Abstract
Infestation by the sheep bot fly Oestrus ovis was firstly reported in a single roe deer from Central Spain in 2022. For assessing the current situation of nasal myiases in this ungulate in this area, the nasopharyngeal cavities of 184 roe deer hunted in Central Spain between January-June 2023 were examined. All larvae were recovered and morphologically identified; in addition, species identification was molecularly confirmed in a subset of specimens. Forty-four roe deer (23.9%; CI 95 17.95–30.74) were positive for different Oestrinae larval stages. Twenty-six animals (14.1%; CI 95 9.44–20.02%) were infested by the roe deer nasal bot fly (Cephenemyia stimulator) with a mean intensity of 35.2 (SD 49.71) larvae/infested animal, and eighteen (9.8%; CI 95 5.90-15.02%) roe deer harboured the sheep bot fly (O. ovis), with a mean intensity of 2.0 (SD 1.33) larvae/infested animal. No mixed infestations by both Oestrinae were found in a single animal. All larval instars (L1, L2 and L3) of both species were identified. Most C. stimulator specimens were located at the nasal turbinates, and a small percentage (3.2%) at the pharynx; all O. ovis larvae were found at the nasal turbinates. Since O. ovis is highly prevalent in sheep and goat flocks from Central Spain, the high sympatry between roe deer and small ruminant populations in the studied area may have increased the risk of cross-infection. Moreover, the finding of mature L3 of O. ovis suggests that this species can complete its life cycle in roe deer. Therefore, monitoring bot flies in sheep and goat flocks as well as in sympatric wild ruminants is strongly recommended for achieving an optimum control of nasal myiases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nose bot flies (Diptera: Oestridae) include nine genera infesting members of the orders Artiodactyla, Perissodactyla and Proboscidea (Scholl et al. 2019). They all belong to the subfamily Oestrinae which differ from other oestrids in that they are larviparous; gravid flies eject first instar larvae into the host nostrils which migrate to sinusal, frontal and/or nasopharyngeal cavities (Colwell 2001; Scholl et al. 2019) causing rhinitis, sinusitis, nasal discharge, and respiratory complications (Allen and Bunch 1982; Dorchies et al. 1998).
The most widely distributed and economically important nasal bot is Oestrus which develops within the nasal cavities and frontal sinuses of domestic and wild Bovidae (Zumpt 1965). Four Oestrus species are currently recognized: O. ovis, O. variolosus, O. aureoargentatus and O. caucasicus (Colwell et al. 2006). Oestrus ovis, known as the sheep bot fly, has a worldwide distribution in domestic sheep and goats, but it has also been described in wild ungulates such as the Alpine ibex (Capra ibex) (Zumpt 1965), the Argali (Ovis ammon) (Zumpt 1965), the Bighorn sheep (Ovis canadensis) (Capelle 1966) and the Asiatic ibex (Capra sibirica) (Sánchez et al. 2017). Human and carnivore infestations by O. ovis have also been occasionally described (Lobato 2011; Zanzani et al. 2016; Sante et al. 2017; Tabuenca-del Barrio et al. 2018).
In Spain, O. ovis prevalence rates in domestic sheep range from 70 to 84% (Alcaide et al. 2003, 2005; Gracia et al. 2010) and it has also been reported in wild sheep and goats such as European mouflon (Ovis aries musimon) (Moreno et al. 1999), Barbary sheep (Ammotragus lervia) (Barroso et al. 2017), and more recently in roe deer (Capreolus capreolus) (Martínez-Calabuig et al. 2023). In addition, a new Oestrus species has been detected in the Iberian ibex (Capra pyrenaica) that was tentatively identified as O. caucasicus (Pérez et al. 1996, 2016).
Cephenemyia (Latreille 1818) and Pharyngomyia (Schiner 1861) are the main genera of nasal bots infecting Cervidae in Holarctic regions. Cephenemyia exclusively parasitize deer from the Cervinae and Odocoileinae subfamilies (Bueno de la Fuente et al. 1998). Four species in this genus (C. ulrichii, C. auribarbis, C. stimulator and C. trompe) infest cervids in the Paleartic region (Morrondo et al. 2021). The roe deer nasal bot, Cephenemyia stimulator, is very prevalent in the European roe deer (Capreolus capreolus), especially in Central Europe (Király and Egri 2007; Morrondo et al. 2021). In Spain, cephenemyiosis is a recent myiasis in roe deer. This species was firstly reported at the beginning of this century in one roe deer imported from France to Central Spain (Notario and Castresana 2001). Since then, cephenemyiosis has experienced a rapid expansion throughout the northern half of the Iberian Peninsula, with prevalences ranging from 31.6 to 62.2% and mean parasite burdens of 16.9–41.2 larvae/animal (Arias et al. 2016; Martínez-Calabuig 2022a).
Pharyngomyia picta, commonly known as deer throat bot fly, has been reported in deer from Europe and Asia (Colwell 2001). In Spain, it was found in the Iberian red deer (Cervus elaphus hispanicus), occurring commonly in mixed infestations with C. auribarbis (Ruiz and Palomares 1993; Bueno-de la Fuente et al. 1998; de la Fuente et al. 2000; Vicente et al. 2004) and it can also infest other sympatric wild cervids as fallow deer (Dama dama) (Ruiz and Palomares 1993). However, mixed infections by Oestrinae were not reported in roe deer, although a case of simultaneous infection by C. stimulator and Lucilia caesar (Diptera: Calliphoridae) has been detected in the nasal cavity of a single roe deer from northern Spain (Martínez-Calabuig et al. 2022b).
Since morphological features are quite similar between different dipteran species, especially among first instar larvae, molecular techniques are increasingly employed for diagnostic and taxonomic investigations of specimens belonging to this order. In this regard, the mitochondrial cytochrome oxidase subunit I (COI) has been used in several studies for assessing the host-specificity, species identification, phylogeny, and diagnosis of oestrid larvae (Otranto and Stevens 2006; Moreno et al. 2015).
Traditionally, oestrids have been considered as highly host-specific; thus, when larvae are introduced into a host widely different from their natural hosts, they do not usually complete their life cycle (Colwell 2006). However, O. ovis does not seem to fit in this theory, in view of the variety of hosts, as mentioned above, that can be infested by this species. In this regard, a recent investigation firstly reported O. ovis in Cervidae, specifically in one roe deer from the north of Guadalajara province in Central Spain (Martínez-Calabuig et al. 2023). It is worth noting that sympatry between wild and domestic ruminants is high in this area. After this first report, a broad survey was designed for providing updated information on the situation of nasal myiases in roe deer from this area.
Materials and methods
Area of study and animals
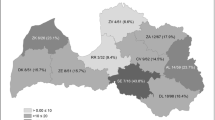
The study was conducted in 35 game reserves located in five provinces of Central Spain (Guadalajara, Burgos, Soria, Segovia, and Zaragoza) from January to June 2023 (Fig. 1; Table 1). This area has a Continental climate characterized by cold winters and hot and dry summers. Precipitation is highly variable within and between years, ranging from scant to moderate and occurring mainly in spring and early summer (San Miguel et al. 2011; Charraza et al. 2018). Temperatures in summer usually exceed 35ºC, favouring the lifecycle of oestrids, whose flies are active when temperatures are above 12–18ºC (Breev et al. 1980). Dominant ecosystems in this area are the Mediterranean forest and grassland environments; the most abundant tree species are the oak (Quercus faginea), the holm oak (Quercus rotundifolia) and the Pyrenean oak (Quercus pyrenaica) (San Miguel et al. 2011).
According to the last official census, the study area comprises the Spanish regions (Castilla-La Mancha, Castilla-León, and Aragón) with the largest ovine populations (MAPA 2022). In addition, the abundance of roe deer in Central Spain is also among the highest of the country, as reflected by the high number of captures (average 7,000 roe deer) authorized in these provinces in the 2021/2022 hunting season (Centenera, unpublished data).
A total of 184 roe deer heads were collected in the field after hunting; they were stored at -20 ºC until processed. Sampling was mainly restricted to January-February for females and to April-June for males for coinciding with the official selective hunting season. Hunters´ reluctance to relinquish the buck trophy resulted in a noticeable disproportion between sexes (170 females and 14 males). The age of roe deer was determined by teeth features (number, shape, and wear of the dental pieces) according to Høye (2006), and three host age categories were considered (< 1 year; 1–5 year; > 6 year).
Larval collection and identification
Roe deer heads were thoroughly examined for nasopharyngeal myiasis according to Martínez-Calabuig (2020). All larvae found in the nasopharyngeal cavities were recovered, rinsed in physiological saline, and preserved in 70% ethanol. The number, location and stage of the larvae were recorded (Table 2). Morphological identification was performed according to Zumpt (1965). First instars were identified according to the shape of the anterior cephalopharyngeal skeleton and the spinulation pattern (Fig. 2a1, b1); identification of L2 and L3 was carried out on the basis of the shape of posterior spiracular plates (Fig. 2a2, b2), antennal lobes disposition and spinulation pattern (Fig. 2a3, b3).
(a) Oestrus ovis larvae. (a1) Detail of the cephalopharyngeal skeleton and the ventral spinulation pattern of a first instar (L1) showing two complete rows of spines in segments 1–4 and an additional incomplete row in the middle of segments 5–11. (a2) Detail of the posterior end of third stage larvae (L3) with D–shaped closed stigmal plates (a3) Detail of the anterior end of a L3 with antennal lobes separated at the basis (white arrow) and the typical spinulation pattern. (b) Cephenemyia stimulator larvae. (b1) Detail of the cephalopharyngeal skeleton and the ventral spinulation pattern of a L1 showing numerous rows of denticles on segments 2–11. (b2) Detail of the posterior end of a L3 with C–shaped stigmal plates (b3) Anterior end with V-shaped antennal lobes (white arrow)
Phylogenetic tree clustering of the partial COI of Oestridae. The tree was obtained using a General Time Reversible substitution model with gamma-shaped rate variation with a proportion of invariable sites (GTR + G + I) with MrBayes software 3.2.7 (Ronquist et al. 2012), using Bayesian inference with Markov Chain Monte Carlo sampling (10,000,000 generations, sampling every 1,000 generations). This analysis involved 39 nucleotide sequences. The nucleotide sequence of Lucillia caesar was used as an outgroup. Isolates obtained in this study or identical to those obtained in the present study are highlighted in bold
Morphological identification of both species was molecularly confirmed by the analysis of a subset of larvae including specimens of the three larval instars. DNA was extracted using a commercial kit (High Pure PCR Template Preparation Kit, Roche Diagnostics GmbH®, Mannheim, Germany) following the manufacturers’ instructions. DNA samples were analysed using a PCR targeting a 688 bp segment of the mitochondrial COI gene of Oestridae as previously described (Otranto et al. 2000); DNA of Hypoderma actaeon and nuclease free water were included as positive and negative controls, respectively.
PCR products were separated by electrophoresis on 1% agarose gels stained with RedSafe (iNtRON Biotechnology®, South Korea) and visualized using a GelDoc Go Imagen System (Bio-Rad Laboratories®, California, USA). Selected fragments were purified and sequenced at STAB VIDA´s laboratories of the Universidade Nova de Lisboa, Portugal. Sequences were aligned and edited using ChromasPro (Technelysium, Brisbane, Australia) and consensus sequences were then scanned against the GenBank database using BLAST.
Phylogenetic analysis
A phylogenetic analysis was carried out using MrBayes 3.2.7 software (Ronquist et al. 2012) by Bayesian approach with Markov Chain Monte Carlo sampling (10,000,000 generations sampling every 1,000 steps). A General Time Reversible substitution model with gamma-shaped rate variation with a proportion of invariable sites (GTR + G + I), was used. The model was selected based on AIC value (Akaike Information Criterion) using the free software jModelTest v.2.1.10 (Darriba et al. 2012). The tree was visualized and edited using FigTree 1.4.3 (http://tree.bio.ed.ac.uk/software/figtree/).
Statistical analysis
Statistical analyses were performed with R statistical package (R v.4.3.2; 3). Variables were grouped and categorised for statistical analysis as represented in Table 1. A logistic regression analysis was performed for assessing the possible influence of different factors (age, sex, province, and month of capture) on the prevalence of O. ovis and C. stimulator. The binomial GLM was fitted with the brglm2 package (Kosmidis 2023). Identification of risk factors affecting the intensity of infestation was assessed using the ANOVA test. Since larval counts were not normal distributed, they were log normalized (natural log + 1) previous to the analysis. Only data from positive animals were used for performing the ANOVA test.
Results
Overall, 44 out of 184 (23.9%; CI 95 17.95–30.74) roe deer heads harboured dipteran larvae (n = 959) in their nasopharyngeal cavity, and the mean number of larvae per infested roe deer was 35.2 (SD 49.71).
Morphological examination allowed the identification of two Oestrinae species: C. stimulator and O. ovis (Fig. 2); these findings were molecularly confirmed. Regarding O. ovis, COI sequences showed a percentage of identity between 98.9 and 99.8% when compared to the deposited sequences NC_059851.1 and AF497767.1 of O. ovis larvae recovered from sheep and goat from Spain and Italy (Otranto et al. 2003; Aleix-Mata et al. 2021). The phylogenetic analysis revealed that our sequences clustered with other O. ovis sequences obtained in Spain, Turkey, and Kyrgyzstan, being clearly separated from a novel Oestrus species detected in an Iberian ibex from Spain (Moreno et al. 2015) (Fig. 3). In addition, the COI sequences of C. stimulator were identical to those (MG763915.1 and NC_059850.1) recovered from roe deer from Spain (Aleix-Mata et al. 2021; Fidalgo et al. 2021); the phylogenetic tree showed that all these sequences clustered with other sequences of C. stimulator recovered from roe deer in our country (de la Fuente et al. 2021). The partial sequences obtained in the present study were deposited under the accession numbers PP078924.1-PP078925.1.
Cephenemyia stimulator was the most prevalent nasal bot fly since 26 animals (14.1%; CI 95 9.44–20.02%) were infested, showing a mean intensity of infestation of 35.2 (SD 49.71) larvae/infested animal; after identification of larval instars, 910 were classified as L1, five as L2 and one as L3 (Table 2). Only eighteen roe deer (9.8%; CI 95 5.90-15.02%) harboured O. ovis, and the mean intensity of infestation was 2 (SD 1.33) larvae/infested animal; the distribution of the different larval stages was 32 L1, 7 L2 and 4 L3. No mixed infestations by both Oestrinae were found in any animal. Table 2 shows a strong predominance of first instars (> 65%) for both species, especially in January and February. Regarding to the location of larvae, all O. ovis specimens (n = 43) were located at the nasal turbinates; those of C. stimulator (n = 916) were also mainly found at the nasal turbinates (n = 884; 96.8%), although a small number was detected at the pharynx (n = 32; 3.2%).
Prevalence and intensity of infestation values considering the different factors analysed are summarized in Table 1. Oestrus ovis was detected in the provinces of Guadalajara, Burgos and Soria with percentages ranging from 6.12 to 14.29%. Moreover, C. stimulator was found in roe deer from Burgos, Soria and Zaragoza, reaching higher prevalences (35.7–57.1%) than O. ovis. It is worth noting that both Oestrinae were detected in roe deer from Burgos and Soria, although no mixed infestations were found in any animal. On the contrary, both species were absent in the eight roe deer hunted in Segovia.
Regarding the sex of the hosts, the prevalence for both species was higher in males than in females. In general, old animals (> 6 year) showed high prevalence values than young adults and calves; however, the logistic model over O. ovis and C. stimulator prevalence did not show any statistical differences when considering the sex, age, month, or province of origin (p > 0.05).
The influence of the month when roe deer were hunted was only significant for the intensity of infestation by C. stimulator (p = 0.004), with higher larval burdens in February than in April.
Discussion
Two different nasal bot flies, C. stimulator and O. ovis are currently affecting roe deer in central Spain. Our results reveal that the prevalence and intensity of C. stimulator in roe deer from this area is much lower (16.3%) than that previously detected by Martínez-Calabuig et al. (2022b) in northern Spain. Nevertheless, our data show high variations between provinces that ranged from 0% in Guadalajara and Segovia to 57.1% in Zaragoza (Fig. 1). Marked differences in the prevalence of C. stimulator among neighboring provinces, such as Zaragoza and Guadalajara or Segovia and Burgos, are probably due to the current irregular distribution of this myiasis within those provinces; thus, all positive animals from Zaragoza were hunted in the northern part of the province, while those closest to the border with the province of Guadalajara were negative. Similar findings were observed in roe deer from Segovia and Burgos since most of the positive animals from Burgos belonged to the northernmost populations located far from Segovia province. In addition, the noticeable prevalence found for the sheep bot fly, O. ovis, suggests that its presence in roe deer was not accidental as it had been hypothesized after its first identification.
Oestrosis is extensively distributed in domestic and wild sheep and goats in Spain (Alcaide et al. 2003, 2005; Gracia et al. 2010). Nevertheless, there have been widespread reports of accidental infestations in other species, including humans and dogs (Lucientes et al. 1997; Moreno et al. 1999; Lobato et al. 2011), suggesting that gravid O. ovis females are not strictly host specific (Colwell 2001). According to our findings, the occurrence of late larval stages (L2-L3) in roe deer suggests that O. ovis could complete its life cycle in Cervidae.
Alcaide et al. (2005) indicated that high O. ovis prevalence and intensity of infection values in its main hosts (domestic small ruminants) may potentially increase the risk of spillover of this parasite to new hosts. High roe deer densities (> 10 animals/100 ha) and the existence of shared habitats with abundant sheep flocks in the area of study may have favoured the interspecific spillover of O. ovis. A similar situation has been previously described for Hypoderma actaeon (Diptera: Oestridae), considered specific for red deer, which has become an emerging myiasis in roe deer in Central Spain (Panadero et al. 2017). Changes in the pattern of distribution of potentially susceptible hosts (red deer/roe deer) in Central Spain may have favoured the spreading of this myiasis to other hosts (Panadero et al. 2020). It is worth noting that some authors (Moreno et al. 1999) consider oestrid flies as host-opportunistic so that their specificity would be very influenced by the availability of adequate host species (Price 1980). Our results would reinforce the lack of host specificity of O. ovis.
Although our study was restricted to the first semester of the year, our results show a strong predominance of first instars for both Oestrinae species from January to April, indicating the existence of a hypobiotical period where larvae delay their development while waiting for the optimal breeding season (Alcaide et al. 2003). Additionally, the existence of a low proportion of L2 and L3 during this period would reveal the end of the quiescence period and the beginning of larval reactivation. However, a year-round sampling, covering the entire life cycle of these parasites is needed for a complete analysis of their chronobiology.
Larval burdens, mainly represented by first instars, were much higher for C. stimulator than for O. ovis. Taking into consideration that L1 represent the insurance of the survival of the parasitic population (Tabouret et al. 2001), those differences could be attributed to the major suitability of roe deer as a host for C. stimulator than for O. ovis. In this sense, several factors promoting larviposition and subsequent larval survival have been related to the suitability of a host for Oestrinae, e.g. host related odour (Poddighe et al. 2010), moistness (Cepeda-Palacios et al. 2000) and structure of the host’s muzzle (Cogley and Anderson 1981), host immune reaction (Tabouret et al. 2003), as well as behavioural responses to avoid larviposition (Anderson 1975).
Oestrids have very different life cycles and adaptations for survival at different sites into the host (Colwell et al. 2006). Early larval stages (L1) of C. stimulator are found in the nasal cavity of roe deer, whereas late stages (L2-L3) are mainly located in the pharyngeal pouches or diverticula (Angulo-Valadez et al. 2010; Martínez-Calabuig 2020). In this study, all O. ovis larvae were only found in the nasal passages of roe deer, whereas in its natural hosts, sheep and goats, larvae are found in the nasal cavity as well as in nasal and frontal sinuses (Angulo-Valadez et al. 2010).
Statistical analysis did not allow identifying any factor significantly affecting the prevalence of infestation by C. stimulator and O. ovis. The absence of significant differences may be due to the low number of roe deer infested within some categories.
Our results also reveal that the geographical distribution of both Oestrinae in roe deer from Central Spain is not homogeneous. Cephenemyia stimulator is a high prevalent myasis which is actually present in three out of the five provinces surveyed in this study, whereas O. ovis was only sporadically found in three provinces. It is worth noting that the infestation by O. ovis was more prevalent in the province of Guadalajara, where C. stimulator is absent, than in Burgos and Soria, where both species coexist; nevertheless, it is worth noting that in the latter provinces, no roe deer was simultaneously parasitized by both species. Further studies to elucidate the influence some biotic (host density) and abiotic (climate, altitude, etc.) factors in the distribution of nasal myiasis are needed.
The absence of co-occurrence within the same individual could be attributed to behavioural changes of roe deer after being attacked by C. stimulator flies. In this sense, Anderson (1975) evidenced that, after a first infection by Cephenemyia spp., experienced deer try to evade larviposition by oestrid females, also reducing the infection success by O. ovis. Although co-occurrence of different oestrids such as P. picta and C. auribarbis are commonly reported in red deer from Europe (Ruiz and Palomares, 1993; de la Fuente et al. 2000; Vicente et al. 2004; Leitner et al. 2016; Miranda et al. 2022), the earlier larviposition by C. auribarbis and its faster larval development compared to P. picta in southern Spain may reflect asynchronous life cycles of both oestrids decreasing the co-occurrence of both sympatric species (Bueno-de la Fuente et al. 1998). In addition, it was reported that the intensity of P. picta in concomitant infections with C. auribarbis was lower than in pure infections (Vicente et al. 2004), providing good evidence of interspecific competence, which could be dealt with by parasites by means of asynchronous life cycles and different maturation periods.
Conclusion
Our data suggest a possible interspecific transmission of O. ovis from domestic ruminants to roe deer in central Spain; this may be due to the high density of this wild ungulate and free ranging sheep which usually share habitats in this area. Thus, this Spanish region may be considered a hotspot for cross-transmission of different Oestridae between Cervidae and Bovidae, as it has been previously reported for H. actaeon. Since wildlife may contribute to the reinfestation of domestic flocks with O. ovis, the efficacy of oestrosis control programs can be compromised. Thus, monitoring strategies must include sheep and goat flocks together with sympatric wild ruminants.
Data availability
The datasets generated during this study will be available upon request.
References
Alcaide M, Reina D, Sánchez J, Frontera E, Navarrete I (2003) Seasonal variations in the larval burden distribution of Oestrus ovis in sheep in the southwest of Spain. Vet Parasitol 118:235–241
Alcaide M, Reina D, Sánchez-López J, Frontera E, Navarrete I (2005) Seroprevalence of Oestrus ovis (Diptera, Oestridae) infestation and associated risk factors in ovine livestock from southwestern Spain. J Med Entomol 42:327–331
Aleix-Mata G, Peréz JM, Sánchez A (2021) The complete mitochondrial genome of Oestrus ovis (Linnaeus, 1758) (Diptera: Oestridae). Mitochondrial DNA Part B Resour 6(7):1847–1848
Allen SD, Bunch TD (1982) Cranial lesions attributable to chronic sinusitis in bighorn sheep. JAVMA 181:1418–1419
Anderson JR (1975) The behaviour of nose bot flies (Cephenemyia apicata and C. Jellisoni) when attacking black-tailed deer (Odocoileus hemionus columbianus) and the resulting reactions of the deer. Can J Zool 53:977–992
Angulo-Valadez CE, Scholl PJ, Cepeda-Palacios R, Jacquiet P, Dorchies P (2010) Nasal bots… a fascinating world! Vet Parasitol 174:19–25
Arias MS, Pajares G, Díez-Baños N, Pérez-Creo A, Prieto A, Díez-Baños P, Morrondo P (2016) Cephenemyiosis, an emergent myiasis in roe deer (Capreolus capreolus) from northwestern Spain. Parasitol Res 115:4605–4610
Barroso P, Ruiz-de-Ybáñez R, Martínez-Carrasco C, Gens MJ, Escribano F, Sánchez A, Pérez JM (2017) First report of oestrosis in aoudad from southeastern Spain. Parasitol Res 116:2053–2055
Breev KA, Zagretdinov RG, Minár J (1980) Influence of constant and variable temperatures on pupal development of the sheep bot fly (Oestrus ovis L). Folia Parasitol 27:359–365
Bueno-de La Fuente ML, Moreno V, Pérez JM, Ruíz-Martínez I, Soriguer RC (1998) Oestrosis in red deer from Spain. J Wildl Dis 34:820–824
Capelle KJ (1966) The occurrence of Oestrus ovis L. (Diptera: Oestridae) in the bighorn sheep from Wyoming and Montana. J Parasitol 52:618–621
Cepeda- Palacios R, Scholl PJ (2000) Factors affecting the larvipositional activity of Oestrus ovis (Diptera:Oestridae). Vet Parasitol 91: 93–105.
Chazarra A, Flórez E, Peraza B, Tohá T, Lorenzo B, Criado E, Moreno JV, Romero R, Botey R (2018) Mapas climáticos De España (1981–2010) y ETo (1996–2010). Agencia Estatal de Meteorología. http://hdl.handle.net/20.500.11765/945
Cogley TP, Anderson JR (1981) Invasion of black-tailed deer by nose bot fly larvae (Diptera: Oestridae: Oestrinae). Int J Parasitol 11:281–286
Colwell DD (2001) Bot flies and warble flies (Order Diptera: family Oestridae). In: Samuel WM, Pybus MJ, Kocan AA (eds) Parasitic diseases of Wild mammals, vol 2. Iowa State University, pp 46–71
Colwell DD, Hall MJR, Scholl PJ (2006) The Oestrid Flies. Biology, Host-parasite Relationships, Impact and Management, CABI International: 359pp
R Core Team (2023) R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
Darriba D, Taboada GL, Doallo R, Posada D (2012) jModelTest 2: more models, new heuristics and parallel computing. Nat Methods 9:772–772
de la Fuente C, San Miguel JM, Santín M, Alunda JM, Domínguez I, López A, Carballo M, González A (2000) Pharyngeal bot flies in Cervus elaphus in Central Spain: Prevalence and Population dynamics. J Parasitol 86:33–37
de la Fuente AM, Caparrós N, Mora-Rodríguez JM, Molina M, Aleix-Mata G, Velarde R, Fidalgo LE, López-Beceiro AM, Lorite P, Boos M, Faure E, Pérez JM, Sánchez A (2021) Characterization of new molecular markers of three botflies parasitizing Cervid hosts. J Med Entomol 58:1463–1469
Dorchies P, Duranton C, Jacquiet P (1998) Pathophysiology of Oestrus ovis infection in sheep and goats: a review. Vet Rec 142:487–489
Fidalgo LE, López-Beceiro A, Martínez-Carrasco C, Caparrós Fontarosa N, Sánchez Baca A, Vila-Pastor M, Barreiro-Vázquez JD, Sarasa M, Pérez JM (2021) Unexpected intracranial location of a Cephenemyia stimulator larva in a roe deer, Capreolus capreolus, revealed by computed tomography. Galemys 33:13–19
Gracia MJ, Lucientes J, Peribáñez MA, Castillo JA, Calvete C, Ferrer LM (2010) Epidemiology of Oestrus ovis infection of sheep in northeast Spain (Mid-ebro Valley). Trop Anim Health Prod 42:811–813
Høye TT (2006) Age determination in roe deer: a new approach to tooth wear evaluated on known age in individuals. Acta Theriol 51:205–214
Király I, Egri B (2007) Epidemiological characteristics of Cephenemyia stimulator (Clark, 1815) larval infestation in European roe deer (Capreolus capreolus) in Hungary. Acta Zool Acad Sci Hung 53:271–279
Kosmidis I (2023) _brglm2: Bias Reduction in Generalized Linear Models_. R package version 0.9, https://CRAN.R-project.org/package=brglm2
Leitner N, Schwarzmann L, Zittra C, Palmieri N, Eigner B, Otranto D, Glawischnig W, Fuehrer HP (2016) Morphological and molecular identification of nasopharyngeal bot fly larvae infesting red deer (Cervus elaphus) in Austria. Parasitol Res 115:4417–4442
Lobato C, Remacha MA, Martínez A, López CM, Díez-Baños P, Panadero R (2011) External ophthalmomyiasis caused by Oestrus ovis in northwestern Spain: A report of a case. European Veterinary Parasitology College, Annual Conference 2011, Zagreb
Lucientes J, Ferrer M, Andres MJ, Peribañez MA, Gracia MJ, Castillo JA (1997) Canine myiasis by sheep bot fly (Diptera: Oestridae). J Med Entomol 34:242–243
MAPA (2022) Caracterización del sector ovino y caprino en España: orientación productiva carne, Subdirección General de Producciones Ganaderas y Cinegéticas, Dirección General de Producciones y Mercados Agrarios. Catálogo de Publicaciones de la Administración General del Estado: https://cpage.mpr.gob.es/, NIPO: 003211284
Martínez-Calabuig N (2020) Prevalencia Y desarrollo larvario de Cephenemyia spp. en corzos del norte de España. Trabajo Fin de Grado, Universidad de Santiago de Compostela, Lugo, Galicia, Spain, p 49
Martínez-Calabuig N, López CM, Díaz P, Remesar S, García-Dios D, Saldaña A, López-Lorenzo G, Díez-Baños P, Morrondo P (2022a) Distribución Y Ciclo biológico De Cephenemyia stimulator. Diptera: Oestridae) en corzos del norte de España. XXII Congreso de la Sociedad Española de Parasitología, pp 5–8. July, Madrid, 277.
Martínez-Calabuig N, Panadero R, Remesar S, García-Dios D, Díaz P, Prieto A, López G, Díez-Baños P, Morrondo P, López C (2022b) Mixed nasopharyngeal myiasis by bots and blowflies in a roe deer (Capreolus capreolus). J Wildl Dis 58:232–234
Martínez-Calabuig N, Remesar S, Varas G, García-Dios D, Saldaña A, Díaz P, Díez-Baños P, Morrondo P, Panadero R (2023) First report of Oestrus ovis infesting roe deer (Capreolus capreolus) in an area with high sympatry between wild and domestic ruminants. Annual meeting of the European Veterinary Parasitology College, Paris, 29–30 de Junio 2023
Miranda R, Serejo J, Pérez JM, Aranha J, Venâncio C, Vieira-Pinto M (2022) First Study of Pharingomyia picta and Cephenemyia auribarbis in wild populations of red deer (Cervus elaphus) in Portugal. Anim (Basel) 26:121896
Moreno V, Pérez JM, Moreno PA, Granados JE, Ruíz-Martínez I, Soriguer RC, de Simon MA (1999) Oestrid myiasis in European mouflon from Spain. J Wildl Dis 35:78–81
Moreno V, Romero-Fernández I, Marchal JA, Beltrán M, Granados JE, Habela MA, Tamadon A, Rakhshandehroo E, Sarasa M, Pérez JM, Sánchez A (2015) Molecular characterization of bot flies, Oestrus spp. (Diptera, Oestridae), from domestic and wild Bovidae hosts. Vet Parasitol 212: 473–477
Morrondo P, Pajares G, Arias MS, Martínez-Calabuig N, Remesar S, García-Dios D, Díaz P, López CM, Panadero R, Díez-Baños P (2021) An update on Cephenemyiosis in the European Roe deer: Emergent Myiasis in Spain. Animals 11:3382
Notario A, Castresana L (2001) Contribution to the knowledge of Cephenemyia stimulator Clark, 1815 (Diptera, Oestridae) in Spain. Folia Venatoria 30–31:325–326
Otranto D, Stevens JR (2006) Molecular Phylogeny and Identification. In: The Oestrid Flies. Biology, Host-parasite Relationships, Impact and Management, Edited by Colwell D.D., Hall M.J.R. and Scholl P.J. CABI International: 51–66
Otranto D, Tarsitano E, Giangaspero A, Puccini V (2000) Differentiation by polymerase chain reaction-restriction fragment length polymorphism of some Oestridae larvae causing myiasis. Vet Parasitol 90:305–313
Otranto D, Traversa D, Guida B, Tarsitano E, Fiorente P, Steven JR (2003) Molecular characterization of the mitochondrial cytochrome oxidase I gene of Oestridae species causing obligate myiasis. Med Vet Entomol 17:307–315
Panadero R, Varas G, Pajares G, Markina F, López C, Díaz P, Pérez-Creo A, Prieto A, Díez-Baños P, Morrondo P (2017) Hypoderma actaeon: an emerging myiasis in roe deer (Capreolus capreolus). Med Vet Entomol 31:94–96
Panadero R, López CM, Remesar S, Cabanelas E, Varas G, Markina F, Díaz P, García-Dios D, Prieto A, Fernández G, Díez-Baños P, Morrondo P (2020) Temporal and spatial spread of Hypoderma actaeon infection in roe deer from peninsular Spain determined by an indirect enzyme-linked immunosorbent assay. Med Vet Entomol 34:44–48
Pérez JM, Granados JE, Soriguer RC, Ruiz-Martínez I (1996) Prevalence and seasonality of Oestrus Caucasicus Grunin, 1948 (Diptera: Oestridae) parasitizing the Spanish ibex, Capra pyrenaica (Mammalia: Artiodactyla). J Parasitol 82:233–236
Pérez JM, Moreno V, Navas J, Vélez de Mendizábal N, Quesada JM, Esteban FJ (2016) A system dynamics model of the population dynamics of Oestrus sp. (Diptera: Oestridae) infesting Iberian ibex, Capra pyrenaica. Ital J Zool 83:130–138
Poddighe S, Dekker T, Scala A, Angioy AM (2010) Olfaction in the female sheep botfly. Naturwissenschaften 97: 827–835.
Price PW (1980) Evolutionary Biology of parasites. Monogr Popul Biol 15:1–237
Ronquist F, Teslenko M, van der Mark P, Ayres DL, Darling A, Höhna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP (2012) MrBayes 3.2: efficient bayesian phylogenetic inference and model choice across a large model space. Syst Biol 61:539–542
Ruíz Martínez I, Palomares F (1993) Occurrence and overlapping of pharyngeal bot flies Pharyngomyia picta and Cephenemyia auribarbis (Oestridae) in red deer of southern Spain. Vet Parasitol 47:119–127
San Miguel A, Perea García-Calvo R, Roig-Gómez S, Fernández M (2011) Bosque y matorral mediterráneo continental (Capítulo 6). Sección III: Evaluación de los tipos operativos de ecosistemas. Informe final EME: Evaluación de los ecosistemas del milenio de España conservación de los servicios de los ecosistemas y la biodiversidad para el bienestar humano. https://ecomilenio.es/informe-de-resultados-eme/1760
Sánchez A, Caparrós N, Ostrowski S, Sarasa M, Pérez JM (2017) Oestrosis in Asiatic Ibex (Capra sibirica): a case report and molecular characterization of larvae. Vet Parasitol 236:55–57
Sante L, Hernández-Porto M, Tinguaro V, Lecuona M (2017) Oftalmomiasis Y miasis nasal por Oestrus ovis en paciente residente en las Islas Canarias con características epidemiológicas poco frecuentes. Enferm Infecc Microbiol Clin 35(7):461–462
Scholl PJ, Colwell DD, Cepeda-Palacios R (2019) Myiasis (Muscoidea, Oestroidea). In: Mullen GR, Durden LA (eds) Medical and Veterinary Entomology, 3rd edn. Elsevier-Academic, San Diego, pp 383–419
Tabouret G, Jacquiet P, Scholl P, Dorchies P (2001) Oestrus ovis in sheep: relative third-instar populations, risks of infection and parasitic control. Vet Res 32:525–531
Tabouret G, Lacroux C, Andreoletti O, Bergeaud JP, Hailu-Tolosa Y, Hoste H, Prevot F, Grisez C, Dorchies P, Jacquiet P (2003) Cellular and humoral local immune responses in sheep experimentally infected with Oestrus ovis (Diptera: Oestridae). Vet Res 34:231–241
Tabuenca-del Barrio L, Mozo-Cuadrado M, Zubicoa-Eneriz A, Plaza-Ramos P (2018) Ocular external myiasis. A series of cases due to larvae Oestrus ovis in Navarra, Spain. Arch Soc Esp Oftalmol (English Edition) 93:567–570
Vicente J, Fierro Y, Martínez M, Gortázar C (2004) Long-term epidemiology, effect on body condition and interspecific interactions of concomitant infection by nasopharyngeal bot fly larvae (Cephenemyia Auribarbis and Pharyngomyia picta, Oestridae) in a population of Iberian red deer (Cervus elaphus hispanicus). Parasitol 129:349–361
Zanzani S, Cozzi L, Olivieri E, Gazzonis A, Manfredi MT (2016) Oestrus ovis L. (Diptera: Oestridae) induced nasal myiasis in a dog from Northern Italy. Vet Med: 5205416
Zumpt F (1965) Myasis in man and animals in the old world. Ed. Butterworths, London, pp 205–229
Acknowledgements
The authors express their gratitude to the Asociación del Corzo Español (ACE) for its collaboration.
Funding
Funding was provided to INVESAGA group by the Program for Consolidating and Structuring Competitive Research Groups (ED431C 2023/04, Xunta de Galicia, Spain). Néstor Martínez-Calabuig has a pre-doctoral FPU grant (FPU21/04523) from the Spanish Ministry of Education and Science.
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature.
Author information
Authors and Affiliations
Contributions
NMC wrote the main manuscript and investigation; RP, PD conceptualization, supervision, review and editing; GV, AS, DGD investigation and methodology, SR, CL prepared figures, supervision, review and editing; PDB, PM conceptualization and funding acquisition.
Corresponding author
Ethics declarations
Ethical approval
Samplings were performed on animal carcasses obtained through legal hunting activities without research purposes, so authorization from the bioethics committee was not required.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Martínez-Calabuig, N., Panadero, R., Varas, G. et al. Prevalence of nasopharyngeal myiasis in roe deer (Capreolus capreolus) from an area with high sympatry between wild and domestic ungulates in Central Spain. Eur J Wildl Res 70, 60 (2024). https://doi.org/10.1007/s10344-024-01814-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10344-024-01814-2