Abstract
Despite enormous efforts, complete animal tuberculosis (TB) eradication has only been achieved in few regions. Epidemiological analyses are key to identify TB risk factors and set up targeted biosecurity measures. Here, we conducted an in-depth characterization of 84 extensive beef cattle farms from a high TB prevalence region in Western Spain, and assessed how farm management and wildlife presence on farms contribute to cattle TB risk. Twenty-six out of 84 variables were associated with cattle farm TB positivity. Farm management variables associated with TB positivity included older cattle, larger herd size, highly fragmented farm structure and greater connectivity between farms. TB-positive farms provided supplemental feed over a higher number of months, used calf feeders, and had higher number of waterholes. Detecting Eurasian wild boar (Sus scrofa), red fox (Vulpes vulpes), European badger (Meles meles), roe deer (Capreolus capreolus), or Egyptian mongoose (Herpestes ichneumon) on cattle farms was also associated with farm TB positivity. The best ordinal regression model indicated that in farms with a large herd size (> 167 animals) the odds of being positive or recurrently positive (versus negative) was 7.34 (95% CI = 2.43–23.51) times higher that of farms with small herd size. Further, for every additional host species detected in the farm premises, the odds of being TB-positive increased 56%. We conclude that both cattle management and wildlife need to be targeted for successful TB control in grazing-based farming systems.
Resumen
A pesar de los enormes esfuerzos, la erradicación completa de la tuberculosis (TB) animal solo se ha logrado en algunas regiones. Los estudios epidemiológicos son fundamentales para identificar factores de riesgo de TB y establecer medidas de bioseguridad específicas. Aquí, realizamos una caracterización exhaustiva de 84 explotaciones de ganado bovino extensivo en una provincia occidental de España con alta prevalencia de TB bovina, y evaluamos cómo el manejo del ganado y la presencia de fauna silvestre contribuían al riesgo de esta enfermedad. De las 84 variables estudiadas, 26 mostraron asociación con la positividad a TB bovina. Entre las variables de manejo destacaron: ganado de mayor edad, rebaños de mayor tamaño, explotaciones altamente fragmentadas y mayor conectividad entre ganaderías. Asimismo, las explotaciones positivas a TB prolongaban la alimentación suplementaria, utilizaban cebaderos para terneros y tenían un mayor número de charcas. La detección de jabalí (Sus scrofa), zorro (Vulpes vulpes), tejón (Meles meles), corzo (Capreolus capreolus) o meloncillo (Herpestes ichneumon) en las ganaderías también se asoció con la positividad a TB. El mejor modelo de regresión ordinal mostró que en explotaciones con un tamaño de rebaño grande (> 167 animales) las probabilidades de ser positivo o recurrentemente positivo (frente a negativo) eran 7,34 (95 % IC = 2,43–23,51) veces superiores, a las de las explotaciones con un tamaño de rebaño pequeño. Además, por cada especie hospedadora susceptible detectada adicionalmente, la probabilidad de que la ganadería fuese positiva a TB aumentaba un 56 %. Concluimos que tanto el manejo del ganado como la interacción con fauna silvestre deben ser considerados para un control exitoso de la TB en explotaciones con sistemas de pastoreo extensivo.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Animal tuberculosis (TB) is a chronic disease caused by Mycobacterium tuberculosis complex (MTC) bacteria, mainly M. bovis and M. caprae (World Organisation for Animal Health 2019). This zoonotic disease has a great impact on the economy, society, and public health of affected countries, involving domestic and wild animals (Barberis et al. 2017). Despite enormous efforts and costs, complete TB eradication has only been achieved in few regions such as Australia, a country with irrelevant MTC non-bovine hosts (Cousins and Roberts 2001). In 2022, the overall TB prevalence of the European Union (EU) cattle herds was 0.61% and 50% of the Member States still reported bovine TB (EFSA and ECDC 2023).
Four main features of MTC epidemiology hamper TB control: the limited accuracy of diagnostic tests (Schiller et al. 2010; Thomas et al. 2021); the ability of MTC to survive in the environment (Courtenay et al. 2006; Allen et al. 2021); its capacity to be transmitted indirectly through water and feed (Palmer et al. 2004; Barasona et al. 2017); and the ability to infect multiple host species, which results in multi-host systems that help maintaining MTC circulation (Barasona et al. 2019; Santos et al. 2020, 2022).
In Spain, TB is still present with an overall 1.40% cattle herd prevalence in 2022 (PNETB 2024). High TB prevalence in wildlife is also observed, particularly in Mediterranean habitats, with estimated infection prevalence reaching up to 50% in Eurasian wild boar (Sus scrofa) and 10% in red deer (Cervus elaphus) in some areas of south-central Spain (Vicente et al. 2013), although prevalence is lower towards the north of the Iberian Peninsula (Gortázar et al. 2017). Implementation of the National Bovine Tuberculosis Eradication Program (Programa Nacional de Erradicacion de Tuberculosis Bovina 2024) has reduced cattle herd prevalence in the last decades. However, “hotspots” remain where MTC infection has become endemic and persists in multi-host communities (Gortázar et al. 2008; Acevedo et al. 2019; EFSA and ECDC 2023). A large proportion of TB-positive cattle herds are in areas where wild hosts are abundant and where grazing-based farming systems predominate (Aranaz et al. 2004; Alvarez et al. 2014).
The Castilla y León autonomous community holds the largest Spanish cattle population (1,454,385 heads in 2022, 23% of the total; MAPA 2023) and is classified as a high TB prevalence region, with an estimated herd prevalence of 2.07% in 2022 (PNETB 2024). A cross-sectional survey revealed that the main driver of TB in cattle is their management [grazing-based farming; communal pastures (Gortázar et al. 2017)]. The study additionally revealed a multi-host maintenance host community and specifically a significant role played by the wild boar.
Several studies have successfully identified TB risks derived from farm management practices, wildlife-livestock interactions, and social aspects, among others (Reilly and Courtenay 2007; Cowie et al. 2014b; Broughan et al. 2016; Marsot et al. 2016; Ciaravino et al. 2021; Milne et al. 2022; Pozo et al. 2024). These epidemiological analyses are key to identify TB risk factors and set up targeted biosecurity measures such as segregating wildlife and cattle at risk points (Barasona et al. 2013) or implementing comprehensive on-farm risk mitigation protocols (Martínez-Guijosa et al. 2021a). Here, we conducted an in-depth characterization of 84 extensive beef cattle farms located in Salamanca, a high TB prevalence province [4.47% in 2022, (MAPA 2022a)] in Western Spain. We assessed how farm management variables (e.g., cattle movements, feeding and watering, wildlife presence on farms, etc.) contribute to cattle TB risk. Based on previous findings, we hypothesized that food and water distribution would be of importance (Cowie et al. 2014a) and that farms with a richer community of susceptible host species would face a stronger challenge regarding cattle TB control (Barasona et al. 2019).
Material and methods
Study area and data sources
The study area was located in Salamanca Province (41°45’ N; 4°46’ W), Castilla y León (Western Spain), along the border with Portugal. According to Regional Veterinary Authorities, TB prevalence in this region was of 4.47% in 2022, reaching 25% in 2021 in some municipalities. A total of 14 municipalities were studied, all of them declared as “special TB incidence” areas in 2022 (BOCYL 2023). The region is characterized by dry pasture landscapes, dotted with evergreen oak trees (Quercus spp.) and bushy undergrowth, that create a fragmented Mediterranean woodland habitat called “dehesa” in Spain and “montado” in Portugal. These dry systems are marked by an annual humidity deficit, combined with a pronounced summer drought lasting from three to five months. Grazing-based, mixed livestock production systems are common in this area and wild ungulates and carnivores are abundant, thereby offering opportunities for inter-species transmission of TB between cattle, non-bovine domestic species, and wildlife (Crispell et al. 2019; Santos et al. 2020).
With the consent of the farmers, a total of 84 extensive beef cattle farms (Fig. 1) were visited between June and December 2021. Data regarding health and management information concerning each farm, namely administrative data, census, farm location, and cattle and farm TB status for the period 2016–2021 was provided by the Regional Veterinary Authorities (see Online Resource 1, Table S1). Additional data on farm characteristics, number of incoming cattle movements (i.e., contacts between farms), number of farms from which animals were received (in-degree), number of animals received per farm and TB status of other farms, was collected through the Integrated Animal Traceability Information System (SITRAN) (MAPA 2022b).
Further information relative to livestock management, type of fencing and number of pasture-plots (hereafter “plots”), feed supplementation, watering sites, hunting situation and wildlife sightings was gathered through a questionnaire (Online Resource 2) and through systematic inspections for wildlife presence indicators during farm visits. The amount of time invested in collecting this information was around 1 h per farm for the interview and 2 h for the wildlife presence indicators. Detailed information about both the questionnaire and the field inspection is provided below.
Questionnaire
The questionnaire was run by the Official Veterinary Services and consisted of approximately 80 open-ended and closed questions (see Online Resource 2). Livestock management questions concerned animals (i.e., domestic species present in the farm, breed, cattle replacement, culling age), habitat configuration (i.e., number of plots in which the land was divided,% of dehesa surface, type of pasture management), feed supplementation (i.e., type of supplementary feed, months in which they administrated feed, storage place) and water availability (i.e., source of water, water troughs used, distribution of waterholes (geological depressions with water in them, normally fed by rainwater)). Wildlife-related questions were regarding wildlife sightings (i.e., direct sightings observed in the farm, periodicity of sightings) and hunting activity (i.e., presence of hunting grounds, hunting exploitation, use of hunting fencing).
Wildlife presence indicators
Watering sites, including both water troughs and waterholes, were mapped and the main waterholes used by cattle (generally one per plot) were inspected. We searched for signs of wildlife presence such as tracks, rootings, droppings, or hairs along the shore of each main waterhole (Online Resource 1, Figure S1).
We also recorded wildlife signs along a randomly chosen line of 1500 m (generally 500 m per plot) per farm. This consisted of 15 linear transects of 100 m length and 1 m width. Each transect was in turn divided into 10 sectors of 10 m. Sign frequency for abundance estimates was defined as the average number of 10 m sectors with signs of a given wildlife species [e.g., wild boar droppings, tracks, and rootings, or European badger (Meles meles) tracks or latrines (Online Resource 1, Figure S1)]. Sign frequencies were used to calculate the frequency-based indirect index of abundance (FBII). To calculate the spatial aggregation index of wild boar, the sign-frequency data was transformed according to the runs test (Acevedo et al. 2007). Only presence/absence was recorded for less abundant wildlife.
Statistical analyses
Initial data exploration was performed to check for variable parametric assumptions and transformation needs. Here, the outcome variable was the ordinal form of each farm TB status based on the number of years the farm was TB-positive (negative = TB0, positive for one year = TB1, and positive for ≥ 2 years or recurrently positive = TB2). The evaluated potential risk factors were grouped into four different categories: farm management (n = 23), feed supplementation (n = 17), watering sites (n = 11), and wildlife presence variables (n = 33) (Online Resource 3). Continuous variables were categorized into terciles or quartiles (e.g., herd size: small (1 – 83), medium (84 – 167) and large (> 167); in-degree: 0 – 3, 2 – 4, 5 – 7, > 7) to facilitate interpretation. Non-parametric tests for non-normally distributed explanatory variables were run to compare TB-positive, recurrently positive, and negative farms. Specifically, Fisher’s tests and Chi-squared tests were used for categorical predictors, whereas the Kruskal-Wallis test followed by post-hoc tests with Bonferroni corrections for multiple comparisons was applied to continuous data. Furthermore, to check for linear relationships between explanatory variables, Spearman’s rank correlation tests were used.
The effect of each potential risk factor on the ordinal form of the TB status was then tested in an univariable ordinal logistic regression model. Risk factors that had a liberal p value < 0.20 in the univariable model were then considered for inclusion in a multivariable model. The likelihood ratio test was applied to estimate the overall statistical significance of the univariable models (Dohoo et al. 2003). To avoid multicollinearity between potential covariables, those who presented a variance inflation factor (VIF) > 5 were orderly excluded from the model (Belsley et al. 2005). The proportional odds assumption was checked using the Brant test (Brant 1990). The final selected risk factors, along with two-way biologically plausible interactions, were then tested in a multivariable ordinal logistic model using a backward selection procedure based on corrected Akaike’s information criterion (AICc) scores (Akaike 1974). Once the best model was selected, predictive capacity, normality, and the absence of residual patterns in data variation were checked (Barbosa et al. 2013; Robin et al. 2011). The predicted probabilities of each category of the TB status for the covariables included in the model were computed. Additionally, we evaluated how the predicted probabilities of membership to each category of TB status changed as the number of hosts species was varied. Finally, the goodness of fit of the final model was checked using the Lipsitz test (Lipsitz et al. 1996). Results in the model were expressed as odds ratios (ORs) and 95% confidence intervals (CIs).
Multiple comparisons, network analyses, graphics and model fitting were conducted using MASS (Venables and Ripley 2002), lsr (Navarro 2015), brant (Schlegel and Steenbergen 2022), and ggplot2 (Wickham 2011) packages. All statistical analyses were conducted using the computing software R, version 4.0.4 (R Core Team 2021).
Results
Based on the information retrieved from the TB eradication program during the six year period (2016–2021), 26/84 were negative farms [31%, TB0, (Online Resource 1, Table S1)], 28/84 were positive farms [33.3%, TB1, (minimum one positive animal diagnosed in the official testing scheme)], whereas 30 were recurrently positive farms [35.7%, TB2, (tested positive for at least two years)]. Information on the evaluated outcome and risk factors can be accessed in Online Resource 3.
Statistically significant differences in TB status were found to occur for 8 out of 23 of the farm management variables analyzed (Online Resource 3). The presence of older cattle (bulls older than 24 months; higher culling age), as well as larger farms with larger herd sizes, higher number of plots, and larger surfaces, stood out in TB-positive status farms. TB1 and TB2 farms had statistically significant increased herd size (median TB1 = 133, IQR = 81–237, and median TB2 = 196, IQR = 125–269, respectively) than TB0 farms (median = 70, IQR = 47–128, p < 0.001, Kruskal Wallis test with Bonferroni corrections). TB1 and TB2 farms used larger land surfaces (median TB1 = 166.0 hectares, IQR = 102–275, and median TB2 = 210.0, IQR = 170.0 – 317.2, respectively), and presented an overall higher fragmented farm structure (farms were divided in a median TB1 = 4 plots, IQR = 3–6.3, and median TB2 = 5.5 plots, IQR = 4–8, respectively), compared to TB0 farms (median = 101 hectares, IQR = 55.5–156 and median of 3 plots, IQR = 2–4.8, p < 0.001, and p = 0.015 Kruskal Wallis test with Bonferroni corrections, respectively). Around a quarter of farms (22.6%, n = 19) held two additional domestic species other than cattle (goat, sheep, and pig), which were not linked to TB status (p = 0.874, Chi-square test).
Farm surface and number of plots were positively correlated (rs = 0.63; p < 0.001). No statistically significant differences regarding the number of properties per farm (distinct land holdings each farmer owns), the use of fencing around farmyard, the percentage of dehesa and leased pastures, seasonal Iberian pig pasturing (“montanera”), the number of breeds, the number of sheep and pigs, or the number of epidemiological links with other farms were found. However, TB1 and TB2 farms were connected to a statistically significant higher in-degree (median TB1 = 5, IQR = 4–8, and median TB2 = 6, IQR = 2–9, respectively) than TB0 farms [median = 4, IQR = 2–4, p = 0.007, Kruskal Wallis test with Bonferroni corrections, (Online Resource 1, Figure S2)]. The relationship between other variables related to the purchase of animals (i.e., the type of animal restocking, the number of incoming movements and animals) and the farm TB status were inconclusive because these models did not converge.
Regarding the feed supplementation variables, a greater proportion of TB-positive status farms provided supplemental feed over a higher number of months (median = 12 months), used straw bundles (59.6%, n = 34/57), and provided feeders to calves both indoor (86%, n = 49/57) and outdoor (94.7%, n = 54/57, Online Resource 3). However, the use of other types of supplemented food, the place of storage, the number of feed types given on the ground, and the use of the ground as feed supplementation placement (82/84 farms used the ground to place -part of- the feed) did not differ between TB0, TB1 and TB2 farms.
TB1 and TB2 farms had higher number of waterholes (median TB1 = 6.0 waterholes/farm, IQR = 4–8.8, and median TB2 = 6.0, IQR = 5–10, respectively), compared to TB0 farms (median = 3.0 waterholes/farm, IQR = 2–5; p = 0.002, Kruskal-Wallis test with Bonferroni corrections, Online Resource 3). During the farm visits, 265/545 waterholes (48.6%) were inspected. Waterholes had a median perimeter of 253 m (IQR = 128–397) in TB2 farms, significantly larger compared to TB0 farms (median = 138, IQR = 98–186, p = 0.002, Kruskal-Wallis test with Bonferroni corrections). Also, the number of plots with waterholes was correlated with the number of waterholes (rs = 0.873; p < 0.001). Additionally, the variables referring to troughs were related to the farm TB status, although no difference in the frequency of use of the latter (continuously or only during dry seasons) was observed. Unlike the variables referring to stagnant water (p < 0.05), we recorded no significant differences in variables related to running water such as rivers or streams.
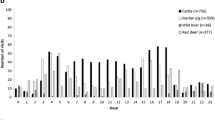
All farms detected at least three wild mammal species regarded as potential MTC hosts in their surroundings (median = 5 different species, IQR = 4–6), which were significantly linked to the farm TB positive statuses (p < 0.001, Kruskal-Wallis test, Online Resource 3). Specifically, detection of wild boar, red fox (Vulpes vulpes), badger, roe deer (Capreolus capreolus), or Egyptian mongoose (Herpestes ichneumon) on cattle farms was significantly related with TB1 and TB2 farms (Fig. 2), although no difference was observed in red deer detection among different TB statuses farms. The median size of the largest recorded wild boar group detected in TB2 farms was 9.5 (IQR = 2–15) and was significantly larger than groups detected in TB0 farms (2.5 IQR = 1–7), but not in TB1 farms (median = 6.5, IQR = 2–14, p = 0.034, Kruskal-Wallis test with Bonferroni corrections). Larger wild boar group sizes correlated with wild boar detection (rs = 0.57; p < 0.001).
Regarding wild boar aggregation indexes, twenty-four farms resulted not significant to the runs test, meaning the sequence was not random and there was aggregation. From these, 5/24 (20.8%) belonged to TB0 farms, 10/24 (41.2%) to TB1 farms, and 9/24 (37.5%) to TB2 farms. Differences were not statistically significant among mean wild boar aggregation and FBII abundance indexes, nor were among the mean badger FBII abundance indexes (p < 0.05, Kruskal-Wallis test, Fig. 3). No significant differences were observed in the remaining wildlife-related variables (Online Resource 3).
Wild boar spatial aggregation indexes (left panel) and wild boar and badger frequency-based indirect indexes (FBII, right) in tuberculosis (TB)-negative (TB0), TB-positive (TB1), and recurrently TB-positive (TB2) farms. Average aggregation indexes are shown only for wild boar for the 24 farms that resulted not random to the runs test statistic. Mean FBII data refers to wild boar and badger indexes (species are indicated with black silhouettes). Whiskered bars indicate standard deviations
Results of the univariable ordinal logistic regression analyses revealed that 22/84 variables related to farm management practices, feed supplementation, watering sites, and wildlife presence were significantly associated with cattle farm TB status (Online Resource 3). After checking for correlated variables and VIF exclusion, 37/84 variables were selected for stepwise regression procedure in the multivariable model (liberal p < 0.2). Based on lower AICc, the final model included farm herd size and the number of suitable MTC host species detected in the farm or its surroundings. Results of the multivariable model revealed a significant positive association between these variables and being in the higher categories of TB status (TB1 or TB2, Table 1). For farms with a large herd size (i.e., > 167 animals), the odds of being TB1 or TB2 (versus TB0) was 7.34 (95% CI = 2.43–23.51) times higher that of farms with small herd size (1–83 animals). Further, for every additional detected host in the farm or its surroundings, the odds of being in the higher categories of the TB status increased 56%. The predicted probability of being a TB2 farm increased linearly in a range of 0.37–0.84 with increasing number of hosts detected in large farms (Fig. 4). Additionally, farms that detected a smaller number of hosts in the farm or its surroundings tended to have high predicted probabilities of being TB0 and these progressively decreased as the number of hosts increased, while the opposite was observed with TB2 farms (Fig. 5).
Left panel a Predicted probability of tuberculosis (TB) status related to suitable Mycobacterium tuberculosis complex (MTC) host species richness (number of species) detected in TB-negative (TB0 -blue-), TB-positive (TB1 -orange-), and recurrently TB-positive (TB2 -red-) farms. Standard errors are displayed. Right panel b proportion of non-bovine hosts observed in each TB status category farms. Grey silhouettes are domestic species (sheep, pig); black silhouettes are wild species (wild boar, red fox, badger, roe deer, mongoose, red deer, fallow deer)
The assumption of proportional odds was checked for the two variables included in the multivariable model and was not violated (p > 0.05), while the assumption of no multicollinearity was met. There were no significant interaction terms and the model fit well the data based on the Lipsitz likelihood-ratio test (p > 0.05).
Discussion
Several studies have investigated the risk factors associated with TB persistence in cattle herds across Castilla y León and neighboring regions (Pozo et al. 2019, 2020, 2024). Nevertheless, factors regarding the interplay of livestock and grazing management, along with the wildlife reservoir community in this grazing-based region, remained largely uncharacterized. The results of this study confirmed our initial hypothesis: supplementation of food and water distribution were relevant for cattle TB and TB was more likely to occur on farms with a richer community of susceptible host species. The novelty is that we had no a priori focus on well-established MTC maintenance hosts, but rather included all susceptible host species in a range of farm-level multi-host communities. We found a close link between host species richness and cattle TB risk.
Effects of farm management on TB positivity
Relevant farm management variables included cattle age, herd sizes, number of plots, and incoming cattle movements. This agrees with previous findings in other regions (Rodríguez-Prieto et al. 2012; Picasso et al. 2017; Clegg et al. 2018). Concerning cattle age, older animals and higher culling age stood out in TB-positive farms. Leaving undetected TB-positive cattle within a herd increases the risk of residual infection and the duration of TB outbreaks (De la Cruz et al. 2019). Previous studies identified residual infections as an important cause of bovine TB breakdowns and active circulation of the pathogen (Gavier-Widén et al. 2009; Guta et al. 2014), which suggests that an earlier culling age might contribute to cattle TB control, as evidenced during the successful eradication campaigns in Australia (Cousins and Roberts 2001). In line with our results, herd size has been identified as a relevant, major TB-risk factor in different studies, where larger cattle herds have greater probability of TB infection and persistence (Martínez-López et al. 2014; Pozo et al. 2020). However, it is uncertain whether this variable functions as a standalone risk factor or serves as a partial summary measure of other factors (Skuce et al. 2012).
Regarding the higher number of plots observed in TB-positive and recurrently positive farms, in Northern Ireland, highly fragmented farms were almost twice as likely to have TB-positive neighbors compared to non-fragmented ones (Milne et al. 2022). Positive herds may spread mycobacteria to other herds by direct contact between boundaries or by drainage of contaminated sewage (Dommergues et al. 2012), among others. Therefore, larger numbers of both neighbors and plots, may facilitate contact with infected herds. The presence of solid fencing in farms was not associated to TB risk, however, although fences restricted the mobility of cattle, they generally allowed wildlife to cross, as evidenced by sightings and indirect indicators. Furthermore, purchasing infected animals can also trigger TB breakdowns (Okafor et al. 2011) and has been suggested as the most likely source of infection in other areas of Spain (Pozo et al. 2019; Ciaravino et al. 2021; Herraiz et al. 2023) and in North-east England (Gilbert et al. 2005; Gopal et al. 2006).
Effects of feed supplementation on TB positivity
TB-positive farms provided supplemental feed over a higher number of months and used more calf feeders and straw bundles (Brook et al. 2013; Barasona et al. 2017). Food sources may become contaminated through saliva or aerosols, particularly in farm or supplementary feed settings, and infection could take place by ingesting infected feed (Santos et al. 2015a; Richomme et al. 2020). In addition, feed provided on the ground and in low feeders can be easily shared between livestock and wildlife (Cowie et al. 2014a; Martínez-Guijosa et al. 2021b), indicating a possible hazard. Although previous studies found that food related risk management may not be a priority (Kukielka et al. 2013; Martínez-Guijosa et al. 2021a), potential cattle-wildlife interactions could be mitigated by using biosecurity measures (BSMs) such as cattle-selective feeders (Balseiro et al. 2019) and exclusion fences (Barasona et al. 2013). However, presence of calf feeding troughs in this study may be explained by the long time periods of movement restriction to which TB-positive livestock are subjected (PNETB 2024). TB-positive farms can only move animals exclusively to slaughterhouses or to be sold to uncertified feedlots, and if not, the herd remains on the farm until animals can be sold to a certified feedlot [estimated period of 18 months, (BOE 2011)], to which farmers may look for alternatives to avoid additional costs (Pérez-Morote et al. 2020) such as fatten the calves in the own farm, therefore associating the presence of calf feeding troughs to the positivity of TB in farms.
Effects of water distribution on TB positivity
Mycobacterium bovis may persist in water for up to two months and high levels of contamination by MTC bacteria (up to 39 MPN/g) can be detected in waterholes (Fine et al. 2011; Santos et al. 2015b), meaning that waterholes can pose a risk too (Herrero-García et al. 2024). In this study, farms testing positive to TB, had higher number of waterholes compared to TB-negative farms (were troughs and alternative water sources were utilized instead), suggesting their relevance in pathogen maintenance, animal aggregation and cross-species interaction, and hence in disease control (Turner et al. 2016; Barasona et al. 2017). Further, larger waterholes were seen at recurrently positive farms, compared to negative farms (1.8 times larger). The study area is characterized by droughts and annual humidity deficit, and hence, animals face resource availability constrains, meaning waterholes may be crucial to animal and wildlife survival (Epaphras et al. 2008; Amoroso et al. 2019), therefore, larger waterholes may be preferred by host species. However, it is not only the number or the size of waterholes that is important, but also the intrinsic characteristics of each waterhole such as their distance to vegetation cover or the presence of macrophytes covering the waterhole (Herrero-García et al. 2024). In contrast to stagnant waters, running waters (rivers and streams) were not significantly associated with TB-status and could involve a lesser risk. This considered, segregating TB host species at waterholes, or deviating access to safer water sources (Barasona et al. 2013; Martínez-Guijosa et al. 2021a), should be evaluated.
Effects of hosts species presence on TB positivity
The process through which M. bovis is transmitted between species is still poorly understood, however there is a possibility for both, direct and indirect transmission, and although its presence in wildlife species is usually introduced by infected livestock herds, the role of wildlife introducing the pathogen into uninfected herds has been suggested (Blanco Vázquez et al. 2021; Swift et al. 2021; Herraiz et al. 2023). We found particularly relevant results regarding cattle contact with wild MTC susceptible hosts and MTC host species richness. Sheep are part of the MTC maintenance communities in specific circumstances (Muñoz-Mendoza et al. 2016; Pozo et al. 2024); and, in some regions, TB prevalence in pigs is comparable to the one observed in cattle (Barandiaran et al. 2021). However, we found an absence of evidence of a statistically significant effect of the numbers of both species, when considered on their own, with respect to TB-status. The role of the wild boar in MTC maintenance at the livestock-wildlife interface is well-known (e.g., Naranjo et al. 2008; Santos et al. 2022; Herrero-García et al. 2023). The aggregation index values found in recurrently positive and negative farms matched those of fenced and open Spanish hunting grounds, respectively (Acevedo et al. 2007). In contrast, the role of badgers is less clear outside Atlantic regions of Europe (Abernethy et al. 2006; Blanco Vázquez et al. 2021; Swift et al. 2021), although a potential association between badger detection and cattle TB prevalence has recently been suggested in northern Spain (Pozo et al. 2024). Although little information exists on badger abundance in Castilla y León, it has been estimated of 5.0 adults/km2 in Atlantic Spain (Acevedo et al. 2014), and of 0.6–1.9 individuals/km2 in other Mediterranean environments (Molina-Vacas et al. 2009). Increasing numbers of badger sightings were associated with a higher probability of TB positivity in our study, suggesting that badgers might indeed contribute to the local MTC host community. Roe deer, red fox and Egyptian mongoose sightings were more frequently recorded in TB-positive farms. This information adds further evidence on the debated roles of roe deer (Balseiro et al. 2009; Hardstaff et al. 2014), red fox (Michelet et al. 2018; Richomme et al. 2020; Pérez de Val. et al. 2022), and Egyptian mongoose (Matos et al. 2016; Ferreras-Colino et al. 2023) in MTC maintenance, at least as -possibly minor- components of complex, species-rich, wildlife-livestock host communities. A review performed by Justus and collaborators in 2024 revealed that the Iberian Peninsula was the region in Europe that most frequently surveyed wild boar, red deer, red fox, and fallow deer in terms of bovine TB prevalence rates, however, upcoming studies may emphasize new species in the role in the disease ecology (Conteddu et al. 2024).
We found that the odds of being in a higher TB status category increased 56% for every additional detected host species, meaning that MTC host species richness represented a risk factor for cattle TB and should be considered in context whenever possible (Gortázar et al. 2023; Justus et al. 2024). This contrasts with findings linking host diversity with lower pathogen diversity (Barroso et al. 2023) but agrees with previous findings of a more likely infection maintenance in richer host communities (Barasona et al. 2019). Interestingly, hunting-related variables were not significant in this study. This contrasts with previous findings in studies at a larger (regional) scale (Gortázar et al. 2017) and with observations from neighboring regions (Vicente et al. 2007), were variables related to the presence of fenced hunting estates or game management posed a risk factor for cattle TB. One plausible explanation is that hunting is not a relevant activity in most of the studied farms.
Concluding remarks and management implications
We conclude that in this high TB prevalence region, cattle management and wildlife are relevant for MTC maintenance, and both need to be targeted in future control efforts. Control measures in cattle would include bolstering the existing testing schemes and movement controls along with additional efforts regarding age-culling, monitoring cleaning and disinfection programs within herds, using dedicated equipment for different areas and enhancing hygienic practices. Farm biosecurity can also be improved by acting on surface-, feed-, water-, and wildlife-related risks Optimizing the use of available floor space by increasing pasture contiguity could minimize exposure and contact between animals allowing more effective monitoring. The diversity of wildlife hosts suggests focusing on reducing the likelihood of indirect interactions with cattle at watering or feeding sites rather than acting on any specific host species, as single-species actions could overlook crucial contributors to disease transmission. Since farmers’ disposition towards TB control and BSM implementation is variable (Preite et al. 2023), informing about the identified risks and involving farmers in decision making might contribute to a better acceptance of the ongoing TB control efforts. These insights could be generalized to similar geographical areas.
Data availability
No datasets were generated or analysed during the current study.
References
Abernethy DA, Denny GO, Menzies FD, McGuckian P, Honhold N, Roberts AR (2006) The Northern Ireland programme for the control and eradication of Mycobacterium bovis. Vet Microbiol 112(2–4):231–237. https://doi.org/10.1016/j.vetmic.2005.11.023
Acevedo P, Vicente J, Höfle U, Cassinello J, Ruiz-Fons F, Gortázar C (2007) Estimation of European wild boar relative abundance and aggregation: a novel method in epidemiological risk assessment. Epidemiol Infect 135(3):519–527. https://doi.org/10.1017/S0950268806007059
Acevedo P, González-Quirós P, Prieto JM, Etherington TR, Gortázar C, Balseiro A (2014) Generalizing and transferring spatial models: A case study to predict Eurasian badger abundance in Atlantic Spain. Ecol Model 275:1–8. https://doi.org/10.1016/j.ecolmodel.2013.12.011
Acevedo P, Prieto M, Quirós P, Merediz I, de Juan L, Infantes-Lorenzo JA, Triguero-Ocaña R, Balseiro A (2019) Tuberculosis epidemiology and badger (Meles meles) spatial ecology in a hot-spot area in Atlantic Spain. Pathogens 8(4):1–18. https://doi.org/10.3390/pathogens8040292
Akaike H (1974) A new look at the statistical model identification. IEEE Trans Automat Contr 19(6):716–723. https://doi.org/10.1109/TAC.1974.1100705
Allen AR, Ford T, Skuce RA (2021) Does Mycobacterium tuberculosis var. bovis survival in the environment confound bovine tuberculosis control and eradication? A Lit Rev Vet Med Int 2021:1–10. https://doi.org/10.1155/2021/8812898
Alvarez J, Perez A, Marques S, Bezos J, Grau A, de la Cruz ML, Romero B, Saez JL, Esquivel MR, Martínez MC, Mínquez O, de Juan L, Domínquez L (2014) Risk factors associated with negative in-vivo diagnostic results in bovine tuberculosis-infected cattle in Spain. BMC Vet Res 10(14):1–10. https://doi.org/10.1186/1746-6148-10-14
Amoroso CR, Kappeler PM, Fichtel C, Nunn CL (2019) Fecal contamination, parasite risk, and waterhole use by wild animals in a dry deciduous forest. Behav Ecol Sociobiol 73:153. https://doi.org/10.1007/s00265-019-2769-6
Aranaz A, De Juan L, Montero N, Sanchez C, Galka M, Delso C, Álvarez J, Domínguez L (2004) Bovine tuberculosis (Mycobacterium bovis) in wildlife in Spain. J Clin Microbiol 42(6):2602–2608. https://doi.org/10.1128/JCM.42.6.2602-2608.2004
Balseiro A, Oleaga A, Orusa R, Robetto S, Zoppi S, Dondo A, Goria M, Gortázar C, Marin J, Domenis L (2009) Tuberculosis in roe deer from Spain and Italy. Vet Rec 164:468–470. https://ria.asturias.es/RIA/handle/123456789/11229
Balseiro A, Oleaga Á, Álvarez Morales LM, González Quirós P, Gortázar C, Prieto JM (2019) Effectiveness of a calf-selective feeder in preventing wild boar access. Eur J Wildl Res 65(38):1–6. https://doi.org/10.1007/s10344-019-1276-4
Barandiaran S, Marfil MJ, Capobianco G, Pérez Aguirreburualde MS, Zumárraga MJ, Eirin ME, Cuerda MX, Winter M, Martínez Vivot M, Perez AM, La Sala LF (2021) Epidemiology of pig tuberculosis in Argentina. Front Vet Sci 8(693082):1–8. https://doi.org/10.3389/fvets.2021.693082
Barasona JA, VerCauteren KC, Saklou N, Gortazar C, Vicente J (2013) Effectiveness of cattle operated bump gates and exclusion fences in preventing ungulate multi-host sanitary interaction. Prev Vet Med 111(1–2):42–50. https://doi.org/10.1016/j.prevetmed.2013.03.009
Barasona JA, Vicente J, Díez-Delgado I, Aznar J, Gortázar C, Torres MJ (2017) Environmental presence of Mycobacterium tuberculosis complex in aggregation points at the wildlife/livestock interface. Transbound Emerg Dis 64(4):1148–1158. https://doi.org/10.1111/tbed.12480
Barasona JA, Gortázar C, de la Fuente J, Vicente J (2019) Host richness increases tuberculosis disease risk in game-managed areas. Microorganisms 7(6):1–7. https://doi.org/10.3390/microorganisms7060182
Barberis I, Bragazzi NL, Galluzzo L, Martini M (2017) The history of tuberculosis: From the first historical records to the isolation of Koch’s bacillus. J Prev Med 58(1):E9–E12
Barbosa AM, Real R, Muñoz AR, Brown JA (2013) New measures for assessing model equilibrium and prediction mismatch in species distribution models. Divers Distrib 19(10):1333–1338. https://doi.org/10.1111/ddi.12100
Barroso P, Relimpio D, Zearra JA, Cerón JJ, Palencia P, Cardoso B, Ferreras E, Escobar M, Cáceres G, López-Olvera JR, Gortázar C (2023) Using integrated wildlife monitoring to prevent future pandemics through one health approach. One Health 16(100479):1–9. https://doi.org/10.1016/j.onehlt.2022.100479
Belsley DA, Kuh E, Welsch RE (2005) Regression diagnostics: identifying influential data and sources of collinearity. John Wiley and Sons, Inc., New York, NY
Blanco Vázquez C, Barral TD, Romero B, Queipo M, Merediz I, Quirós P, Armenteros JA, Juste R, Domínguez L, Domínguez M, Casais R, Balseiro A (2021) Spatial and temporal distribution of Mycobacterium tuberculosis complex infection in Eurasian badger (Meles meles) and cattle in Asturias. Spain Animals 11(5):1–14. https://doi.org/10.3390/ani11051294
BOE (2011) Boletín Oficial del Estado, Ministerio de Medio Ambiente, y Medio Rural y Marino. https://www.boe.es/boe/dias/2011/06/16/pdfs/BOE-A-2011-10458.pdf. Accessed 15 Mar 2024
BOCYL (2023) Boletín Oficial de Castilla y León, Consejería de Agricultura, Ganadería y Desarrollo Rural. https://s1.ppllstatics.com/lagacetadesalamanca/www/items/binrepository/2023/bocyl-d-22022023-17_1-4400709_20230222090745.pdf. Accessed 23 Nov 2023
Brant R (1990) Assessing proportionality in the proportional odds model for ordinal logistic regression. Biometrics 46(4):1171–1178. https://doi.org/10.2307/2532457
Brook RK, Vander Wal E, van Beest FM, McLachlan SM (2013) Evaluating use of cattle winter feeding areas by elk and white-tailed deer: implications for managing bovine tuberculosis transmission risk from the ground up. Prev Vet Med 108(2–3):137–147. https://doi.org/10.1016/j.prevetmed.2012.07.017
Broughan JM, Maye D, Carmody P, Brunton LA, Ashton A, Wint W, Alexander N, Naylor R, Ward K, Goodchild AV, Hinchliffe S, Eglin RD, Upton P, Nicholson R, Enticott G (2016) Farm characteristics and farmer perceptions associated with bovine tuberculosis incidents in areas of emerging endemic spread. Prev Vet Med 129:88–98. https://doi.org/10.1016/j.prevetmed.2016.05.007
Ciaravino G, Laranjo-González M, Casal J, Sáez-Llorente JL, Allepuz A (2021) Most likely causes of infection and risk factors for tuberculosis in Spanish cattle herds. Vet Rec 189(2):1–11. https://doi.org/10.1002/vetr.140
Clegg TA, Good M, Hayes M, Duignan A, McGrath G, More SJ (2018) Trends and predictors of large tuberculosis episodes in cattle herds in Ireland. Front Vet Sci 5(86):1–12. https://doi.org/10.3389/fvets.2018.00086
Conteddu K, English HM, Byrne AW, Amin B, Griffin LL, Kaur P, Morera-Pujol V, Murphy KJ, Salter-Townshend M, Smith AF, Ciuti S (2024) A scoping review on bovine tuberculosis highlights the need for novel data streams and analytical approaches to curb zoonotic diseases. Vet Res 55(1):64. https://doi.org/10.1186/s13567-024-01314-w
Courtenay O, Reilly LA, Sweeney FP, Hibberd V, Bryan S, Ul-Hassan A, Newman C, Macdonald DW, Delahay RJ, Wilson GJ, Wellington EMH (2006) Is Mycobacterium bovis in the environment important for the persistence of bovine tuberculosis? Biol Lett 2(3):460–462. https://doi.org/10.1098/rsbl.2006.0468
Cousins DV, Roberts JL (2001) Australia’s campaign to eradicate bovine tuberculosis: The battle for freedom and beyond. Tuberculosis 81(1–2):5–15. https://doi.org/10.1054/tube.2000.0261
Cowie CE, Beck BB, Gortazar C, Vicente J, Hutchings MR, Moran D, White PC (2014a) Risk factors for the detected presence of Mycobacterium bovis in cattle in south central Spain. Eur J Wildl Res 60:113–123. https://doi.org/10.1007/s10344-013-0757-0
Cowie CE, Marreos N, Gortázar C, Jaroso R, White PC, Balseiro A (2014b) Shared risk factors for multiple livestock diseases: A case study of bovine tuberculosis and brucellosis. Vet Sci Res J 97(3):491–497. https://doi.org/10.1016/j.rvsc.2014.09.002
Crispell J, Benton CH, Balaz D, De Maio N, Ahkmetova A, Allen A, Biek R, Presho EL, Dale J, Hewinson G, Lycett SJ, Nunez-Garcia J, Skuce RA, Trewby H, Wilson DJ, Zadoks RN, Delahay RJ, Kao RR (2019) Combining genomics and epidemiology to analyse bi-directional transmission of Mycobacterium bovis in a multi-host system. Elife 8(e45833):1–36. https://doi.org/10.7554/eLife.45833
De la Cruz ML, Pozo P, Grau A, Nacar J, Bezos J, Perez A, Dominguez L, Saex JS, Minguez O, de Juan L, Alvarez J (2019) Assessment of the sensitivity of the bovine tuberculosis eradication program in a high prevalence region of Spain using scenario tree modeling. Prev Vet Med 173(104800):1–10. https://doi.org/10.1016/j.prevetmed.2019.104800
Dohoo I, Martin W, Stryhn H (2003) Veterinary Epidemiologic Research. Charlottetown. AVC Inc., Prince Edward Island, Canada
Dommergues L, Rautureau S, Petit E, Dufour B (2012) Network of contacts between cattle herds in a French area affected by bovine tuberculosis in 2010. Transbound Emerg Dis 59(4):292–302. https://doi.org/10.1111/j.1865-1682.2011.01269.x
EFSA (European Food Safety Authority), ECDC (European Centre for Disease Prevention and Control) (2023) The European union one health 2022 zoonoses report. EFSA J 20(12):112–116. https://doi.org/10.2903/j.efsa.2023.8442
Epaphras AM, Gereta E, Lejora IA, Ole Meing’ataki GE, Ng’umbi G, Kiwango Y, Mwangomo E, Semanini F, Vitalis L, Bazoli J, Mtahiko MGG, (2008) Wildlife water utilization and importance of artificial waterholes during dry season at Ruaha National Park. Tanzania Wetl Ecol Manag 16:183–188. https://doi.org/10.1007/s11273-007-9065-3
Ferreras-Colino E, Descalzo E, Romero B, Lozano FJ, Gortázar C, Ferreras P (2023) Mycobacterium bovis in Egyptian mongoose. Spain ZPHOAH 70(2):1–4. https://doi.org/10.1111/zph.13033
Fine AE, Bolin CA, Gardiner JC (2011) Kaneene JB (2011) A study of the persistence of Mycobacterium bovis in the environment under natural weather conditions in Michigan. USA Vet Med Int 765430:1–12. https://doi.org/10.4061/2011/765430
Gavier-Widén D, Cooke MM, Gallagher J, Chambers MA, Gortázar C (2009) A review of infection of wildlife hosts with Mycobacterium bovis and the diagnostic difficulties of the ‘no visible lesion’ presentation. NZVJ. https://doi.org/10.1080/00480169.2009.36891
Gilbert M, Mitchell A, Bourn D, Mawdsley J, Clifton-Hadley R, Wint W (2005) Cattle movements and bovine tuberculosis in Great Britain. Nature 435:491–496. https://doi.org/10.1038/nature03548
Gopal R, Goodchild A, Hewinson G, de la Rua DR, Clifton-Hadley R (2006) Introduction of bovine tuberculosis to north-east England by bought-in cattle. Vet Rec 159(9):265–271. https://doi.org/10.1136/vr.159.9.265
Gortázar C, Torres MJ, Vicente J, Acevedo P, Reglero M, de la Fuente J, Negro JJ, Aznar-Martín J (2008) Bovine tuberculosis in Doñana Biosphere Reserve: the role of wild ungulates as disease reservoirs in the last Iberian lynx strongholds. PLoS One 3(7):e2776. https://doi.org/10.1371/journal.pone.0002776
Gortázar C, Fernández-Calle LM, Collazos-Martínez JA, Mínguez-González O, Acevedo P (2017) Animal tuberculosis maintenance at low abundance of suitable wildlife reservoir hosts: A case study in northern Spain. Prev Vet Med 146:150–157. https://doi.org/10.1016/j.prevetmed.2017.08.009
Gortázar C, de la Fuente J, Perelló A, Domínguez L (2023) Will we ever eradicate animal tuberculosis? Ir Vet J 76(Suppl 1):24. https://doi.org/10.1186/s13620-023-00254-9
Guta S, Casal J, Napp S, Saez JL, Garcia-Saenz A, Perez de Val B, Romero R, Alvarez J, Allepuz A (2014) Epidemiological investigation of bovine tuberculosis herd breakdowns in Spain 2009/2011. PLoS One 9(8):e104383. https://doi.org/10.1371/journal.pone.0104383
Hardstaff JL, Marion G, Hutchings MR, White PC (2014) Evaluating the tuberculosis hazard posed to cattle from wildlife across Europe. Res Vet Sci 97:S86–S93. https://doi.org/10.1016/j.rvsc.2013.12.002
Herraiz C, Vicente J, Gortázar C, Acevedo P (2023) Large scale spatio-temporal modelling of risk factors associated with tuberculosis exposure at the wildlife-livestock interface. Prev Vet Med 220:106049. https://doi.org/10.1016/j.prevetmed.2023.106049
Herrero-García G, Acevedo P, González-Quirós P, Prieto M, Romero B, Amado J, Queipo MA, Gortázar C (2023) Balseiro A (2023) Tuberculosis Epidemiology and Spatial Ecology at the Cattle-Wild Boar Interface in Northern Spain. Transbound Emerg Dis 2147191:1–11. https://doi.org/10.1155/2023/2147191
Herrero-García G, Barroso P, Preite L, Relimpio D, Vaz-Rodrigues R, Balseiro A, Gortázar C (2024) Waterhole Characteristics in Tuberculosis Positive and Negative Beef Cattle Farms from Endemic Regions in Spain. Rangel Ecol Manag 92:50–58. https://doi.org/10.1016/j.rama.2023.09.008
Justus W, Valle S, Barton O, Gresham A, Shannon G (2024) A review of bovine tuberculosis transmission risk in European wildlife communities. Mammal Rev 0305–1838:1–16. https://doi.org/10.1111/mam.12347
Kukielka E, Barasona JA, Cowie CE, Drewe JA, Gortazar C, Cotarelo I, Vicente J (2013) Spatial and temporal interactions between livestock and wildlife in South Central Spain assessed by camera traps. Prev Vet Med 112(3–4):213–221. https://doi.org/10.1016/j.prevetmed.2013.08.008
Lipsitz SR, Fitzmaurice GM, Molenberghs G (1996) Goodness-of-fit tests for ordinal response regression models. J Royal Stat Soc Series C Appl Stat 45:175–190. https://doi.org/10.2307/2986153
MAPA (Ministry of Agriculture, Fisheries and Food) (2022a). Prevalencias provincials 2022. https://www.mapa.gob.es/es/ganaderia/temas/sanidad-animal-higiene-ganadera/prevalenciasprovinciales2022_tcm30-653230.pdf. Accessed 27 Jan 2023
MAPA (Ministry of Agriculture, Fisheries and Food) (2022b). Sistema Integral de Trazabilidad Animal (SITRAN). https://www.mapa.gob.es/es/ganaderia/temas/trazabilidad-animal/registro/default.aspx. Accessed 27 Jan 2023
MAPA (Ministry of Agriculture, Fisheries and Food) (2023). Caracterización del sector vacuno de carne: datos 2022. https://www.mapa.gob.es/es/ganaderia/temas/produccion-y-mercados-ganaderos/2023informecaracterizacionsectorvacunodecarne_datos2022_pub2_tcm30-553721.pdf. Accessed 15 Jan 2023
Marsot M, Béral M, Scoizec A, Mathevon Y, Dur B, Courcoul A (2016) Herd-level risk factors for bovine tuberculosis in French cattle herds. Prev Vet Med 131:31–40. https://doi.org/10.1016/j.prevetmed.2016.07.006
Martínez-Guijosa J, Lima-Barbero JF, Acevedo P, Cano-Terriza D, Jiménez-Ruiz S, Barasona JA, Boadella M, García-Bocanegra I, Gortázar C, Vicente J (2021a) Description and implementation of an On-farm Wildlife Risk Mitigation Protocol at the wildlife-livestock interface: Tuberculosis in Mediterranean environments. Prev Vet Med 191(105346):1–10. https://doi.org/10.1016/j.prevetmed.2021.105346
Martínez-Guijosa J, López-Alonso A, Gortázar C, Acevedo P, Torres MJ, Vicente J (2021b) Shared use of mineral supplement in extensive farming and its potential for infection transmission at the wildlife-livestock interface. Eur J Wildl Res 67(3):1–9. https://doi.org/10.1007/s10344-021-01493-3
Martínez-Lopez B, Barasona JA, Gortazar C, Rodriguez-Prieto V, Sanchez-Vizcaino JM, Vicente J (2014) Farm-level risk factors for the occurrence, new infection or persistence of tuberculosis in cattle herds from South-Central Spain. Prev Vet Med 116:268–278. https://doi.org/10.1016/j.prevetmed.2013.11.002
Matos AC, Figueira L, Martins MH, Pinto ML, Matos M, Coelho AC (2016) New insights into Mycobacterium bovis prevalence in wild mammals in Portugal. Transbound Emerg Dis 63(5):e313–e322. https://doi.org/10.1111/tbed.12306
Michelet L, De Cruz K, Hénault S, Tambosco J, Richomme C, Réveillaud É, Gares H, Moyen JL, Boschiroli ML (2018) Mycobacterium bovis infection of red fox. France Emerg Infect Dis 24(6):1151–1153. https://doi.org/10.3201/eid2406.180094
Milne G, Graham J, McGrath J, Kirke R, McMaster W, Byrne AW (2022) Investigating farm fragmentation as a risk factor for bovine tuberculosis in cattle herds: a matched case-control study from Northern Ireland. Pathogens 11(3):1–18. https://doi.org/10.3390/pathogens11030299
Molina-Vacas G, Bonet-Arboli V, Rt-Plaza ER, Rodríguez-Teijeiro JD (2009) Spatial ecology of European badgers (Meles meles) in mediterranean habitats of the north-eastern Iberian peninsula. i: home range size, spatial distribution and social organization. Vie et Milieu 59(2):223–232
Muñoz-Mendoza M, Romero B, Del Cerro A, Gortázar C, García-Marín JF, Menéndez S, Moruelo J, de Juan L, Sáez JL, Delahay RJ, Balseiro A (2016) Sheep as a potential source of bovine TB: epidemiology, pathology and evaluation of diagnostic techniques. Transbound Emerg Dis 63(6):635–646. https://doi.org/10.1111/tbed.12325
Naranjo V, Gortazar C, Vicente J, de La Fuente J (2008) Evidence of the role of European wild boar as a reservoir of Mycobacterium tuberculosis complex. Vet Microbiol 127(1–2):1–9. https://doi.org/10.1016/j.vetmic.2007.10.002
Navarro D (2015) Learning statistics with R: A tutorial for psychology students and other beginners. (Version 0.6). University of New South Wales, Sydney, Australia. R package version 0.5.1. https://learningstatisticswithr.com. Accessed 10 Jan 2024
Okafor CC, Grooms DL, Bruning-Fann CS, Averill JJ (2011) Kaneene JB (2011) Descriptive epidemiology of bovine tuberculosis in Michigan (1975–2010): lessons learned. Vet Med Int 874924:1–13. https://doi.org/10.4061/2011/874924
Palmer MV, Waters WR, Whipple DL (2004) Shared feed as a means of deer-to-deer transmission of mycobacterium bovis. J Wildl Dis 40(1):87–91. https://doi.org/10.7589/0090-3558-40.1.87
Pérez de Val B, Perea C, Estruch J, Solano-Manrique C, Riera C, Sanz A, Vidal E, Velarde R (2022) Generalized tuberculosis due to Mycobacterium caprae in a red fox phylogenetically related to livestock breakdowns. BMC Vet Res 18(1):1–6. https://doi.org/10.1186/s12917-022-03454-7
Pérez-Morote R, Pontones-Rosa C, Gortáza C, Muñoz-Cardona ÁI (2020) Quantifying the economic impact of bovine tuberculosis on livestock farms in South-Western Spain. Animals 10(12):2433. https://doi.org/10.3390/ani10122433
Picasso C, Alvarez J, VanderWaal KL, Fernandez F, Gil A, Wells SJ, Perez A (2017) Epidemiological investigation of bovine tuberculosis outbreaks in Uruguay (2011–2013). Prev Vet Med 138:156–161. https://doi.org/10.1016/j.prevetmed.2017.01.010
PNETB (Programa Nacional de Erradicación de Tuberculosis Bovina) (2024) Programa Nacional de Erradicacion de Tuberculosis Bovina 2023 (Infección por el complejo Mycobacterium tuberculosis) https://www.mapa.gob.es/es/ganaderia/temas/sanidad-animal-higiene-ganadera/programatb2024_tcm30-671966.pdf. Accessed 16 Feb 2024
Pozo P, VanderWaal K, Grau A, de la Cruz ML, Nacar J, Bezos J, Perez A, Minguez O, Alvarez J (2019) Analysis of the cattle movement network and its association with the risk of bovine tuberculosis at the farm level in Castilla y Leon. Spain Transbound Emerg Dis 66(1):327–340. https://doi.org/10.1111/tbed.13025
Pozo P, Romero B, Bezos J, Grau A, Nácar J, Sáez JL, Mínguez O, Álvarez J (2020) Evaluation of risk factors associated with herds with an increased duration of bovine tuberculosis breakdowns in Castilla y Leon, Spain (2010–2017). Front VetSci 25(7):545328. https://doi.org/10.3389/fvets.2020.545328
Pozo P, Isla J, Asiain A, Navarro D, Gortázar C (2024) Contribution of herd management, biosecurity, and environmental factors to the risk of bovine tuberculosis in a historically low prevalence region. Animal 18(3):101105. https://doi.org/10.1016/j.animal.2024.101105
Preite L, Barroso P, Romero B, Balseiro A, Gortázar C (2023) Struggling to improve farm biosecurity: do free advice and subsidies hit the target? Prev Vet Med 212(105839):1–4. https://doi.org/10.1016/j.prevetmed.2023.105839
R Core Team (2021) R: a language and environment for statistical computing. r foundation for statistical computing, Vienna, Austria. https://www.R-project.org/
Reilly LA, Courtenay O (2007) Husbandry practices, badger sett density and habitat composition as risk factors for transient and persistent bovine tuberculosis on UK cattle farms. Prev Vet Med 80(2–3):129–142. https://doi.org/10.1016/j.prevetmed.2007.02.002
Richomme C, Réveillaud E, Moyen JL, Sabatier P, De Cruz K, Michelet L, Boschiroli ML (2020) Mycobacterium bovis infection in red foxes in four animal tuberculosis endemic areas in France. Microorganisms 8(7):1070. https://doi.org/10.3390/microorganisms8071070
Robin X, Turck N, Hainard A, Tiberti N, Lisacek F, Sanchez JC, Müller M (2011) pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinform 12(77):1–8. https://doi.org/10.1186/1471-2105-12-77
Rodríguez-Prieto V, Martínez-López B, Barasona JA, Acevedo P, Romero B, Rodriguez-Campos S, Gortázar C, Sánchez-Vizcaíno JM, Vicente J (2012) A Bayesian approach to study the risk variables for tuberculosis occurrence in domestic and wild ungulates in South Central Spain. BMC Vet Res 8(148):1–13. https://doi.org/10.1186/1746-6148-8-148
Santos N, Almeida V, Gortázar C, Correia-Neves M (2015a) Patterns of Mycobacterium tuberculosis-complex excretion and characterization of super-shedders in naturally-infected wild boar and red deer. Vet Res 46(129):1–10. https://doi.org/10.1186/s13567-015-0270-4
Santos N, Santos C, Valente T, Gortázar C, Almeida V, Correia-Neves M (2015b) Widespread environmental contamination with Mycobacterium tuberculosis complex revealed by a molecular detection protocol. PLoS One 10(11):e0142079. https://doi.org/10.1371/journal.pone.0142079
Santos N, Richomme C, Nunes T, Vicente J, Alves PC, de la Fuente J, Correia-neves M, Boschiroli ML, Delahay R, Gortázar C (2020) Quantification of the animal tuberculosis multi- host community offers insights for control. Pathogens 9(6):421. https://doi.org/10.3390/pathogens9060421
Santos N, Colino EF, Arnal MC, de Luco DF, Sevilla I, Garrido JM, Fonseca E, Valente AM, Balseiro A, Queirós J, Almeida V, Vicente J, Gortázar C, Alves PC (2022) Complementary roles of wild boar and red deer to animal tuberculosis maintenance in multi-host communities. Epidemics 41(100633):1–11. https://doi.org/10.1016/j.epidem.2022.100633
Schiller I, Oesch B, Vordermeier HM, Palmer MV, Harris BN, Orloski KA, Buddle BM, Thacker TC, Lyashchenko KP, Waters WR (2010) Bovine tuberculosis: A review of current and emerging diagnostic techniques in view of their relevance for disease control and eradication. Transbound Emerg 57(4):205–220. https://doi.org/10.1111/j.1865-1682.2010.01148.x
Schlegel B, Steenbergen M (2022) R package brant: test for parallel regression assumption. https://mirrors.nics.utk.edu/cran/web/packages/brant/brant.pdf. Accessed 10 Jan 2024
Skuce RA, Allen AR, McDowell SW (2012) Herd-level risk factors for bovine tuberculosis: a literature review. Vet Med Int 2012:621210. https://doi.org/10.1155/2012/621210
Swift BMC, Barron ES, Christley R, Corbetta D, Grau-Roma L, Jewell C, O’Cathail C, Mitchell A, Phoenix J, Prosser A, Rees C, Sorley M, Verin R, Bennett M (2021) Tuberculosis in badgers where the bovine tuberculosis epidemic is expanding in cattle in England. Sci Rep 11(1):20995. https://doi.org/10.1038/s41598-021-00473-6
Thomas J, Balseiro A, Gortázar C, Risalde MA (2021) Diagnosis of tuberculosis in wildlife: a systematic review. Vet Res 52(31):1–23. https://doi.org/10.1186/s13567-020-00881-y
Turner WC, Kausrud KL, Beyer W, Easterday WR, Barandongo ZR, Blaschke E, Cloete CC, Lazak J, Van Ert MN, Ganz HH, Turnbull PCB, Stenseth NC, Getz WM (2016) Lethal exposure: an integrated approach to pathogen transmission via environmental reservoirs. Sci Rep 6:27311. https://doi.org/10.1038/srep27311
Venables WN, Ripley BD (2002) Modern applied statistics with S, 4th edition. Springer, New York. ISBN 0–387–95457–0. https://www.stats.ox.ac.uk/pub/MASS4/. Accessed 10 Jan 2024
Vicente J, Hofle U, Garrido JM, Fernandez-de-Mera IG, Acevedo P, Juste R, Barral M, Gortazar C (2007) Risk factors associated with the prevalence of tuberculosis-like lesions in fenced wild boar and red deer in south central Spain. Vet Res 38:451–464
Vicente J, Barasona JA, Acevedo P, Ruiz-Fons JF, Boadella M, Diez-Delgado I, Beltran-Beck B, González-Barrio D, Queirós J, Montoro V, de la Fuente J, Gortazar C (2013) Temporal trend of tuberculosis in wild ungulates from Mediterranean Spain. Transbound Emerg 60(s1):92–103. https://doi.org/10.1111/tbed.12167
Wickham H (2011) ggplot2. Wiley Interdiscip Rev Comput Stat 3(2):180–185. https://doi.org/10.1002/wics.147
World Organisation for Animal Health (2019) OIE-Listed diseases, Bovine tuberculosis. https://www.woah.org/en/what-we-do/animal-health-and-welfare/animal-diseases/. Accessed 27 Feb 2023
Acknowledgements
We would like to thank the colleagues at the IREC (UCLM, Spain), Animal Health Service of the Junta de Castilla y León, veterinarians of Grupo EULEN, farmers, and all those who participated in the fieldwork and data collection.
Funding
This work is a result of the I+D+i research projects PLEC2021-008113, funded by the Spanish MCIN/AEI/https://doi.org/10.13039/501100011033/ and the European Union NextGeneration EU/PRTR (Ganadería con una sola salud: monitoreo Ambiental mitigación de riesgos para la producción ganadera Segura y sostenible y la conservación…); PID2022-141906OB-C21, funded by MCIN /AEI/https://doi.org/10.13039/501100011033 / FEDER una manera de hacer Europa (Efectos de la ganadería extensiva de rumiantes sobre la biodiversidad y las comunidades de vectores artrópodos y patógenos); PID2022-141906OB-C22, funded by MCIN /AEI/https://doi.org/10.13039/501100011033 / FEDER una manera de hacer Europa (Efectos de la ganadería extensiva de rumiantes sobre la biodiversidad y las comunidades de vectores artrópodos y patógenos); B2021/006949, funded by Junta de Castilla y León (Aplicación piloto de técnicas de secuenciación masiva del ADN del complejo Mycobacterium tuberculosis para cuantificar las interacciones fauna silvestre-ganado bovino); regional funds PCTI 2018–2020 (GRUPIN: IDI2018-000237); and 2023-GRIN-34227 (Consolidado - SANIDAD Y BIOTECNOLOGIA). Gloria Herrero-García is funded by Junta de Castilla y León and FSE (contract ref LE036-20); Rita Vaz-Rodrigues is funded by Universidad de Castilla-La Mancha (UCLM) and ESF (contract ref 2022/20675); Pilar Pozo is funded by Juan de la Cierva Formación 2021, funded by the Ministerio de Ciencia e Innovación, Agencia Estatal de Investigación and NextGenerationEU/PRTR (contract ref FJC2021-046805-I); Patricia Barroso is funded by a Juan de la Cierva post-doc fellowship (contract ref DC2022-049103-I); and Alberto García-Rodríguez is funded by the EU-NextGenerationEU funds through the 2021–2023 Margarita Salas call for the requalification of the Spanish university system, convened by the Universidad de León.
Author information
Authors and Affiliations
Contributions
Conceptualization: C.G.; Methodology: P.B., A.G., O.M., A.B., C.G.; Formal analysis and investigation: G.H.G., R.V.R., P.P., P.B., D.R., J.N., A.G., O.M., A.G.-R., A.B., C.G.; Resources: A.G., O.M., A.B., C.G.; Writing - original draft preparation: G.H.G., R.V.R., C.G.; Writing - review and editing: G.H.G., R.V.R., P.P., P.B., D.R., J.N., A.G., O.M., A.G.-R., A.B., C.G.; Supervision: P.P., A.G., O.M., A.B., C.G.; Project Administration: A.G., O.M., A.B., C.G. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval
No ethical approval by an Institutional Animal Care and Use Committee was deemed necessary.
Human ethics and consent to participate
Not applicable.
Competing interests
Patricia Barroso is one of the editors of the special issue “Management opportunities at the wildlife-livestock interface” and Christian Gortázar is the editor in chief of the European Journal of Wildlife Research.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Herrero-García, G., Vaz-Rodrigues, R., Pozo, P. et al. Farm management practices and host species richness associated with higher likelihood of tuberculosis positive farms in Western Spain. Eur J Wildl Res 70, 81 (2024). https://doi.org/10.1007/s10344-024-01833-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10344-024-01833-z