009). Generally, attached CCA grow very slowly, ranging from 10 (Littler et al. 1991) to 5200 µm yr−1 (Adey and Vassar 1975), while the average is at about 500 µm yr−1 (Schäfer et al. 2011), strongly depending on parameters like CCA species, geographical area, water depth and turbidity. Several growth rate estimations in the older literature have to be handled with care because of the varying methods and sometimes questionable assumptions (Foster 2001). However, more recent studies found interesting correlations between CCA growth rates and various environmental parameters, such as temperature (Adey et al. 2013), kelp cover (Halfar et al. 2011) and sea ice cover (Leclerc et al. 2022b). Interestingly, there are no quantitative estimates of growth rates or carbonate production in fossil CCA. This would be an interesting field, as the preservation quality of CCA is often very high and growth rate quantification could serve as a valuable tool for palaeoenvironmental analyses.
An important feature especially in those species producing thick crusts is the succession of growth increments (Fig. 6), which have been used extensively as a recorder for (palaeo-) environmental conditions, employing geochemical methods (Hetzinger et al. 2013; Teichert et al. 2020b; Leclerc et al. 2022a) and structural analyses (Ragazzola et al. 2013; Schlüter et al. 2021). The growth increments in CCA commonly are seen in the banding patterns that result from development of cells responding to alternating seasonal environmental parameters. For example, specimens of Boreolithothamnion glaciale from northern Norway show a distinct bright-dark rhythmicity as a response to the extreme seasonality in that area (Freiwald and Henrich 1994), whereas the darker bands seem to be caused by a reduced calcification during the (darker) winter time of the year (Freiwald 1993). In this context, it is key to correctly assign CCA growth increments to the timespan over which they developed. While most studies refer to annual resolutions, staining experiments have highlighted the existence of lower order growth increments that resemble weekly (Kamenos et al. 2008) or lunar cycles (Agegian 1981). However, other studies on tropical (Sletten et al. 2017), Mediterranean (Hetzinger et al. 2023) and Arctic (Williams et al. 2018b) rhodoliths have shown that these different orders of banding are irregular and therefore likely not related to weekly or lunar cycles, but rather to turning and temporary burial of one side of a rhodolith. Moreover, it has to be considered that CCA thalli can feature non-seasonal growth interruptions, also known as hiati, which are further outlined below. Such hiati form when a once dead algal thallus is recolonized by another CCA thallus, potentially from the same species as the underlying dead thallus. In that case, the recognition of such a hiatus may be difficult and, in any case, the amount of time between dieback of the old and recolonization with the new thallus is unknown (Schlüter et al. 2021).
Examples of growth increments in CCA, visible as an alternation of darker layers formed during the winter season and brighter layers formed during the summer season. a Reflected light microscopy image showing the growth increments of the rhodolith-forming CCA Sporolithon nodosum from 5 m water depth at Whangaparaoa peninsula, New Zealand. b Transmitted light microscopy image of the rhodolith-forming CCA Lithothamnion cf. corallioides from the island of Fuerteventura (courtesy of Axel Munnecke, FAU Erlangen-Nürnberg). c Transmitted light microscopy image of a fossil specimen of the CCA Phymatolithon calcareum from the Miocene of the Latium-Abruzzi platform, central Apennines, Italy (courtesy of Axel Munnecke, FAU Erlangen-Nürnberg)
Free-living CCA
Many features described for the attached CCA apply to the free-living CCA as well, but with the main difference that the substrate these CCA are growing on is mobile or that a substrate is lacking at all. According to Woelkerling (1988), free-living CCA can form in two ways: (1) from either encrusting portions or protuberances of attached or unattached individuals which have broken off but continue to grow or (2) from thalli which have completely enveloped small stones, mollusc shells or other materials and then continue to grow around this central nucleus. The thallus morphology itself can vary as in the case of the attached CCA except that no forms with unconsolidated filaments are known, i.e. there are free-living crustose forms with or without protuberances, forms composed entirely of branched protuberances and free-living taeniform thalli (Woelkerling 1988).
There has been a great number of terms assigned to free-living CCA and several research articles and reviews have suggested various schemata of the correct application of these terms over the past decades (Bosence 1983b; Woelkerling et al. 1993; Foster 2001; Aguirre et al. 2017; Riosmena-Rodríguez 2017). The most commonly used terms today are rhodolith, maërl, coated grain and coralline algal nodule. Of these, the term “coralline algal nodule” is very unspecific and remains undefined scientifically and could potentially been applied to any free-living CCA structure, so I discourage its further use for the sake of clarity. Other terms for free-living CCA in the literature that are no longer considered appropriate are rhodolite, a term that has priority for a variety of garnet (Binda 1973), rhodoid, a term that has been rejected for its unsuitable etymology (Bosence 1983b), oncolith, a term that is defined to include microbes, cyanobacteria, green algae and encrusting foraminifera but not CCA (Flügel 2010), and macroid, a term that describes nodules that indeed include coralline red algae but other encrusting organisms as well (Hottinger 1983). Maërl sensu stricto is a Breton word for unattached, branched and loose-lying CCA that can be alive or dead and that commonly occur in extensive deposits or algal gravels off the northwest coast of France (Crouan and Crouan 1867; Riosmena-Rodríguez 2017). The term maërl is correct when used in this context, but it should not be applied for occurrences elsewhere in the world. Exceptionally, and if one focuses on the similarity to maërl sensu stricto, it might be useful to use the terms “maërl-type” or “maërl facies”. The two terms commonly applicable to free-living CCA all over the world and in recent and fossil occurrences as well are rhodoliths and coated grains. The discrimination between both terms is easy and straightforward: a free-living CCA that lacks a nucleus is always referred to as a rhodolith, whereas a free-living CCA with a nucleus (organic or inorganic) is referred to as a rhodolith when the volume of the structure is composed mostly (> 50%) of CCA tissue, otherwise the application of the term coated grain is correct. However, as the term coated grain can include a variety of coatings, I propose to use the term “CCA-coated grain” to refer to free-living CCA structures that are composed < 50% of CCA tissue. Of course, this is not a static condition and a CCA-coated grain can easily become a rhodolith by further growth of the involved CCA specimens or by the accretion of additional individuals (Fig. 7).
Successive development of a CCA-coated grain to a rhodolith. a CCA-coated grain with thin encrustations of Boreolithothamnion glaciale which not even fully cover a lithoclastic nucleus. b Scanning electron microscopy image showing the growing margin of a B. glaciale specimen that colonizes a lithoclastic nucleus. c CCA-coated grain with thicker crusts of B. glaciale which envelope a lithoclastic nucleus; white margins (meristematic areas) mark borders between several CCA individuals. d Fully grown rhodolith in which the CCA skeleton outweighs the lithoclastic nucleus. All samples have been collected in Mosselbukta, northern coast of Spitsbergen, Norway
For a refined descriptive identification of rhodoliths and CCA-coated grains, the scheme defined by Bosence (1983b) is straightforward and still applicable. Free-living CCA can consist of one or several CCA specimens, species, genera, etc., therefore called monospecific or multispecific. Size descriptions are most easily done by measuring the long (L), intermediate (I) and short (S) axis of each rhodolith/CCA-coated grain, applying the equation for the volume of an ellipsoid
Regarding the shape of a specific rhodolith, this has long been used as a proxy for the hydrodynamic or sedimentological regime in which a rhodolith developed (Bosence 1983c). However, this is meaningful only for rhodoliths lacking a nucleus or when the rhodolith tissue mass is several magnitudes higher than that of the nucleus. Otherwise, the nucleus shape will be reflected in the rhodolith shape, potentially without a causal relation to the hydrodynamic or sedimentological regime, as it has been observed especially for polar environments (Teichert et al. 2014). Respecting this, a shape description for rhodoliths and CCA-coated grains can be meaningful. The most basic way is the calculation of the general sphericity, using the equation by Sneed and Folk (1958):
and it is also possible to apply the pebble shape diagram developed by the same authors to make a distinction between spheroidal, discoidal and ellipsoidal shapes within a ternary diagram. Regarding ternary rhodolith shapes, there is also a straightforward plotting tool developed by Graham and Midgley (2000).
The features regarding growth successions and hiati in CCA mentioned above become even more important in free-living CCA, as with movement, conditions within specific parts of the rhodolith/CCA-coated grain or within the whole structure can change significantly. In a cross section of a rhodolith (Fig. 8), it is often possible to identify a variety of sessile, and vagile, organisms alternating with CCA tissue, potentially allowing for the reconstruction of changing environmental conditions. Likewise, changing growth conditions can result in hiati within the CCA tissue (Fig. 9), a fact that deserves attention when it comes to analyses that depend on a correct age estimation of the involved CCA.
Cross section of a resin-embedded rhodolith mainly built by Boreolithothamnion glaciale from 40 m water depth, Mosselbukta, northern coast of Spitsbergen, Norway. The rhodolith harbours a great variety of sessile and vagile organisms, including a barnacles, b bryozoans, c bivalves, d serpulids and e polychaetes. Whereas bryozoans and serpulids tend to colonize the surface of a rhodolith when it is facing downwards, barnacles colonize the upward facing side, thus potentially enabling the reconstruction of the developmental history of a rhodolith. White scale bars represent 1 cm
Growth interruptions (hiati) of unknown lapse of time within CCA specimens. a Two hiati (arrows) in a micro-CT reconstruction of a recent Boreolithothamnion glaciale protuberance from Mosselbukta, northern coast of Spitsbergen, Norway, visible as black (low density) interruptions in the bright-dark alternation of cells. b Two hiati in a thin section of recent Lithothamnion cf. corallioides from the island of Fuerteventura (courtesy of Axel Munnecke, FAU Erlangen-Nürnberg); left arrow shows the border between the eroded surface of an older CCA and a successional individual with larger cells which curve upwards; right arrow shows the border between an older CCA first overgrown by a bryozoan and later by a successional CCA individual. c Reflected light microscopy of two hiati in a recent rhodolith mainly built by B. glaciale from Mosselbukta, northern coast of Spitsbergen, Norway; lower arrow shows the border between strongly altered CCA material and an intact CCA individual, pointing to a potentially long time interval incorporated in the hiatus. Upper arrow points to a hiatus of potentially shorter duration, as both CCA individuals appear unscathed
Attached and free-living CCA in the fossil record
The “first appearance” of CCA
When investigating the first appearance of CCA in the fossil record, it quickly becomes clear that there are two “schools of thought”, the “Early-Cretaceous-School” and the “Pre-Cretaceous-School”. Additionally, the taxonomic position of the so-called Solenoporaceans, some of which are treated as potential ancestors of the Corallinophycidae (Riding 1993), has created confusion, an issue that is outlined below.
According to the Early-Cretaceous-School, the earliest taxa commonly mentioned to be the first “confirmed” occurrences of CCA in the fossil record are Sporolithon sp. (Valanginian of Greece) mentioned in Chatalov et al. (2015), as well as Archaeolithothamnium rude (Hauterivian of Spain) mentioned in Arias et al. (1995), a genus that was later identified as Sporolithon (Ghosh and Maithy 1996). Interestingly, both articles do not include a comprehensive description of these specimens that would render them “confirmed” occurrences from a taxonomist’s perspective. On the other hand, there is the Pre-Cretaceous-School with several accounts of older CCA occurrences, e.g. from the Triassic (Anisian) of Hungary (Senowbari-Daryan and Velledits 2007) and Turkey (Senowbari-Daryan and Link 2007), as well as from the Silurian (Wenlockian) of Gotland, Sweden (Brooke and Riding 1998, 2000; Teichert et al. 2019) and the Ordovician (Floian) of Wales (Riding et al. 1998). Accepting the Silurian fossils as CCA, Graticula gotlandica would be the first representative of the order Sporolithales and Aguirrea fluegelii would be the first representative of the order Corallinales.
A strong refutation commonly raised against the pre-Mesozoic CCA occurrences is the question why for a clade with a very rich (I deliberately do not say “complete”) fossil record back to the Lower Cretaceous, the abundance of CCA fossils should suddenly decrease within the older strata. On the other hand, it has not been analysed to what extent this change in occurrence frequency is biased. Probably, this can only be improved by an open-minded taxonomic reassessment of type material as well as a comprehensive compilation of the CCA literature in a geoscience-specific database like the PBDB, in order to enable the analysis of refined origination and extinction patterns.
Regarding their phylogeny, the most recent and comprehensive work on the evolutionary history of CCA given by Peña et al. (2020) concludes that the crown group diversification of CCA started in the Lower Jurassic and sped up during the Lower Cretaceous, as inferred from a multilocus time-calibrated phylogeny. However, the age calibration points for the most recent common ancestors (MRCA) of the two orders Hapalidiales and Sporolithales have been set in very narrow intervals (116 ± 0.66 Ma and 137.63 ± 1.23 Ma, respectively) and the clock model has been set a fixed local clock model, a fact that to some extent predetermines the outcome of such an analysis. While this approach is based on the strong evidence that the fossil record of the clades used to date the nodes in this phylogeny is reasonably complete, this does end up in the argumentation outlined above. A potential improvement would be the application of models that account for incomplete sampling, as implemented in the fossilized birth–death model for the analysis of stratigraphic range data under different speciation modes (Stadler et al. 2018) and to review whether the application of such a more realistic model would yield comparable results.
The Elianellaceans (formerly known as Solenoporaceans)
There are few algal groups in the fossil record that cause more confusion than the Solenoporaceans. This starts with name of the group, ranging from “Solenoporaceans” over “Solenopores”, “solenoporacean red algae” to “Solenoporaceae”, the last one implying an affiliation of these organisms to one single family. To shed some light on the status and composition of the group, Brooke and Riding (1998) gave a very comprehensive overview. They highlighted the most important point, namely, Solenoporaceans are a heterogenous group based on an aggregation of disparate taxa, some of which may not be algal. However, the confusion about the group culminated when Riding (2004) highlighted that the type species, Solenopora spongioides, of the eponymous genus Solenopora is a chaetetid sponge, not an alga. In his study, Riding (2004) pointed out very clearly that the name Solenopora has been used to include species not congeneric with the species S. spongioides and that the whole group is likely to be heterogenous. Nevertheless, this publication caused other authors to conclude that representatives of the whole genus Solenopora might not be algae at all (Barden et al. 2015). As a first step towards better knowledge and organization of these fossil organisms, Granier and Dias-Brito (2016) introduced the family Elianellaceae to replace the family Solenoporaceae, because the type of the latter is currently ascribed to the animal kingdom. They tentatively assigned the Elianellaceae to the order Rhodogorgonales, thus rendering them to be a member of the subclass Corallinophycidae, to which CCA also belong, an approach that is also accepted by AlgaeBase. Therefore, and to avoid future confusion, I propose to address these issues in future studies involving specimens originally assigned to the “Solenoporaceans”.
Regarding the differences between (truly algal) organisms originally assigned to the Solenoporaceans and representatives of the Corallinales, Hapalidiales and Sporolithales, four features have been regarded as especially important. These are cell size (generally larger than in CCA), thallus differentiation (less expressed than in CCA and no epithallus), reproductive organs as well as the presence of tiny cell fusions and pit connections (Braga et al. 1993; Brooke and Riding 1998). Regarding the lack of fossilized reproductive organs, it was suggested that these were external and uncalcified (Pia 1927; Steneck 1983). Several of these differences in morphological features (Figs. 10, 11) were potentially responsible for the extinction of the group. After the first appearance during the Cambrian and times of maximum abundances during the Ordovician and the Jurassic, the group declined following a peak in the Jurassic, became rare in the early Paleogene and finally went extinct in the Miocene (Steneck 1983). This was probably caused by an intensification in herbivory (discussed in greater detail below), especially because of the lack of an epithallus, resulting in an unprotected meristem, and the uncalcified reproductive structures as well as their putative position on the outer thallus surface, making them readily edible for grazing organisms (Steneck 1983).
Distinct morphological features of the Elianellaceans. a Thalli of Solenopora jurassica from the Bathonian (Middle Jurassic) of Gloucestershire, England, with potentially seasonal bandings according to Wright (1985). b Thalli of another specimen of S. jurassica from the same outcrop, clearly showing the large cells typical for members of the Elianellaceans. The white arrow indicates a drilling track and the white rectangle refers to the magnification in c, highlighting the absence of thallus differentiation and an epithallus. d Thallus of Graticula gotlandica from the Wenlockian (Lower Silurian) of Gotland, Sweden, alike without thallus differentiation. White arrow indicates enlarged cells, so-called trichocytes, and black arrow indicates structures that have been regarded as potential calcified sporangial compartments (Brooke and Riding 1998). All thin sections courtesy of Axel Munnecke, FAU Erlangen-Nürnberg
Schematic comparison of anatomical features classically used to discriminate members of the Elianellaceans from representatives of the Corallinales, Hapalidiales and Sporolithales. Within these, Elianellaceans commonly feature larger cells, poor thallus differentiation including the lack of an epithallus, uncalcified/unprotected reproductive organs, and a lack of tiny cell fusions and pit connections. Figure is based on Fig. 4 in Steneck (1983)
CCA as palaeoenvironmental and facies indicators
Since the pioneering studies on the utility of attached and free-living CCA for facies interpretations (Bosence 1983b, c; Flügel 2010), their applicability for the interpretation of palaeoenvironments has been refined in many ways. As CCA are photoautotrophic organisms, their occurrence in a specific environment can be attributed to sufficient light levels. However, is has to be considered that (1) CCA have a special photosystem that expands their bathymetric range down to water depths of more than 290 m (Littler et al. 1991), depending on the turbidity of the water column and (2) that, because of their skeletal stability, the thalli might have been transported to deeper water layers after their death, a factor that especially free-living CCA like rhodoliths are prone to. Therefore, attached CCA appear to be more suitable when it comes to the interpretation of the palaeobathymetry.
CCA are resilient to changes in salinity (Wilson et al. 2004) as long as the conditions stay marine (Schoenrock et al. 2018) and even one freshwater CCA has been described (Ragazzola et al. 2020), so they are regarded as not suitable for salinity reconstructions. Regarding ocean temperature, it has been considered that boundaries of the biogeographical regions occupied by CCA today are associated with isotherms and reveal a clear latitudinal diversity gradient (Lüning 1990); however, more recent attempts point to more complicated patterns with 62 distinct provinces across 11 realms, whereat the central Indo-Pacific and temperate Australasia proved to be the most diverse realms (Rebelo et al. 2021). There is evidence that patterns related to ocean temperature were also existing during geological times. Trends within various taxonomic groups of CCA, especially during the Cenozoic, appear to reflect climate change (Aguirre et al. 2000), but because of the existing taxonomic issues with fossil CCA, such interpretations must be handled with care.
However, facies shifts from coral- to CCA-dominated types can be used to document regional and global changes. For example, the global shift from coral- to rhodolith-dominated carbonate communities during the Burdigalian to Tortonian has been related to global changes during the Miocene (Halfar and Mutti 2005). These observations imply that rhodolith facies types develop under much broader nutrient and temperature conditions than coral reef facies and also benefit from decreasing ocean temperatures. Another factor potentially leading to a replacement of corals reefs by CCA-dominated facies types is sea-level rise, as it has been observed, e.g. in Miocene strata of the tropical Pacific (Bourrouilh-Le Jan and Hottinger 1988), because the photosystem of many CCA is adapted to extreme low-light conditions (Gantt 1990). Also, the mode of facies replacement by CCA-dominated types depends on various factors, especially regarding the formation of CCA frameworks. These require low sediment input, a specific taxonomic composition with taxa that are able to grow directly on fine-grained soft substrates, as well as substrate stability as a function of substrate composition and hydrodynamic energy (Rasser and Piller 2004).
When it comes to the reconstruction of palaeoenvironments and facies conditions, free-living CCA like rhodoliths are used much more often than attached CCA. When assessing rhodoliths, internal and external algal growth morphology, rhodolith external shape and inner arrangement as well as organism assemblages forming the rhodoliths can yield valuable information on the palaeoenvironmental conditions, a relation that has first been pursued by Bosellini and Ginsburg (1971). Most commonly, rhodoliths have been used in this context to reconstruct water energy and several studies reported that discoidal rhodoliths rather form in calm waters whereas discoidal and spheroidal shapes are more commonly associated with higher-energy environments, resulting from frequent turnover of the rhodoliths (Aguirre et al. 2017 and references therein). However, several studies have shown that rhodolith turnover does not only result from water movement, but also, and in some cases more often, from benthic organisms in search of food (Marrack 1999; Wisshak et al. 2019). Also, the shape of a rhodolith can be inherited from the nucleus it formed around (Teichert et al. 2014), a factor that diminishes with increasing age of the rhodolith by the accretion of more and more CCA thallus material (Fig. 12). Despite the problems involved, rhodoliths are a good indicator for hydrodynamics as they require a specific balance between enough water energy to keep them free from fine sediments and to overturn them frequently enough to keep them free from fouling epibionts and sufficiently calm conditions to avoid excessive breakage and abrasion of their outer parts. This abrasion of the outer parts again can be used for interpretations of the hydrodynamic regime as well, as the branching density of rhodoliths can also correspond to water energy (Bosence 1976), implying that a higher water energy leads to more densely growing protuberances that might additionally show higher degrees of abrasion. Also, it has been shown that the percentage of constructional voids among algal thalli in rhodoliths increases in calm waters (Aguirre et al. 2017 and references therein).
Different effects of nuclei on rhodolith shape visualized in micro-CT reconstructions. a Large nucleus (highlighted in red) in a rhodolith (at the borderline to a CCA-coated grain) made up Boreolithothamnion glaciale from Mosselbukta, northern coast of Spitsbergen, Norway. The shape of the nucleus significantly affects the shape of the rhodolith, rendering it unusable as a proxy for hydrodynamic energy. b Small nucleus (highlighted in red) in a rhodolith made up by Sporolithon nodosum from Whangaparaoa peninsula, New Zealand. The shape of the nucleus is largely overprinted by thick CCA crust, thus making this rhodolith a good proxy for the prevailing hydrodynamic energy
Aside the shape of rhodoliths, also their taxonomic composition can be used to infer the water depth. For example, Braga and Martín (1988) showed that rhodoliths from various water depths consist of different species assemblages. This context is especially useful when it comes to the interpretation of Quaternary deposits, because most of the species from that geological timespan are extant today and can be used for calibration purposes. For older strata, especially from the Mesozoic and before, the taxonomic issues outlined above need to be considered. To avoid the problems involved in CCA taxonomy, another approach recently applied on extant rhodoliths is interesting: the composition of not only CCA species can change with water depth, but also of organisms additionally involved in the formation of a rhodolith can change significantly. Moreover, it has been shown that the frequency of growth interruptions (hiati) within the algal thalli forming a rhodolith significantly increases with water depth (Schlüter et al. 2021), which is probably a function of less overturning and therefore of more frequent dieback of the downward facing parts of the rhodoliths.
In all these analyses, it should always be considered that rhodoliths are unattached structures that can be transported between various water depths and from one depositional environment to another (Nalin et al. 2008; Basso et al. 2009). Moreover, because of their longevity, rhodoliths might also record shifts in the palaeoenvironmental conditions, even if they stay in place. Therefore, all features commonly available from rhodolith analysis (shape, protuberance growth patterns, taxonomical composition regarding CCA and other organisms, etc.) should be considered in a holistic approach when it comes to palaeoenvironmental and facies interpretations.
CCA as carbonate factories
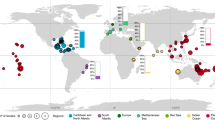
While it has been shown recently that CCA might contribute even more than corals to reef carbonate production in specific areas (Cornwall et al. 2023), the minimal coverage of CCA in past and present environments in two major reviews on carbonate factories (Schlager 2003; Reijmer 2021) highlights the potential underestimation of CCA in that context. For recent CCA communities, however, there is a substantial amount of data on their CaCO3 production rates spanning from shallow to deep water environments and from the tropics to the polar regions and in several araeas, CCA contribute significantly to carbonate sedimentation (Table 2, Fig. 13). Production rates vary by two orders of magnitude. It must be considered though that the completely different approaches in calculating the production rates and the handling of miscellaneous CCA species from different water depths and biogeographic zones make it difficult to compare the results. Nevertheless, a multiple regression analysis of the values in Table 2 (using mean values where applicable) shows that the CaCO3 production rates significantly increase towards lower latitudes while not correlating significantly with water depth (Table 3 and Fig. 14). The increase in production rates towards lower latitudes is further visualized in Fig. 15, co-equally highlighting the considerable data gap for the Southern Hemisphere.
The variety of methods applied to calculate the CaCO3 production rates of CCA include, but are not restricted to direct measurements of CCA calcification rates with in situ growth experiments using total alkalinity anomaly, buoyant weighing and the use of isotopes (see Cornwall et al. 2023 and references therein) as well as the counting of presumed annual growth increments in relation to thallus weight (Schäfer et al. 2011; Teichert and Freiwald 2014). Of course, direct measurements are only applicable on recent CCA occurrences. Fossil material can only be analysed via the indirect method of age estimation against thallus weight, nevertheless with a potential error of density change as a consequence of early or late diagenetic processes.
Such measurements on specific CCA thalli then have to be coupled with area of coverage estimations in the respective area, ideally including CCA rugosity. For recent environments, this can be done by scuba diving, encompassing open, sheltered and cryptic surfaces (Cornwall et al. 2023), underwater video footage (Teichert et al. 2014) or remote sensing via acoustic mapping (Hetzinger et al. 2006). For fossil material, the application of surface measurements is difficult as the conditions in outcrops rather represent perpendicular sections, which of course is an advantage when it comes to thickness measurements of, e.g. rhodolith beds. Interestingly, there are extensive data on the thickness of rhodolith beds from the geological past (e.g. Nalin et al. 2008; Nebelsick et al. 2013), but none for recent rhodolith beds. The density of attached and unattached CCA in geological sections can be semi-quantitatively determined by applying charts for visual comparison of the proportion of fossil CCA components per rock volume (Kidwell and Holland 1991). For the sake of greater accuracy, however, quantitative methods like point-counting should be used preferentially.
Real quantitative studies on CCA as carbonate factories are sparse for recent environments, especially when it comes to the evaluation of larger areas. While estimations for the shelf of eastern Brazil with CaCO3 production rates of 0.025 Gt yr−1 (Amado Filho et al. 2012) are based on relatively robust assumptions, global models incorporate vast uncertainties (van der Heijden and Kamenos 2015). In this context, it also has to be highlighted that there is a difference between gross CaCO3 production rates and an actual carbonate budget (i.e. net CaCO3 production rates), as carbonate is not only produced but also removed via chemical dissolution and physical and biogenic erosion.
For the geological past, quantitative studies on CCA as carbonate factories are absent with one exception for the Miocene (Halfar and Mutti 2005), highlighting that facies dominated by CCA reached peak abundances and commonly replaced coral reef environments from the Burdigalian to the early Tortonian, accompanied by a decline in other carbonate-producing phototrophs, a process that was reasoned by globally cooler temperatures following a climatic optimum. In this context, a quantitative analysis including the hitherto described CCA occurrences in the geological past and their waxing and waning over time would be most desirable.
The ecological roles of CCA
CCA and herbivory
Evolutionary changes in herbivore abundance, diversity and ability to excavate calcareous substrata occurred independently in three major herbivore groups: molluscs, sea urchins and fish. Less significant clades are annelids and crustacean arthropods. The developments in herbivory have escalated dramatically since the mid-Mesozoic era to the extent that herbivory in the recent is more intense that at any time in the geological past (Steneck 1983). With regard to the three most important groups (molluscs, echinoids, fish), there are three functionally different categories of their herbivorous effects:
-
1.
Herbivores incapable of denuding primary substrata (often called browsers) with little effect on the algal community structure.
-
2.
Herbivores that can denude primary substrata but cannot excavate calcareous substrata.
-
3.
Herbivores capable of excavating calcareous substrata.
Within the molluscs, there are two groups capable of both denuding and excavating calcareous substrata, the chitons (Polyplacophora Gray, 1821, Fig. 16), which are known already from the Cambrian period and the true limpets (Patellidae Rafinesque, 1815), occurring first in the Triassic. Despite belonging to different clades, these molluscs have several convergent adaptations like robust buccal muscles and a unique dentition which enable them to excavate calcareous substrata (Steneck 1983).
Two representatives of the most important CCA-associated herbivore groups, grazing on a rhodolith made up by Boreolithothamnion glaciale, northern coast of Spitsbergen, Norway. Both, the chiton Tonicella rubra (left side) and the echinoid Strongylocentrotus sp. (right side) are capable of denuding and excavating calcareous substrata. Photograph has been taken in an aquarium by Solvin Zankl, Kiel, shortly after retrieving the sample with a dredge
Regarding echinoids (Fig. 16), these radiated during the Mesozoic era and their average plucking and excavating abilities improved dramatically during that time (Hopkins and Smith 2015). Compared to these post-Palaeozoic echinoids, the earlier echinoids had a less effective apparatus, so it seems unlikely that they were effective herbivores of macroalgae or that they could excavate calcareous substrata (Steneck 1983). All of the modern characters in echinoid feeding apparatuses were present since the late Cretaceous (Kroh and Smith 2010; Hopkins and Smith 2015), which is supported by their trace fossil record. Their graze marks are unknown in pre-Jurassic deposits and the oldest extensively echinoid-excavated area is known from the late Cretaceous (Bromley 1975).
Although reef-grazing fish have existed for nearly 400 million years, specialized detritivores feeding on macroalgae have only been known since the Miocene (Bellwood et al. 2014). The only fish clade, however, that is capable of excavating calcareous substrata and that is sufficiently abundant to have a significant impact on CCA are the parrot fishes (Scarinae Rafinesque, 1810).
Despite the disturbance-related character of herbivory, it seems obvious that CCA as well as the Elianellaceans probably always required a distinct amount of herbivory to remain free of epiphytes (Steneck 1983) and other organisms that colonize CCA (Fig. 17). The extinct Elianellaceans, like the CCA, were calcareous and had a reduced exposure to the environment due to their prostrate growth and their CaCO3-surrounded cells, probably putting them under the same competitive disadvantages in the presence of more productive fouling epiphytes when it comes to growth speed and area coverage (Steneck 1983). Calcification in algae is widely thought to be an adaptation that reduces the impact of herbivory, including the assumption that the incorporation of CaCO3 in the tissue would reduce the nutritional value of the algae. However, on a per-volume (i.e. per-mouthful) basis, CCA can be just as calorie-rich as fleshy algae, as both calcium carbonate and water comprise non-nutritious components of calcified and non-calcified algal thalli, respectively (Maneveldt and Keats 2008). It has been shown that CCA have a high organic content, similar to those of other encrusting and turf-forming algae, and an even higher organic content than foliose algae (Maneveldt et al. 2006). Also, it has been shown experimentally that the herbivory-reducing benefits of calcification likely rather depend on CCA thallus morphology than just on the relative amount of CaCO3 incorporation in the algal tissue (Martone et al. 2021).
Examples of organisms that commonly colonize the surface of CCA, recorded by scanning electron microscopy. Samples were collected in Mosselbukta, northern coast of Spitsbergen, Norway. The cellular structure in the background of all images consists of the epithallial tissue of Boreolithothamnion glaciale, and the organisms present are a–c several species of diatoms and d a large foraminifera
Experimental removal of herbivores leads to a reduced growth of CCA in favour of fleshy algae (Stachowicz and Hay 1996), not only by domination of the fleshy algae, but also due to the entrapment of sediment, as a consequence of the absence of bioturbation, which can smother and kill the CCA (Belliveau and Paul 2002).
Notwithstanding the obvious need for a distinct intensity of herbivory in CCA and Elianellaceans as well, the successive evolution of the feeding abilities among herbivores resulted in fundamentally different outcomes for both of the calcareous algal clades. After two times of maximum abundance and diversity during the Ordovician and the Jurassic, Elianellaceans became rare in the early Paleogene and became extinct in the Miocene (Steneck 1983; Edwards et al. 1993). Moreover, there is a proportional increase of wounds in Elianellaceans fossils after the middle Jurassic and none of the Cenozoic fossils are without wounds, whereas CCA from the same geological formations reveal only insignificant proportions of wounded thalli (Steneck 1983). The CCA, on the other hand, are more diverse today than ever before in earth history (Aguirre et al. 2000). This probably resulted from the fact that the Elianellaceans missed several morphological features of the CCA that are regarded to be of fundamental importance when it comes to withstand grazing pressure:
-
1.
The epithallus, which is a layer of one or several rows of cells that overlies and thereby protects the meristem.
-
2.
Fusion cells in conjunction with primary and secondary pit connections that enable the lateral and vertical transport of photosynthates and other materials within the algal thallus which are necessary for wound healing.
-
3.
Conceptacles, which are enclosed cavities that contain both asexual and sexual reproductive structures and which can be sunken below the thallus surface, thus offering further protection.
The lack of these features in the Elianellaceans contributed to their decline and their final extinction in the Miocene. However, the CCA also experienced severe turnovers as a consequence of herbivore radiation and evolution. This is especially true when it comes to the ability of CCA to support the growth of and to stabilize coral reefs, as the evolution of herbivores deteriorated this capability several times (Teichert et al. 2020a). Moreover, the evolution of herbivores has led to specific phenotypic distributions of CCA, depending on which herbivore groups are present in a respective ecosystem and how abundant they are (Steneck 1986).
The two important components of herbivory are the amount of biomass removed in a single bite (grazing intensity) and the number of such bites per unit of time (grazing frequency). Grazing intensity varies as a function of body size, feeding apparatus and foraging behaviour and can be measured via the bite depth, e.g. using scanning electron microscopy. The three grazer clades outlined above exert significantly different grazing intensities on CCA (Steneck 1986, 1990), ranging from chitons and limpets (7–10 µm bite depth) over echinoids (88 µm bite depth) to parrot fish (288 µm bite depth and more, see also Bonaldo et al. (2011)). Indeed, thin CCA crusts grow fastest, and with a protective, potentially multi-layered epithallus and sunken conceptacles, they are well protected against low-intensity grazing as exerted by chitons and limpets. With increasing abundances of echinoids, niches are successively occupied by branching CCA. The twig-like morphologies of branching CCA prevent echinoids from denuding the CCA thallus and confine this process to the tips of the branches (Steneck 1983). As CCA are able to transfer nutrients within their thallus, these superficial grazing wounds can be rapidly healed if sufficient nutrient reservoirs are present in other, ungrazed parts of an algal specimen. Meristems and conceptacles engulfed in the thallus may be another adaptation pertinent to the relatively low impact of echinoid grazing, as this is a plausible strategy to protect the reproductive and growth structures of the CCA. The more intense grazing pressure exerted by the parrot fishes, which bite CCA to an average depth of 288 μm and are able to just eat the tips of branched CCA completely (Steneck and Adey 1976; Tâmega and Figueiredo 2019) may have resulted in a greater abundance of CCA with very thick crusts. Parrot fish species can even have a dietary specialization on CCA, potentially because of the CCA-inhabiting cyanobacteria, so this relationship has also been discussed in a co-evolutionary context (Nicholson and Clements 2022).Thick-crust CCA possess larger nutrient reservoirs making them capable to recover from grazing exerted by parrot fish and there are studies that report on the survival of actually fish-consumed pieces of CCA crusts (Tâmega et al. 2016).
Where fish and echinoids co-occur as grazers, it has to be considered that these not only control the abundance of fleshy algae and CCA by exerting feeding pressure, but may also feature linkages between each other (Burkepile and Hay 2010), i.e. fish feed on the echinoids and keep their abundance on a stable level. In this context, it has been shown that fishing can result in phase shifts in the grazing community with subsequent effects on the CCA and other algal groups, when echinoids become excessively abundant and thereby change the CCA community structure (O'Leary and McClanahan 2010; Hind et al. 2019).
The respective level of adaptation of different CCA morphologies towards various intensities and frequencies of herbivory mirrors the biogeographical and local distribution of CCA, resulting in the following patterns (summarized in Fig. 18). In tropical regions, the strongly branched CCA dominate the shallowest subtidal zones, become absent in intermediate water depths (as a consequence of increased parrot fish presence) and more common with increasing water depth again. The thick (> 500 µm) CCA crusts in contrast are rare in the most shallow and deep waters but common in the intermediate water depths which are subject to intense parrot fish grazing (Steneck 1986; Ladd et al. 2021). This trend looks different in non-tropical areas, because the parrot fishes do not play a role and co-equally, the branched morphologies offer good protection against the frequent echinoids. In both regions, tropical and non-tropical, the thin (≤ 500 µm) crusts become more common towards deeper waters, because they can easier maintain their metabolism at the prevailing dysphotic light levels.
CCA in reefs
When the CCA are handled in undergraduate palaeobiology classes, they are often highlighted as a group that is “important for reefs”. Reefs, broadly defined as laterally confined limestone structures built by the growth or metabolic activity of sessile benthic aquatic organisms (Kiessling 2003), comprise a large array of constructional styles and biota, and grew and grow in a variety of environments. Regarding recent coral reefs, CCA play a key role in their construction in several aspects. They are not only primary producers and contributors of calcareous sediment (Cornwall et al. 2023) but are also considered as “the glue that holds coral reefs together” (Castro and Huber 2010). Recently, it has also been shown that there is a strong correlation between the presence of CCA and the success of coral reefs throughout the last 150 million years (Teichert et al. 2020a). The reasons for this are manifold, as coral reefs can benefit from CCA in various ways: relating to the reef ridge, the stony pavement made up by the algae protects the ridge from onrushing waves and also consolidates the reef flats behind the ridges (Johansen 1981). With reference to the whole reef, CCA reinforce the structure created by corals, fill cracks, bind together much of the sand, dead corals and debris, and thereby create a stable substrate and reduce reef erosion (Nelson 2009). Larval settlement, metamorphosis and recruitment of several coral species are strictly determined by chemosensory recognition of specific signal molecules uniquely available in specific CCA species (Morse and Morse 1996). Of course, also the CCA can benefit from reef growth in terms of ecological niches provided, thus implying a mutual benefit (Teichert et al. 2020a). The situation appears to be different for a closely related group of algae, the Peyssonneliales Krayesky, Fredericq & Norris 2009. As only few species calcify, they are less impacted by ocean acidification and rapidly increase in abundance in coral reefs, thereby not strengthening coral reefs but fostering a phase transition from corals to algae (Edmunds et al. 2023).
The importance of CCA for coral reef thriving becomes especially evident on geological timescales. During the last 150 million years, the evolution of novel herbivore groups destabilized the interaction between CCA and coral reef growth at least three times. The first crisis during the Selandian–Thanetian corresponded with a marked increase in the rate of morphological evolution in echinoids, with a net trend towards improved mobility and feeding ability also on CCA (Hopkins and Smith 2015). The two other crises, during the Serravallian and over the Zanclean–Piacenzian boundary, can be attributed to the evolution of parrot fish, whereas the Serravallian was the time of parrot fish origination and the Zanclean–Piacenzian was the time of parrot fish lineage diversification (Choat et al. 2012; Bellwood et al. 2014). All these crises have been overcome by the CCA relatively fast, by the morphological innovations outlined above.
While the importance of CCA for coral reef construction is clear and reasonable, it is not easy to determine the actual significance of CCA in relation to the corals quantitatively, especially when it comes to carbonate budget estimation. Depending on the particular research focus, even the analysis of the same reef structure can illustrate seemingly completely different reef settings. For example, the description of a reef from the Nago Limestone (Late Eocene) type locality in northern Italy tends to be a CCA-dominated structure when analysed by an algal expert (Bassi 1998) or a true coral reef structure in an article focusing on corals (Bosellini 1998). According to Cornwall et al. (2023), there are numerous calcifiers which contribute to coral reef carbonate budgets, but the contributions of these particular groups are not necessarily equivalent to the maintenance of the reef structure. Having said this, some calcifying taxa predominantly add to the integral reef framework structures while other taxa primarily contribute to the reef sediments.
Regarding CCA in coral reefs, Cornwall et al. (2023) highlighted that their carbonate production can be spatially and temporally high, while it is still difficult to quantitively assess their contribution on a global scale. Strikingly, temporal shifts in environmental conditions or major disturbances to the corals like bleaching or storm events can cause previously coral-dominated reefs to change to CCA-dominated structures (Cornwall et al. 2023), as CCA often are more resilient against, e.g. fluctuating temperature and light levels (Wilson et al. 2004). Indeed, there is a great variety of fossil and recent CCA reef frameworks, as for example CCA biostromes from the Miocene of Malta, CCA reefs from the Eocene of north-eastern Spain and recent CCA reefs of Bermuda (Bosence 1983a). That said, the ongoing climate change is likely to drive further decline in coral health, suggesting that CCA may become increasingly important contributors to the carbonate production in reefs as well as to the provision of ecological niches in the Anthropocene (Cornwall et al. 2023).
Rhodolith beds as reef equivalents
Biodiversity in marine ecosystems depends on habitat heterogeneity (Zajac et al. 2013), which is commonly increased by so-called ecosystem engineers, i.e. organisms that modify, maintain or destroy habitats (Jones et al. 1994). With that definition rhodoliths are globally important ecosystem engineers that develop rigid structures through their calcified skeletal growth and provide ecospace in the form of three-dimensional habitat complexity (Jardim et al. 2022). There are many examples showing the positive impact of rhodoliths for general biodiversity (Teichert 2014; Straube et al. 2024) or specific faunal elements, including economically important species like scallops or juvenile cod (Kamenos et al. 2004). Whereas both rhodoliths and corals can provide habitat by creating three-dimensionality through their sheer skeletal growth, the main difference is that while corals are able to create real buildups (bioherms), rhodolith beds mainly extend laterally (biostromes), because they are not attached to a fixed substratum and the stability of the corresponding buildups is subject to the angle of slope. Among other things, this implies that while coral reefs can balance sea-level rise up to a specific level, rhodolith beds cannot. However, this apparent disadvantage of the rhodoliths is counterbalanced by the special photosystem of the CCA (Gantt 1990) that enables their thriving down to water depths of more than 290 m (Littler et al. 1991). Generally, rhodolith beds appear to be much more resilient against changing environmental factors than coral reefs (Wilson et al. 2004; Cornwall et al. 2023; Krieger et al. 2023); however, this still has to be tested quantitatively, especially regarding the current threats of ocean acidification (Cornwall et al. 2022).
Compared to tropical coral reefs, rhodolith beds can be found globally in many different climate zones, water depths and environmental settings (Foster 2001), with a significant latitudinal diversity gradient from the tropics to the poles regarding the rhodolith-forming CCA species (Lüning 1990). This implies, of course, that rhodoliths can act as habitat suppliers in regions where corals could not thrive at all. Despite the evidence that rhodoliths may be less affected than corals by global change, it has to be considered that on a global scale, different rhodolith-forming taxa with their various adaptations might react completely differently, with very specific outcomes for particular geographical regions. For a better comparability between the habitat-forming role of rhodolith beds and coral reefs as well as their reaction to major environmental changes, it would be extremely useful to analyse these patterns globally and over geological timescales.
Outlook
Research on CCA and rhodoliths is still gaining momentum and the number of publications released every year is increasing, handling a great variety of ecological, biological and palaeontological questions. Amongst the understanding of specific contexts, this provides us with the great opportunity to focus on quantitative studies in order to answer questions on a global scale and over prolonged time series. In this context, it would be very useful to combine the knowledge of ecological and palaeontological studies on CCA, last but not least to make significant predictions on how CCA-dominated environments will be impacted by global change in the long run and how this might affect the associated organisms. To further increase the quality and usability of the existing data, it would yet be mandatory to clarify several taxonomic issues, especially at the borderline between recent and palaeontological research, which could be achieved by the inclusion of analyses on the nanoscale.
Data availability
All data used to quantify the role of CCA as carbonate factories can be retrieved from Table 2.
References
Adey WH, Vassar JM (1975) Colonization, succession and growth rates of tropical crustose coralline algae (Rhodophyta, Cryptonemiales). Phycologia 14:55–69. https://doi.org/10.2216/i0031-8884-14-2-55.1
Adey HA, Halfar J, Williams B (2013) The coralline genus Clathromorphum Foslie emend. Adey: biological, physiological, and ecological factors controlling carbonate production in an Arctic-Subarctic climate archive. Smithson Contrib Mar Sci 40:1–42
Agegian CR (1981) Growth of the branched coralline alga, Porolithon gardineri (Foslie) in the Hawaiian Archipelago. In: of the Fourth International Coral Reef Symposium, Manila, pp 419–423
Aguirre J, Riding R, Braga JC (2000) Diversity of coralline red algae: origination and extinction patterns from the Early Cretaceous to the Pleistocene. Paleobiology 26:651–667
Aguirre J, Braga JC, Bassi D (2017) Rhodoliths and rhodolith beds in the rock record. In: Riosmena-Rodríguez R, Nelson W, Aguirre J (eds) Rhodolith/Maërl beds: a global perspective. Springer International Publishing, Cham, pp 105–138
Amado Filho GM, Moura LG, Bastos AC, Salgado LT, Sumida PY, Guth AZ, Francini-Filho RB, Pereira Filho GH, Abrantes DP, Brasileiro PS, Bahia RG, Leal RN, Kaufman L, Kleypas JA, Farina M, Thompson FL (2012) Rhodolith beds are major CaCO3 bio-factories in the tropical south west Atlantic. PLoS ONE 7:6. https://doi.org/10.1371/journal.pone.0035171
Arias C, Masse J-P, Vilas L (1995) Hauterivian shallow marine calcareous biogenic mounds: SE Spain. Palaeogeogr Palaeoclimatol Palaeoecol 119:3–17
Auer G, Piller WE (2020) Nanocrystals as phenotypic expression of genotypes—an example in coralline red algae. Sci Adv 6:eaay2126. https://doi.org/10.1126/sciadv.aay2126
Barden HE, Behnsen J, Bergmann U, Leng MJ, Manning PL, Withers PJ, Wogelius RA, van Dongen BE (2015) Geochemical evidence of the seasonality, affinity and pigmenation of solenopora jurassica. PLoS ONE 10:e0138305. https://doi.org/10.1371/journal.pone.0138305
Bassi D (1998) Coralline algal facies and their palaeoenvironments in the Late Eocene of Northern Italy (Calcare di Nago, Trento). Facies 39:179–201. https://doi.org/10.1007/BF02537016
Bassi D, Woelkerling WJ, Nebelsick JH (2000) Taxonomic and biostratigraphical re-assessments of Subterraniphyllum Elliott (Corallinales, Rhodophyta). Palaeontology 43:405–425. https://doi.org/10.1111/j.0031-0239.2000.00133.x
Basso D (2012) Carbonate production by calcareous red algae and global change. Geodiversitas 34:13–33
Basso D, Nalin R, Nelson CS (2009) Shallow-water Sporolithon rhodoliths from North Island (New Zealand). Palaios 24:92–103
Belliveau SA, Paul VJ (2002) Effects of herbivory and nutrients on the early colonization of crustose coralline and fleshy algae. Mar Ecol Prog Ser 232:105–114
Bellwood DR, Hoey AS, Bellwood O, Goatley CHR (2014) Evolution of long-toothed fishes and the changing nature of fish–benthos interactions on coral reefs. Nat Commun 5:3144. https://doi.org/10.1038/ncomms4144
Bickford D, Lohman DJ, Sodhi NS, Ng PKL, Meier R, Winker K, Ingram KK, Das I (2007) Cryptic species as a window on diversity and conservation. Trends Ecol Evol 22:148–155. https://doi.org/10.1016/j.tree.2006.11.004
Binda PL (1973) Form and internal structure of recent algal nodules (Rhodolites) from Bermuda: a discussion. J Geol 81:238–238. https://doi.org/10.1086/627842
Blomberg SP, Garland T Jr, Ives AR (2003) Testing for phylogenetic signal in comoarative data: behavioral traits are more labile. Evolution 57:717–745. https://doi.org/10.1111/j.0014-3820.2003.tb00285.x
Bonaldo RM, Krajewski JP, Bellwood DR (2011) Relative impact of parrotfish grazing scars on massive Porites corals at Lizard Island, Great Barrier Reef. Mar Ecol Prog Ser 423:223–233
Bosellini FR (1998) Diversity, composition and structure of Late Eocene shelf-edge coral associations (Nago Limestone, Northern Italy). Facies 39:203–225. https://doi.org/10.1007/BF02537017
Bosellini A, Ginsburg RN (1971) Form and internal structure of recent algal nodules (Rhodolites) from Bermuda. J Geol 79:669–682. https://doi.org/10.1086/627697
Bosence D (1976) Ecological studies on two unattached coralline algae from western Ireland. Palaeontology 19:365–395
Bosence D (1980) Sedimentary facies, production rates and facies models for recent coralline algal gravels, Co., Galway Ireland. Geol J 15:91–111
Bosence D (1983a) Coralline algal reef frameworks. J Geol Soc Lond 140:365–376. https://doi.org/10.1144/gsjgs.140.3.0365
Bosence D (1983b) Description and classification of Rhodoliths (Rhodoids, Rhodolites). In: Peryt TM (ed) Coated grains. Springer, Berlin, Heidelberg, pp 217–224
Bosence D (1983c) The occurrence and ecology of recent Rhodoliths—a review. In: Peryt TM (ed) Coated grains. Springer, Berlin, Heidelberg, pp 225–242
Bosence D, Wilson J (2003) Maerl growth, carbonate production rates and accumulation rates in the northeast Atlantic. Aquat Conserv 13:S21–S31
Bourrouilh-Le Jan FG, Hottinger LC (1988) Occurrence of rhodolites in the tropical Pacific—a consequence of Mid-Miocene paleo-oceanographic change. Sed Geol 60:355–367. https://doi.org/10.1016/0037-0738(88)90130-3
Braga JC, Martín JM (1988) Neogene coralline-algal growth-forms and their palaeoenvironments in the Almanzora river valley (Almeria, S.E. Spain). Palaeogeogr Palaeoclimatol Palaeoecol 67:285–303
Braga JC, Bosence D, Steneck R (1993) New anatomical characters in fossil coralline algae and their taxonomic implications. Palaeontology 36:535–547
Bromley RG (1975) Comparative analysis of fossil and recent echinoid bioerosion. Palaeontology 18:725–739
Brooke C, Riding R (1998) Ordovician and Silurian coralline red algae. Lethaia 31:185–195
Brooke C, Riding R (2000) Graticula and its derivates, replacement name for the alga Craticula Brooke & Riding non Grunow. Lethaia 33:82
Burkepile DE, Hay ME (2010) Impact of herbivore identity on algal succession and coral growth on a Caribbean Reef. PLoS ONE 5:e8963. https://doi.org/10.1371/journal.pone.0008963
Canals M, Ballesteros E (1997) Production of carbonate particles by phytobenthic communities on the Mallorca-Menorca shelf, northwestern Mediterranean Sea. Deep Sea Res Part II 44:611–629. https://doi.org/10.1016/S0967-0645(96)00095-1
Castro P, Huber ME (2010) Marine biology. McGraw-Hill, New York
Chatalov A, Bonev N, Ivanova D (2015) Depositional characteristics and constraints on the mid-Valanginian demise of a carbonate platform in the intra-Tethyan domain, Circum-Rhodope Belt, northern Greece. Cretac Res 55:84–115. https://doi.org/10.1016/j.cretres.2015.02.001
Chisholm JRM (2000) Calcification by crustose coralline algae on the northern Great Barrier Reef, Australia. Limnol Oceanogr 45:1476–1484
Choat JH, Klanten OS, Van Herwerden L, Robertson DR, Clements KD (2012) Patterns and processes in the evolutionary history of parrotfishes (Family Labridae). Biol J Lin Soc 107:529–557. https://doi.org/10.1111/j.1095-8312.2012.01959.x
Cornwall CE, Diaz-Pulido G, Comeau S (2019) Impacts of ocean warming on coralline algal calcification: meta-analysis, knowledge gaps, and key recommendations for future research. Front Mar Sci. https://doi.org/10.3389/fmars.2019.00186
Cornwall CE, Harvey BP, Comeau S, Cornwall DL, Hall-Spencer JM, Peña V, Wada S, Porzio L (2022) Understanding coralline algal responses to ocean acidification: meta-analysis and synthesis. Glob Change Biol 28:362–374. https://doi.org/10.1111/gcb.15899
Cornwall CE, Carlot J, Branson O, Courtney TA, Harvey BP, Perry CT, Andersson AJ, Diaz-Pulido G, Johnson MD, Kennedy E, Krieger EC, Mallela J, McCoy SJ, Nugues MM, Quinter E, Ross CL, Ryan E, Saderne V, Comeau S (2023) Crustose coralline algae can contribute more than corals to coral reef carbonate production. Commun Earth Environ 4:105. https://doi.org/10.1038/s43247-023-00766-w
Crouan PL, Crouan HM (1867) Florule du Finistère contenant les descriptions de 360 espèces nouvelles de sporogames, de nombreuses observations et une synonymic des plantes cellulaires et vasculaires qui croissent spontanément dans ce département, accompagnées de trente-deux planches où est représentée l’organographie, faite sur l'état vif, des fruits et des tissus de 198 genres d’algues avec la plante grandeur naturelle ou réduite plus une planche supplémentaire où sont figures 24 champignons nouveaux. Friedrich Klincksieck & J.B. et A. Lefournier, Paris & Brest
de Carvalho RT, Wendt CHC, Willemes MJ, Bahia RdG, Farina M, Salgado LT (2022) Ontogeny and early steps of the calcification process in coralline algae lithophyllum corallinae (Florideophyceae Rhodophyta). Front Mar Sci. https://doi.org/10.3389/fmars.2022.900607
Dean AJ, Steneck RS, Tager D, Pandolfi JM (2015) Distribution, abundance and diversity of crustose coralline algae on the Great Barrier Reef. Coral Reefs 34:581–594. https://doi.org/10.1007/s00338-015-1263-5
Edmunds PJ, Schils T, Wilson B (2023) The rising threat of peyssonnelioid algal crusts on coral reefs. Curr Biol 33:R1140–R1141. https://doi.org/10.1016/j.cub.2023.08.097
Edwards D, Baldauf J, Bown PR, Dorning KJ, Feist M, Gallagher LT, Grambast-Fessard N, Hart MB, Powell AJ, Riding R (1993) Algae. In: Benton MJ (ed) The fossil record 2. Chapman and Hall, London, pp 15–40
Edyvean RGJ, Ford H (1984) Population biology of the crustose red algae Lythophyllum incrustans Phil. 2. A comparison of populations from three areas of Britain. Biol J Lin Soc 23:353–363
Flügel E (2010) Microfacies of carboate rocks—analysis interpretation and application. Springer, Berlin, Heidelberg
Foster MS (2001) Rhodoliths: between rocks and soft places. J Phycol 37:659–667
Freiwald A (1993) Subarktische Kalkalgenriffe im Spiegel hochfrequenter Meeresspiegelschwankungen und interner biologischer Steuerungsprozesse. Kiel University
Freiwald A, Henrich R (1994) Reefal coralline algal build-ups within the arctic circle: morphology and sedimentary dynamics under extreme environmental seasonality. Sedimentology 41:963–984
Fusco G, Minelli A (2010) Phenotypic plasticity in development and evolution: facts and concepts. Philos Trans R Soc b: Biol Sci 365:547–556. https://doi.org/10.1098/rstb.2009.0267
Gabrielson PW, Hughey JR, Diaz-Pulido G (2018) Genomics reveals abundant speciation in the coral reef building alga Porolithon onkodes (Corallinales, Rhodophyta). J Phycol 54:429–434. https://doi.org/10.1111/jpy.12761
Gagnon P, Matheson K, Stapleton M (2012) Variation in rhodolith morphology and biogenic potential of newly discovered rhodolith beds in Newfoundland and Labrador (Canada). Bot Mar 55:85–99
Gantt E (1990) Pigmentation and photoacclimation. In: Cole KM, Sheath RG (eds) Biology of the red algae. Cambridge University Press, Cambridge, pp 203–219
Gherardi DFM (2004) Community structure and carbonate production of a temperate rhodolith bank from Arvoredo Island, southern Brazil. Braz J Oceanogr 52:207–224
Ghosh AK, Maithy PK (1996) On the present status of coralline red alga Archaeolithothamnium Roth. from India. J Palaeosci 45:64–70. https://doi.org/10.54991/jop.1996.1220
Graham DJ, Midgley NG (2000) Graphical representation of particle shape using triangular diagrams: an Excel spreadsheet method. Earth Surf Proc Land 25:1473–1477
Granier B, Dias-Brito D (2016) On the fossil alga Elianella elegans Pfender & Basse, 1948, and its so-called lookalikes, with description of Elianella brasiliana n.sp. Revision of the Juliette Pfender Collection. Part 1. Carnets De Géologie 16:213–229
Guiry MD, Guiry GM (2023) AlgaeBase. National University of Ireland, Galway
Halfar J, Mutti M (2005) Global dominance of coralline red-algal facies: A response to Miocene oceanographic events. Geology 33:481–484
Halfar J, Williams B, Hetzinger S, Steneck R, Lebednik PA, Winsborough C, Omar A, Chan P, Wanamaker AD Jr (2011) 225 years of Bering Sea climate and ecosystem dynamics revealed by coralline algal growth-increment widths. Geology 39:579–582
Hetzinger S, Halfar J, Riegl B, Godinez-Orta L (2006) Sedimentology and acoustic mapping of modern rhodolith facies on a non-tropical carbonate shelf (Gulf of California, Mexico). J Sediment Res 76:670–682
Hetzinger S, Halfar J, Zack T, Mecking JV, Kunz BE, Jacob DE, Adey HA (2013) Coralline algal Barium as indicator fot 20th century northwestern North Atlantic surface ocean freshwater variability. Sci Rep 3:1–8
Hetzinger S, Grohganz M, Halfar J, Hathorne E, Ballesteros E, Kersting DK (2023) Elemental cycles in the coralline alga Neogoniolithon hauckii as a recorder of temperature variability in the Mediterranean Sea. Front Mar Sci. https://doi.org/10.3389/fmars.2023.1151592
Hind KR, Starko S, Burt JM, Lemay MA, Salomon AK, Martone PT (2019) Trophic control of cryptic coralline algal diversity. Proc Natl Acad Sci 116:15080–15085. https://doi.org/10.1073/pnas.1900506116
Hinojosa-Arango G, Riosmena-Rodriguez R (2004) Influence of rhodolith-forming species and growth-form on associated fauna of rhodolith beds in the central west Gulf of California, México. Mar Ecol 25:109–127
Hopkins MJ, Smith AB (2015) Dynamic evolutionary change in post-Paleozoic echinoids and the importance of scale when interpreting changes in rates of evolution. Proc Natl Acad Sci 112:3758–3763. https://doi.org/10.1073/pnas.1418153112
Hottinger L (1983) Neritic macroid genesis, an ecological approach. In: Peryt TM (ed) Coated grains, 1983//, Berlin, Heidelberg. Springer, Berlin Heidelberg, pp 38–55
Jardim VL, Gauthier O, Toumi C, Grall J (2022) Quantifying maerl (rhodolith) habitat complexity along an environmental gradient at regional scale in the Northeast Atlantic. Mar Environ Res 181:105768. https://doi.org/10.1016/j.marenvres.2022.105768
Jeong SY, Nelson WA, Sutherland JE, Peña V, Le Gall L, Diaz-Pulido G, Won BY, Cho TO (2021) Corallinapetrales and corallinapetraceae: a new order and family of coralline red algae including Corallinapetra gabrielii comb. nov. J Phycol 57:849–862. https://doi.org/10.1111/jpy.13115
Johansen HW (1981) Coralline algae, a first synthesis. CRC Press, Boca Raton
Jones CG, Lawton JH, Shachak M (1994) Organisms as ecosystem engineers. Oikos 69:373–386. https://doi.org/10.2307/3545850
Kamenos NA, Moore PG, Hall-Spencer J, Donnan D (2004) Maerl: its value as a habitat for commercial species. Shellfish News 18:8–9
Kamenos NA, Cusack M, Moore PG (2008) Coralline algae are global palaeothermometers with bi-weekly resolution. Geochim Cosmochim Acta 72:771–779
Kidwell SM, Holland SM (1991) Field description of coarse bioclastic fabrics. Palaios 6:426–434. https://doi.org/10.2307/3514967
Kiessling W (2003) Reefs. In: Middleton GV, Church MJ, Coniglio M, Hardie LA, Longstaffe FJ (eds) Encyclopedia of sediments and sedimentary rocks. Springer Netherlands, Dordrecht, pp 557–560
Krieger EC, Taise A, Nelson WA, Grand J, Le Ru E, Davy SK, Cornwall CE (2023) Tolerance of coralline algae to ocean warming and marine heatwaves. PLOS Clim 2:e0000092. https://doi.org/10.1371/journal.pclm.0000092
Kroh A, Smith AB (2010) The phylogeny and classification of post-Palaeozoic echinoids. J Syst Paleontol 8:147–212. https://doi.org/10.1080/14772011003603556
Ladd MC, Winslow EM, Burkepile DE, Lenihan HS (2021) Corallivory varies with water depth to influence the growth of Acropora hyacinthus, a reef-forming coral. Ecosphere 12:e03623. https://doi.org/10.1002/ecs2.3623
Leclerc N, Halfar J, Hetzinger S, Chan PTW, Adey W, Tsay A, Brossier E, Kronz A (2022a) Suitability of the coralline alga clathromorphum compactum as an arctic archive for past sea ice cover. Paleoceanogr Paleoclimatol 37:e2021PA004286. https://doi.org/10.1029/2021PA004286
Leclerc N, Halfar J, Porter TJ, Black BA, Hetzinger S, Zulian M, Tsay A (2022b) Utility of dendrochronology crossdating methods in the development of Arctic coralline red algae clathromorphum compactum growth increment chronology for sea ice cover reconstruction. Front Mar Sci. https://doi.org/10.3389/fmars.2022.923088
Littler MM, Littler DS (2013) The nature of crustose coralline algae and their interactions on reefs. Smithson Contrib Mar Sci 39:199–212
Littler MM, Littler DS, Blair SM, Norris JN (1985) Deepest known plant life discovered on an uncharted seamount. Science 227:57–59. https://doi.org/10.1126/science.227.4682.57
Littler MM, Littler DS, Hanisak MD (1991) Deep-water rhodolith distribution, productivity, and growth history at sites of formation and subsequent degradation. J Exp Mar Bio Ecol 150:163–182
López Correa M, Teichert S, Ragazzola F, Cazorla Vázquez S, Engel FB, Hurle K, Mazzoli C, Kuklinski P, Raiteri G, Lombardi C (2023) Structural and Geochemical Assessment of the Coralline Alga Tethysphytum antarcticum from Terra Nova Bay, Ross Sea Antarctica. Minerals 13:215
Lüning K (1990) Seaweeds. Their environment biogeography and ecophysiology. Wiley Interscience, London
Maneveldt GW, Keats DW (2008) Effects of herbivore grazing on the physiognomy of the coralline alga Spongites yendoi and on associated competitive interactions. Afr J Mar Sci 30:581–593. https://doi.org/10.2989/AJMS.2008.30.3.11.645
Maneveldt GW, Wilby D, Potgieter M, Hendricks MGJ (2006) The role of encrusting coralline algae in the diets of selected intertidal herbivores. J Appl Phycol 18:619–627. https://doi.org/10.1007/s10811-006-9059-1
Marrack EC (1999) The relationship between water motion and living rhodolith beds in the southwestern Gulf of California, Mexico. Palaios 14:159–171. https://doi.org/10.2307/3515371
Martin S, Castets M-D, Clavier J (2006) Primary production, respiration and calcification of the temperate free-living coralline alga Lithothamnion corallioides. Aquat Bot 85:121–128
Martone PT, Schipper SR, Froese T, Bretner J, DeMong A, Eastham TM (2021) Calcification does not necessarily protect articulated coralline algae from urchin grazing. J Exp Mar Bio Ecol 537:151513. https://doi.org/10.1016/j.jembe.2021.151513
McCoy SJ, Kamenos NA (2015) Coralline algae (Rhodophyta) in a changing world: integrating ecological, physiological, and geochemical responses to global change. J Phycol 51:6–24. https://doi.org/10.1111/jpy.12262
McCoy SJ, Pueschel CM, Cornwall CE, Comeau S, Kranz SA, Spindel NB, Borowitzka MA (2023) Calcification in the coralline red algae: a synthesis. Phycologia 62:648–666. https://doi.org/10.1080/00318884.2023.2285673
Morse ANC, Morse DE (1996) Flypapers for Coral and Other Planktonic LarvaeNew materials incorporate morphogens for applications in research, restoration, aquaculture, and medicine. Bioscience 46:254–262. https://doi.org/10.2307/1312832
Mude SN, Kundal P (2010) A note on rare structure of fossil coralline algae from the southwest coast of India. J Geol Soc India 75:380–382. https://doi.org/10.1007/s12594-010-0033-9
Nalin R, Nelson CS, Basso D, Massari F (2008) Rhodolith-bearing limestones as transgressive marker beds: fossil and modern examples from North Island, New Zealand. Sedimentology 55:249–274. https://doi.org/10.1111/j.1365-3091.2007.00898.x
Nebelsick J, Bassi D, Lempp J (2013) Tracking paleoenvironmental changes in coralline algal-dominated carbonates of the Lower Oligocene Calcareniti di Castelgomberto formation (Monti Berici, Italy). Facies 59:133–148. https://doi.org/10.1007/s10347-012-0349-6
Nelson WA (2009) Calcified macroalgae—critical to coastal ecosystems and vulnerable to change: a review. Mar Freshw Res 60:787–801
Nelson WA, Sutherland JE, Farr TJ, Hart DR, Neill KF, Kim HJ, Yoon HS (2015) Multi-gene phylogenetic analyses of New Zealand coralline algae: corallinapetra Novaezelandiae gen. et sp. nov. and recognition of the Hapalidiales ord. nov. J Phycol 51:454–468. https://doi.org/10.1111/jpy.12288
Nicholson GM, Clements KD (2022) Scarus spinus, crustose coralline algae and cyanobacteria: an example of dietary specialization in the parrotfishes. Coral Reefs 41:1465–1479. https://doi.org/10.1007/s00338-022-02295-y
O’Leary JK, McClanahan TR (2010) Trophic cascades result in large-scale coralline algae loss through differential grazer effects. Ecology 91:3584–3597. https://doi.org/10.1890/09-2059.1
Peña V, Vieira C, Braga JC, Aguirre J, Rösler A, Baele G, De Clerck O, Le Gall L (2020) Radiation of the coralline red algae (Corallinophycidae, Rhodophyta) crown group as inferred from a multilocus time-calibrated phylogeny. Mol Phylogenet Evol 150:106845. https://doi.org/10.1016/j.ympev.2020.106845
Pia J (1927) Thallophyta. In: Hirmer M (ed) Handbuch der Palaeobotanik, Band 1: Thallophyta, Bryophyta, Pteridophyta. Oldenbourg, München, pp 31–136
Pigliucci M, Murren CJ, Schlichting CD (2006) Phenotypic plasticity and evolution by genetic assimilation. J Exp Biol 209:2362–2367. https://doi.org/10.1242/jeb.02070
Potin P, Floc’h JY, Augris C, Cabioch J (1990) Annual growth rate of the calcareous red algae Lithothamnion corallioides (Corallinales, Rhodophyta) in the Bay of Brest France. Hydrobiologia 204(205):263–267
Ragazzola F, Foster LC, Form AU, Büscher J, Hansteen TH, Fietzke J (2013) Phenotypic plasticity of coralline algae in a High CO2 world. Ecol Evol 3:3436–3446. https://doi.org/10.1002/ece3.723
Ragazzola F, Foster LC, Jones CJ, Scott TB, Fietzke J, Kilburn MR, Schmidt DN (2016) Impact of high CO2 on the geochemistry of the coralline algae Lithothamnion glaciale. Sci Rep 6:20572. https://doi.org/10.1038/srep20572
Ragazzola F, Kolzenburg R, Zekonyte J, Teichert S, Jiang C, Žuljević A, Caragnano A, Falace A (2020) Structural and elemental analysis of the freshwater, low-mg calcite coralline Alga Pneophyllum cetinaensis. Plants 9:1089. https://doi.org/10.3390/plants9091089
Rasser MW, Piller WE (2004) Crustose algal frameworks from the Eocene Alpine Foreland. Palaeogeogr Palaeoclimatol Palaeoecol 206:21–39
Rebelo A, Johnson ME, Rasser MW, Silva L, Melo CS, Ávila SP (2021) Global biodiversity and biogeography of rhodolith-forming species. Front Biogeogr 13:e50646. https://doi.org/10.21425/F5FBG50646
Reijmer JJG (2021) Marine carbonate factories: review and update. Sedimentology 68:1729–1796. https://doi.org/10.1111/sed.12878
Riding R (1993) Calcareous algae. In: Kearey P (ed) The encyclopedia of solid earth sciences. Blackwell Scientific Publications, Oxford, pp 78–81
Riding R (2004) Solenopora is a chaetetid sponge, not an alga. Palaeontology 47:117–122. https://doi.org/10.1111/j.0031-0239.2004.00351.x
Riding R, Cope JCW, Taylor PD (1998) A coralline-like red alga from the lower Ordovician of Wales. Palaeontology 41:1069–1076
Rindi F, Braga JC, Martin S, Peña V, Le Gall L, Caragnano A, Aguirre J (2019) Coralline algae in a changing Mediterranean Sea: how can we predict their future, if we do not know their present? Front Mar Sci. https://doi.org/10.3389/fmars.2019.00723
Riosmena-Rodríguez R (2017) Natural history of Rhodolith/Maërl beds: their role in near-shore biodiversity and management. In: Riosmena-Rodríguez R, Nelson W, Aguirre J (eds) Rhodolith/Maërl beds: a global perspective. Springer International Publishing, Cham, pp 3–26
Rösler A, Perfectti F, Peña V, Braga JC (2016) Phylogenetic relationships of Corallinaceae (Corallinales, Rhodophyta): taxonomic implications for reef-building corallines. J Phycol 52:412–431. https://doi.org/10.1111/jpy.12404
Russell BD, Thompson JI, Falkenberg LJ, Connell SD (2009) Synergistic effects of climate change and local stressors: CO2 and nutrient-driven change in subtidal rocky habitats. Glob Change Biol 15:2153–2162. https://doi.org/10.1111/j.1365-2486.2009.01886.x
Savini A, Basso D, Alice Bracchi V, Corselli C, Pennetta M (2012) Maerl-bed mapping and carbonate quantification on submerged terraces offshore the Cilento peninsula (Tyrrhenian Sea, Italy). Geodiversitas 34:77–98 (22)
Schäfer P, Fortunato H, Bader B, Liebetrau V, Bauch T, Reijmer JJG (2011) Growth rates and carbonate production by coralline red algae in upwelling and non-upwelling settings along the Pacific coast of Panama. Palaios 26:420–432
Schlager W (2003) Benthic carbonate factories of the Phanerozoic. Int J Earth Sci 92:445–464. https://doi.org/10.1007/s00531-003-0327-x
Schlüter M, Pyko I, Wisshak M, Schulbert C, Teichert S (2021) Growth interruptions in arctic rhodoliths correspond to water depth and rhodolith morphology. Minerals 11:538
Schoenrock KM, Bacquet M, Pearce D, Rea BR, Schofield JE, Lea J, Mair D, Kamenos N (2018) Influences of salinity on the physiology and distribution of the Arctic coralline algae, Lithothamnion glaciale (Corallinales, Rhodophyta). J Phycol 54:690–702. https://doi.org/10.1111/jpy.12774
Senowbari-Daryan B, Link M (2007) New occurrence of the red alga Norithamnium madoniensis Senowbari-Daryan, Keupp, Abate & Vartis-Matarangas, 2002 (Corallinales, Rhodophyta) in the Anisian reef boulders from Karaburun Peninsula, Turkey. J Alpine Geol 48:1–6
Senowbari-Daryan B, Velledits F (2007) Aggtecella, a new genus of Corallinales (Rhodophyta) from the Anisian of the Aggtelek-Rudabánya Mountains, NE Hungary. Facies 53:401–407
Sissini MN, Koerich G, de Barros-Barreto MB, Coutinho LM, Gomes FP, Oliveira W, Costa IO, de Castro Nunes JM, Henriques MC, Vieira-Pinto T, Torrano-Silva BN, Oliveira MC, Le Gall L, Horta PA (2022) Diversity, distribution, and environmental drivers of coralline red algae: the major reef builders in the Southwestern Atlantic. Coral Reefs 41:711–725. https://doi.org/10.1007/s00338-021-02171-1
Sletten HR, Andrus CFT, Guzmán HM, Halfar J (2017) Re-evaluation of using rhodolith growth patterns for paleoenvironmental reconstruction: an example from the Gulf of Panama. Palaeogeogr Palaeoclimatol Palaeoecol 465:264–277. https://doi.org/10.1016/j.palaeo.2016.10.038
Smith AM, Sutherland JE, Kregting L, Farr TJ, Winter DJ (2012) Phylomineralogy of the coralline red algae: correlation of skeletal mineralogy with molecular phylogeny. Phytochemistry 81:97–108
Sneed ED, Folk RL (1958) Pebbles in the lower Colorado river, Texas. A study in particle morphogenesis. J Geol 66:114–150
Stachowicz JJ, Hay ME (1996) Facultative mutualism between an herbivorous crab and a coralline alga: advantages of eating noxious seaweeds. Oecologia 105:377–387. https://doi.org/10.1007/BF00328741
Stadler T, Gavryushkina A, Warnock RCM, Drummond AJ, Heath TA (2018) The fossilized birth-death model for the analysis of stratigraphic range data under different speciation modes. J Theor Biol 447:41–55. https://doi.org/10.1016/j.jtbi.2018.03.005
Stearn CW, Scoffin TP, Martindale W (1977) Calcium carbonate budget of a fringing reef on the West Coast of Barbados Part 1: zonation and productivity. Bull Mar Sci 27:479–510
Steneck RS (1983) Escalating herbivory and resulting adaptive trends in calcareous algal crusts. Paleobiology 9:44–61
Steneck RS (1986) The ecology of coralline algal crusts: convergent patterns and adaptive strategies. Annu Rev Ecol Syst 17:273–303
Steneck RS (1990) Herbivory and the evolution of nongeniculate coralline algae (Rhodophyta, Corallinales) in the North Atlantic and North Pacific. In: Garbary DJ, South GR (eds) Evolutionary biogeography of the marine algae of the North Atlantic. Springer, pp 107–129
Steneck RS, Adey WH (1976) The role of environment in control of morphology in Lithophyllum congestum, a Caribbean Algal Ridge Builder. Bot Mar 19:197–215. https://doi.org/10.1515/botm.1976.19.4.197
Straube E, Neumann H, Wisshak M, Mathes G, Teichert S (2024) Bayesian analysis of biodiversity patterns via beam trawl versus video transect—a comparative case study of Svalbard rhodolith beds. Biodivers Conserv 33:1099–1123. https://doi.org/10.1007/s10531-024-02788-y
Tâmega FTS, Figueiredo MAO (2019) Colonization, growth and productivity of crustose coralline algae in sunlit reefs in the Atlantic Southernmost Coral Reef. Front Mar Sci. https://doi.org/10.3389/fmars.2019.00081
Tâmega FTS, Figueiredo MAO, Ferreira CEL, Bonaldo RM (2016) Seaweed survival after consumption by the greenbeak parrotfish, Scarus trispinosus. Coral Reefs 35:329–334. https://doi.org/10.1007/s00338-015-1373-0
Teichert S (2014) Hollow rhodoliths increase Svalbard’s shelf biodiversity. Sci Rep 4:6972. https://doi.org/10.1038/srep06972
Teichert S, Freiwald A (2014) Polar coralline algal CaCO3-production rates correspond to intensity and duration of the solar radiation. Biogeosciences 11:833–842. https://doi.org/10.5194/bg-11-833-2014
Teichert S, Woelkerling WJ, Rüggeberg A, Wisshak M, Piepenburg D, Meyerhöfer M, Form A, Büdenbender J, Freiwald A (2012) Rhodolith beds (Corallinales, Rhodophyta) and their physical and biological environment at 80° 31ʹ N in Nordkappbukta (Nordaustlandet, Svalbard Archipelago, Norway). Phycologia 51:371–390. https://doi.org/10.2216/11-76.1
Teichert S, Woelkerling WJ, Rüggeberg A, Wisshak M, Piepenburg D, Meyerhöfer M, Form A, Freiwald A (2014) Arctic rhodolith beds and their environmental controls. Facies 60:15–37. https://doi.org/10.1007/s10347-013-0372-2
Teichert S, Woelkerling W, Munnecke A (2019) Coralline red algae from the Silurian of Gotland indicate that the order Corallinales (Corallinophycidae, Rhodophyta) is much older than previously thought. Palaeontology 62:599–613. https://doi.org/10.1111/pala.12418
Teichert S, Steinbauer M, Kiessling W (2020a) A possible link between coral reef success, crustose coralline algae and the evolution of herbivory. Sci Rep 10:17748. https://doi.org/10.1038/s41598-020-73900-9
Teichert S, Voigt N, Wisshak M (2020b) Do skeletal Mg/Ca ratios of Arctic rhodoliths reflect atmospheric CO2 concentrations? Polar Biol 43:2059–2069. https://doi.org/10.1007/s00300-020-02767-3
Twist BA, Cornwall CE, McCoy SJ, Gabrielson PW, Martone PT, Nelson WA (2020) The need to employ reliable and reproducible species identifications in coralline algal research. Mar Ecol Prog Ser 654:225–231
van der Heijden LH, Kamenos NA (2015) Reviews and syntheses: calculating the global contribution of coralline algae to total carbon burial. Biogeosciences 12:6429–6441. https://doi.org/10.5194/bg-12-6429-2015
Violle C, Navas M-L, Vile D, Kazakou E, Fortunel C, Hummel I, Garnier E (2007) Let the concept of trait be functional! Oikos 116:882–892. https://doi.org/10.1111/j.0030-1299.2007.15559.x
Walker D, Woelkerling WJ (1988) Quantitative study of sediment contribution by epiphytic coralline red algae in seagrass meadows in Shark Bay, Western Australia. Mar Ecol Prog Ser 43:71–77
Williams S, Adey W, Halfar J, Kronz A, Gagnon P, Bélanger D, Nash M (2018a) Effects of light and temperature on Mg uptake, growth, and calcification in the proxy climate archive Clathromorphum compactum. Biogeosciences 15:5745–5759. https://doi.org/10.5194/bg-15-5745-2018
Williams S, Halfar J, Zack T, Hetzinger S, Blicher M, Juul-Pedersen T (2018b) Comparison of climate signals obtained from encrusting and free-living rhodolith coralline algae. Chem Geol 476:418–428. https://doi.org/10.1016/j.chemgeo.2017.11.038
Wilson S, Blake C, Berges JA, Maggs CA (2004) Environmental tolerances of free-living coralline algae (maerl): implications for European marine conservation. Biol Conserv 120:279–289
Wisshak M, Neumann H, Rüggeberg A, Büscher J, Linke P, Raddatz J (2019) Epibenthos dynamics and environmental fluctuations in two contrasting Polar carbonate factories (Mosselbukta and Bjørnøy-Banken, Svalbard). Front Mar Sci 6:667. https://doi.org/10.3389/fmars.2019.00667
Woelkerling WJ (1988) The coralline red algae. Oxford University Press
Woelkerling WJ, Irvine LM, Harvey AS (1993) Growth-forms in non-geniculate coralline red algae (Corallinales, Rhodophyta). Aust Syst Bot 6:277–293
Wright VP (1985) Seasonal banding in the alga solenopora jurassica from the middle jurassic of gloucestershire England. J Paleontol 59:721–732
Zajac RM, Vozarik JM, Gibbons BR (2013) Spatial and temporal patterns in macrofaunal diversity components relative to sea floor landscape structure. PLoS ONE 8:e65823
Acknowledgements
I am very thankful to Wolfgang Kiessling (FAU Erlangen-Nürnberg, Germany) for his encouragement and support in compiling this review as well as to Wendy Nelson (University of Auckland, New Zealand) for her valuable comments and suggestions, which improved the quality of the manuscript draft significantly. Moreover, I am grateful to Christian Schulbert (FAU Erlangen-Nürnberg) for micro-CT reconstructions, Max Wisshak (Senckenberg am Meer, Germany) and Solvin Zankl (Kiel) for the provision of underwater CCA images as well as to Axel Munnecke (FAU Erlangen-Nürnberg, Germany) for the provision of CCA thin-sections. I sincerely appreciate the valuable comments and suggestions of the editor, Maurice E. Tucker, as well as the two anonymous reviewers, which helped me to improve the quality of the manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares no conflict of interest.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Teichert, S. Attached and free-living crustose coralline algae and their functional traits in the geological record and today. Facies 70, 8 (2024). https://doi.org/10.1007/s10347-024-00682-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10347-024-00682-1