Abstract
Density and abundance estimates are critical to effective wildlife management and are essential for monitoring population trends and setting effective quotas for harvesting. Management of roan (Hippotragus equinus) and sable (H. niger) antelopes in Mudumu National Park (MNP), Namibia, is challenging because they are elusive, naturally unmarked, and believed to occur at low densities. The species are threatened by habitat fragmentation, human population growth, and illegal hunting, and reliable density and abundance estimates have not been quantified, hampering management and conservation plans. Our objective was to estimate roan and sable densities and abundances using the time in front of the camera model (TIFC) and the Poisson-binomial N-mixture model (PB), respectively. We also evaluated the effects of environmental and ecological variables on roan and sable abundance. We used data from two camera trap surveys conducted between March and September 2021 in the MNP. Results showed that the TIFC model provided low-density estimates of 1.62 (95% CI 1.61–1.64) roans/km2 and 2.46 (95% CI 2.42–2.50) sables/km2, consistent with trends reported in Africa where these species occur at low densities. In addition, the total abundance of roans and sables in the MNP from the PB model were 57 and 242, respectively. Higher roan abundance occurred in sites with higher grass cover. This study provides the first accurate camera trap-derived density and abundance estimates for roan and sable in the MNP, which will be critical for developing comprehensive conservation programs and strategies that are likely to reduce the risk of extinction for both species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Effective and efficient wildlife management requires knowledge of a system through state variables such as density and abundance estimates (Williams et al. 2002; Rowcliffe et al. 2008; Nakashima et al. 2017). Estimating animal density and abundance is crucial for monitoring population trends and identifying factors that influence those trends including setting effective quotas for harvesting (Mace et al. 2008; Warbington and Boyce 2020; Finn et al. 2023). To collect sufficient data for population monitoring, techniques must be tailored to the species of interest, taking into account habitat configuration, species distribution, and seasonality (Warbington and Boyce 2020).
Aerial surveys have been used worldwide to monitor ungulates at the landscape level (Marshal et al. 2016; Western and Mose 2021; Davis et al. 2022). Aerial methods have advantages over ground-based surveys, such as collecting count data at the landscape scale within a relatively short sampling period and accessing remote environments where accessibility either by foot or vehicle is limited (Marshal et al. 2016; Western and Mose 2021). Despite these advantages, aerial techniques are subject to several potential sources of bias, including observer effects, weather conditions, and species-specific characteristics (Schlossberg et al. 2016; Davis et al. 2022). In addition, aerial surveys are expensive and logistically challenging, especially in densely vegetated habitats where animals can evade detection (Nakashima et al. 2017; Gilbert et al. 2021; Harris et al. 2020). A consequence from these sources of error is misguided conservation management strategies (Davis et al. 2022).
Alternatives to aerial surveys for ungulates include distance sampling and non-invasive camera traps (Nakashima et al. 2017; Gilbert et al. 2021; Pal et al. 2021; Warbington and Boyce 2020). Camera trapping is an efficient remote tool that uses infrared and motion to detect animals crossing through the camera field-of-view (O’Connell et al. 2011; Burton et al. 2015). Camera traps reduce the amount of fieldwork required to collect quantitative data, as they operate 24 h a day for several weeks at a relatively low labor cost (O’Connell et al. 2011; Burton et al. 2015). Despite these benefits, challenges remain in terms of using camera trap datasets to accurately and precisely estimate the density and abundance of unmarked species (Gilbert et al. 2021; Becker et al. 2022). Various approaches have been developed and applied specifically to estimate the density and abundance of unmarked species, including time in front of the camera model (TIFC; Warbington and Boyce 2020; Becker et al. 2022), the random encounter and staying time model (Nakashima et al. 2017), Poisson-binomial N-mixture model (Royle 2004), and the random encounter model (Rowcliffe et al. 2008). Considering their infancy, there is a need to explore these estimators’ suitability for unmarked antelopes in various habitats and contexts.
In this study, we applied the TIFC and PB models to estimate the density and abundance of roan and sable. TIFC is a straightforward method and is useful for data-poor species because it makes no assumptions about home range size and is not mathematically challenging (Warbington and Boyce 2020). TIFC provided results comparable to aerial surveys when sources of potential bias are accounted for, such as removing prolonged periods when animals were investigating the camera (Becker et al. 2022). Another abundance estimator suitable for unmarked ungulates is the PB model (Royle 2004; Nakashima 2019). The PB model is a hierarchical model that partitions observed replicated count data into the true ecological state and the observation state (Royle 2004; Dail and Madsen 2011). These characteristics of the TIFC and PB models lend them to be convenient methods compared to other statistical demography frameworks such as capture-recapture, which require intensive effort and can be difficult to implement (Royle 2004; Dail and Madsen 2011).
Roan (Hippotragus equinus Desmarest, 1804) and sable (Hippotragus niger Harris, 1838) antelopes are elusive, naturally unmarked, and widely distributed in Africa (Ansell 1971; Martin 2003; Kimanzi 2011). Both species are water-dependent and generally stay within 2 to 5 km of water (Martin 2003; Kimanzi 2011; Havemann et al. 2016). Both antelopes occur naturally in the northeastern part of Namibia, including Mudumu National Park (MNP), an area receiving over 400 mm of rainfall (Martin 2003). Density and abundance trends of roan and sable populations at MNP were unknown, but recent estimates using aerial survey data showed that roan had an increasing trend and sable a stable trend from 1980 to 2019 (Nauyoma 2023, submitted) and unpublished distance sampling data suggest low population sizes (Supplementary Information 2, Table S2; Martin 2003; Namibian Association of CBNRM Support Organisations (NACSO) 2023). Threats to roan and sable in the MNP include population isolation due to the international veterinary fence between Namibia and Botswana, and the ramifications of increasing human encroachment to the Park (i.e., illegal hunting and inter-competition with cattle) (Martin 2003; Naidoo et al. 2022). To mitigate these threats, introductions from these populations led to the establishment of other populations outside their natural range throughout the country, such as the roan and sable populations in Etosha National Park (ENP) and Waterberg Plateau National Park (WPNP) in the late 1970s and early 1980s (Martin 2003). The ones at ENP are now extinct (Turner et al. 2022) and Alfeus (2022) showed a negative population growth for the roan and a zero-population growth for the sable at WPNP. Other important factors reported to increase decline in roan and sable populations include habitat change, rainfall variability with associated droughts and floods, anthrax, illegal hunting, and predation by African lions (Panthera leo) (Kimanzi 2011; Havemann et al. 2016). Both antelopes are listed as species of Least Concern (IUCN Species Survival Commission Antelope Specialist Group 2017). However, this classification may be due to insufficient abundance and distribution data (IUCN Species Survival Commission Antelope Specialist Group 2017). In fact, accurate abundance estimates of roan and sable are lacking for most populations, although it is widely accepted that most populations across these species range are on a declining trend (Havemann et al. 2016).
Specific objectives were to (1) estimate the density and abundance of unmarked roan and sable populations by applying the TIFC and PB models, respectively, to camera trap data from the MNP and (2) evaluate the effects of environmental and ecological variables on roan and sable abundance in native populations based on camera trap data.
Materials and methods
Study area
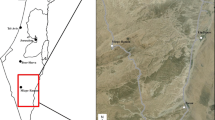
The study was conducted in the Mudumu National Park (MNP) (c. 1010 km2; 18.0965° S, 23.5252° E) (Fig. 1), located in northeastern Namibia. The Park is bordered in the West by the Kwando River and Botswana and also by five communal conservancies. The Park is part of the Kavango Zambezi Transfrontier Conservation Area (KAZA TFCA), where it is a migratory route and serves as a source of wildlife species for adjacent conservancies (Brennan et al. 2020; Stoldt et al. 2020). The area has three seasons: wet (January–May), dry (June–September), and hot-dry (October–January) (Leggett 2006). Average annual rainfall ranges between 740 and 1000 mm and average annual temperature ranges from 5 to 35 ˚C (Schlettwein et al. 1991; Mendelsohn and Roberts 1997). Vegetation falls under the tree and shrub savanna biome composed of tall and high closed and tall open woodlands as well as tall open and high closed grassland. Tall grass species such as Aristida stipitata and A. meridionalis occur in the study area, and both roans and sables hide their calves in these tall grasses during the calving period (Martin 2003; Kimanzi 2011). Other large mammals in the Park include the elephant (Loxodonta africana), giraffe (Giraffa camelopardalis), eland (Taurotragus oryx), African buffalo (Syncerus caffer), Burchell’s zebra (Equus burchelli), blue wildebeest (Connochaetes taurinus), tsessebe (Damaliscus lunatus), and impala (Aepyceros melampus). Predators include the African lion, leopard (Panthera pardus), spotted hyena (Crocuta crocuta), cheetah (Acinonyx jubatus), African wild dog (Lycaon pictus), caracal (Caracal caracal), and black-backed jackal (Canis mesomelas) (Fabiano et al. 2020).
Study design
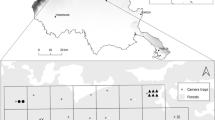
A regular grid with a cell size of 2 × 2 km was superimposed on the MNP vegetation layer similar to other studies of ungulates using camera traps (Gray 2017; Fabiano et al. 2020; Alfeus 2022). We applied a stratified sampling scheme according to vegetation structure in two spatial sampling Blocks each of the 25 grid cells (Fig. 1). Inter-camera trap stations distance was 2 km similar to a study of other ungulates (Phumanee et al. 2020). Due to the limited number of cameras, we used a rotational sampling approach where 24 cameras were deployed in Block B during the wet season (March to May 2021) and 25 cameras in Block A during the dry season (June to September 2021) (e.g., Phumanee et al. 2020). The difference in sampling effort between blocks was due to inaccessibility caused by flooding to one site. Cameras were operational for an average of 43 (11 ± standard deviation) and 47 (24 ± standard deviation) consecutive days in Blocks B and A, respectively. These sampling periods are similar to those from other studies on ungulates using camera traps (e.g., McCollum et al. 2018; Phumanee et al. 2020).
Camera trap surveys
We used Panthera v 7 incandescent-flash cameras with passive infrared sensors (Camera Trap V7 User Guide 2020). A single camera was mounted facing north or south at 1 m above the ground on the tree nearest to the center of a grid cell (Alfeus 2022). Cameras were set to take three photographs within 0.25 s. Cameras could only take one photo at night after each detection, with a 15-s interval between images, and recorded date and time of each photo taken (Camera Trap V7 User Guide 2020). Effective detection distance was set to 10 m, and we placed cameras in an open 10 m field-of-view with fewer tree trunks or thick patches of trees or shrubs.
Site covariates
Based on the patterns of spatial distribution of roan and sable study by Nauyoma (2023, submitted), two site covariates (grass cover and termite mounds) were hypothesized to influence roan abundance in the wet season (Block B) and two site covariates (grass cover and risk of predation) in the dry season (Block A). In addition, two site covariates (shrub and tree species diversity and grass cover) were hypothesized to influence sable abundance in both wet and dry seasons. Given our relatively small sample size, our model could not include detection covariates, although some abundance covariates we considered in this study may also have influenced the detection of the two species. To estimate tree and shrub species diversity (henceforth vegetation diversity) and grass cover, we collected data following the nested sampling design with three plots (i.e., 1 by 1 m, 10 by 10 m and 30 by 30 m quadrats) around each camera trap (Stohlgren et al. 1995). All live shrubs and trees within the 10 by 10 m and 30 by 30 m quadrats were identified and counted, respectively. We defined a juvenile shrub (≤ 0.5 m) and adult shrub (< 4 m tall) as woody plants, with multiple stems and a juvenile tree (≤ 4 m) and adult tree (> 4 m tall) as woody plants with one or few main stems (Gaillard et al. 2018; Le Roux et al. 2018). We applied the Shannon–Wiener Index to compute vegetation diversity, which we used as a proxy for forage availability, hiding cover from predators, and habitat structure (García-Marmolejo et al. 2015). Grass cover was estimated by a single observer as the percentage of the 1 by 1 m quadrat covered by grasses (Singh and Buckingham 2015). Grass cover was used as a proxy of forage availability. For termite mounds, we counted all mounds within a 50-m radius of each camera trap location (Anderson et al. 2016; McCollum et al. 2018). Termite mounds are ungulate hotspots because of their natural salts and for supporting stoloniferous grasses, forbs and shrubs (Mobæk et al. 2005; Phumanee et al. 2020). Risk of predation was based on the detection of either the African lion, leopard, spotted hyena, African wild dog, cheetah, caracal, or black-backed jackal at a camera station. Thus, we hypothesized that both ungulates abundance will be higher on sites with higher vegetation diversity (García-Marmolejo et al. 2015; Ampoorter et al. 2019), forage availability (Martin 2003; Havemann et al. 2016) and termite mounds (Mayengo et al. 2020) and lower in sites with higher predator occurrence (Sinclair 1985; Harrington et al. 1999).
Data analysis
The time in front of the camera (TIFC) model
We estimated roan and sable densities using the TIFC model. The assumptions of TIFC are (i) cameras are randomly placed relative to animal movement, (ii) animals are not attracted to or repelled by the cameras, and (iii) complete detection of animals in at least part of the camera field-of-view (Warbington & Boyce 2020; Becker et al. 2022). Density estimates were calculated as follows:
The number of individuals was the number of animals on each photograph. Time in field-of-view was determined as the total time an animal spent in front of the camera in seconds, derived from the time recorded in the photos, and the total camera operating time in seconds was determined by converting total operating days to seconds (Becker et al. 2022). Cameras were active for half day during the deployment and retrieval days, and for 24 h for the remaining surveying days. The area of field-of-view (surveyed by the camera), \(s\), was calculable as
where α is the angle of the field-of-view and r the maximum distance (10 m) animals can be detected from the camera (Warbington and Boyce 2020). The α was set to 65.8 degrees (Camera Trap V7 User Guide 2020). The area of detection of the camera, s, for r = 10 m was 57.39 m. We combined density estimates for roan and sable in Blocks A and B (Rich et al. 2019). Our study was designed to minimize violations of TIFC model assumptions and all TIFC model calculations were performed in Microsoft Excel.
Poisson-binomial N-mixture model (PB)
The assumptions of the PB model are (i) population is closed between successive counts, (ii) no heterogeneity on detection probability, (iii) abundance at the sampling unit is Poisson distributed, (iv) sampling sessions are independent, and (v) no false-positive errors (Chaudhuri et al. 2022). Our data collection lasted less than 9 months, which allowed us to avoid violating the closure assumption because roan and sable have a gestation period of nine months (Martin 2003).
To prevent false positives, we identified and counted individuals based on individual characteristics. Both roan and sable males tend to have larger and longer horns relative to adult females (Fig. 2; Estes 1991; Kimanzi 2011; Josling et al. 2019). Additional traits used were broken horn, and age class (adults and sub-adults based on height) (Fig. 3). Furthermore, the likelihood of false positives was reduced because in more than 90% of the detections, roans, and sables had a single detection per day across the entire block. There is a distinct dimorphism in sables: older males have shiny black coats, while females are dark brown and subadults and juveniles of both sexes tend to be a lighter shade of brown (Fig. 3; Martin 2003).
The PB modeling approach
To estimate roan and sable abundance from camera trapping count data per block, we used the PB model implemented in the unmarked package (Fiske and Chandler 2011), R v 3.2.2 (R Development Core Team 2022). Derivation is based on true ecological state and observation (or detection) state (Royle 2004; Fiske and Chandler 2011):
-
1.
The true ecological state. The species has a local abundance in i sites (Ni) with latent abundance that follows the Poisson distribution with parameter, λi.
$${N}_{i}\sim {\text{Poisson}}(\lambda )$$(3) -
2.
Observation state. The repeated counts, cij, of the local population, Ni, follow a binomial distribution with parameter Ni and success parameter pij.
$${C}_{ij}\cdot |\cdot {N}_{i}\sim {\text{Binomial}} ({N}_{i},{p}_{ij})$$(4)
As suggested by Royle (2004), we determined Ni per block by multiplying the mean abundance (λ) per camera trap station by the number of sites per block. Abundance covariates and detectability covariates were modeled using the log-linear function according to Royle (2004) and Fiske and Chandler (2011):
where p is detection probability at site “i” and occasion “j.” We modeled abundance as a function by varying all possible combinations of covariates for roan and sable including null models (Chaudhuri et al. 2022). For roan in Blocks B and A, we fitted four candidate models with the most parametrized models being λ (termite mounds + grass cover) ρ(“.”) and (risk of predation + grass cover) ρ(“.”), respectively. For sable in Blocks B and A, we fitted four models with the most parametrized model being λ(grass cover + vegetation diversity) ρ(“.”) and λ(vegetation diversity + grass cover) ρ(“.”), respectively. ρ(“.”) indicates a constant detection probability. The goodness-of-fit of the global models was assessed using the parboot () function of the unmarked package (MacKenzie and Nichols 2004). Model identifiability was assessed using K, which is the upper bound for discrete integration used by maximum likelihood to get the right estimates of detection probability and abundance (Kéry 2018). We showed that models were valuable by being insensitive to three values of K (K = 100, 150, and 200) as the Akaike Information Criterion corrected for small sample size (AICc) remained the same (Kéry 2018).
We used the AICc to rank candidate models based on the differences in the AICc values (ΔAICc) (Burnham and Anderson 2002). Models having ΔAICc ≤ 2 were considered to be strongly supported by the data (Burnham and Anderson 2002). In such cases, we applied model averaging with shrinkage using the modavgshrink () function in AICcmodavg package (Campos-Cerqueira et al. 2021). We estimated 95% confidence intervals for the regression coefficients of covariates in the best or averaged model and considered intervals that did not overlap zero to signal a strong impact on roan and sable abundance (Burnham and Anderson 2002; Lamichhane et al. 2020). All continuous variables were found not to be highly associated (Pearson ≥ 0.45) hence retained (Supplementary Information 1, Table S1).
Results
The time in front of the camera (TIFC) model
Three camera sites were excluded because of camera theft. A sampling effort of 46 camera traps resulted in 2054 camera trap nights and a total camera deployment time is presented in Table 1. There were 283 independent photographs of roan from 23 survey stations and 222 independent photographs of sable from 20 survey stations (Table 1). TIFC model density estimates showed that MNP had fewer roans than sables (Fig. 4d).
Mean abundance (and 95% confidence intervals (CI)) of roan (a) and sable (b) per Blocks B and A, total abundance (c) from the Poisson-binomial N-mixture model, and density estimates (and 95% CI) of roan and sable (d) from the time in front of the camera model in the Mudumu National Park, northeastern Namibia
Poisson-binomial N-mixture model
A sampling effort of 1024 camera trap nights across 24 camera sites in the wet season (Block B) was achieved while 1030 camera trap nights across 22 camera sites (Block A; three sites were excluded) in the dry season was achieved. The roan was detected at 13 survey stations in the wet season and at 10 in the dry season. The sable was detected at nine survey stations in the wet season and at 11 in the dry season. The estimated detection probability for roan in the wet and dry seasons were ρ = 0.03, 95% CI = 0.02–0.06 and ρ = 0.03, 95% CI = 0.02–0.05, respectively. The estimated detection probability for sable in the wet and dry seasons were ρ = 0.02, 95% CI = 0.01–0.04 and ρ = 0.005, 95% CI = 0.002–0.02, respectively. The mean roan abundance estimates per Block was the same between Blocks B and A (Fig. 4a), but mean sable abundance was higher in Block A than in Block B (Fig. 4b). Overall roan and sable abundance in Blocks B and A were 57 and 242, respectively (Fig. 4c, Table 2).
Influence of site covariates on roan and sable abundance
In both Blocks B and A, higher roan abundance occurred in sites with higher grass cover (\(\underset{\_}{\beta }\) = 0.03, 95% CI = 0.01–0.05, Fig. 5a) and (\(\underset{\_}{\beta }\) = 0.01, 95% CI = − 0.01–0.02, Fig. 5b), respectively. In Block B, higher sable abundance occurred in sites with higher vegetation diversity (\(\underset{\_}{\beta }\) = 0.91, 95% CI = − 0.38–2.19, Fig. 5c). In turn, in Block A, higher sable abundance occurred in sites with lower grass cover (\(\underset{\_}{\beta }\) = − 0.02, 95% CI = − 0.04–0.00, Fig. 5d) and lower vegetation diversity (\(\underset{\_}{\beta }\) = − 0.30, 95% CI = − 1.1–0.50, Fig. 5e). Roan abundance was influenced by grass cover in Block B and may not have been in Block A as AICc ≤ 2 between the null and next model (Table 3). Sable abundance was influenced by vegetation diversity in Block B as well in Block A in addition to grass cover (Table 4).
Model-averaged abundance estimates of roan as a function of grass cover in Blocks B (a) and A (b); abundance of sable as a function of vegetation diversity in Block B (c); grass cover (d) in Block A; and vegetation diversity (e) in Block A in the Mudumu National Park, northeastern Namibia. All relationships were obtained from abundance models with ΔAICc < 2. Shaded regions indicate 95% confidence intervals
Discussion
Here, we provide the first camera trapping-based density and abundance estimates of roan and sable within their natural range at Mudumu National Park in northeastern Namibia, while the derived abundance and density estimates based on the TIFC and PB models were in general low which is consistent with the trend reported across Africa (Estes 1991; Grant et al. 2002; Martin 2003; Havemann et al. 2016). The density of 1.62 (95% CI 1.61–1.64) roan/km2 and 2.46 (95% CI 2.42–2.50) sable/km2 in the MNP are significantly lower than density estimates of both species reported in other national parks along their distribution, which range from 3.32 to 108.25 roan/km2 and 23.76 sable/km2 in Kruger National Park (KNP), South Africa (Supplementary Information 2, Table S2; Grant et al. 2002; Oladipo et al. 2019). Our study supports other unpublished work in the MNP finding that roan and sable appear to occur naturally at low densities and abundances (Supplementary Information 2, Table S2; Martin 2003; NACSO, 2023), even though roan had an increasing population trend and the sable a stable trend from 1980 to 2019 (Nauyoma 2023, submitted). Our study points to important relationships between habitat covariates and abundance for both species. The lower density and abundance estimates for both roan and sable in this study could be the result of several factors. No previously published density and abundance estimates for roan and sable were available, leaving open the possibility that they may occur naturally at low densities and abundances in the MNP. Some previous studies of these species outside Namibia have also shown low densities and abundances in their natural range (Supplementary Information 2, Table S2; Allsopp 1979; Van Lavieren and Esser 1980; Milligan et al. 1982). The ecological carrying capacity, K, of the MNP is unknown, so it is impossible to determine the size of the roan and sable populations that can be supported indefinitely by the resources available in the MNP. This is an area for future research. In addition, these large mammals are K-selected species, meaning that their population size will increase until it gradually reaches ecological K, where it will remain for several years (Bowyer et al. 2014; McCullough 1999). K-selected species have long gestation periods of several months, slow maturation, and thus provide extended parental care to their offspring, almost all of which survive and have few offspring (Bowyer et al. 2014; McCullough 1999).
The PB model showed higher roan abundance in sites with higher grass cover, supporting a similar finding by Nauyoma (2023, submitted), that roans in the MNP were more likely to occupy sites with higher grass cover. Overall, our finding is consistent with previous studies in other parts of roan range that showed a positive relationship between roan occupancy and grassland for foraging (Joubert 1976; Perrin and Taolo 1998; Schuêtte et al. 1998; Harrington et al. 1999; Martin 2003; Kimanzi 2011). The presence of tall grass species such as A. stipitata and A. meridionalis may also contribute to increased calf survival as both roan and sable hide their calves in these tall grasses during the calving period, which occurs at any time of the year (Martin 2003; Kimanzi 2011). This finding is backed by roan being predominantly a grazer (Martin 2003; Havemann et al. 2016).
We found higher sable abundance in sites with higher vegetation diversity during the wet season. Variation in vegetation suggests that sable may be browsing during the rainy season and possibly avoiding interspecific competition with roan, which was more abundant in areas with higher grass cover (Martin 2003). In addition, Martin (2003) observed that sables are unable to cope with superabundant grass in good rainfall years and grass swards are underutilized, resulting in favorable conditions for tick irruptions. In contrast, we found higher sable abundance in areas with lower grass cover and lower vegetation diversity in the dry season. Predators, such as leopards and lions, are known to avoid areas with less vegetation cover considering their ambush predation type suggesting that sables may have avoided predator encounters in the dry season (Owen-smith 2019). In addition, higher roan abundance in sites with higher grass cover, as opposed to higher sable abundance in sites with lower grass cover, may indicate a lack of resource competition between the two antelope species in the MNP. Bianchi (1991) suggested that the distribution of sable in the Masebe Nature Reserve in South Africa may be related to avoidance of competition with other grazers such as impala, or to avoidance of predators. Overlap in resource use between ungulates increases interspecific competition for resources such as food, which affects reproduction and overall intrinsic growth rate (births, deaths, immigration and emigration) (Murray and Illius 2000; Mishra et al. 2004). Resource competition is an important density-dependent factor that has been shown to control population size in ungulates (Skogland 1985; Mishra et al. 2004; Bowyer et al. 2014).
The PB model produced low detection probabilities (< 0.5), which are known to produce biased abundance estimates than other methods such as capture-recapture and distance sampling (Couturier et al. 2013). We consider that the low detection probabilities of roan and sable that we obtained in the MNP are a result of the uncommonness of these species in the study area. The assumptions of the TIFC and PB models were met, and we therefore interpreted the abundance estimates as a measure of absolute abundance rather than relative abundance (Gilbert et al. 2021; Chaudhuri et al. 2022; Becker et al. 2022). Despite the limitations of these methods, we encourage the widespread use of the TIFC and PB models to fill knowledge gaps about rare roan, sable, and other species inhabiting densely vegetated or remote terrain in Namibia and elsewhere in the world to help steer their preservation and management.
Conclusion
The density estimates of roan and sable in the MNP are lower than density estimates reported elsewhere in Africa (KNP and Kainji Lake National Park in Nigeria), backing the species national conservation status. It is clear from our study that roan abundance is most likely driven by higher grass cover, suggesting that this resource may contribute to the persistence of roan. Therefore, potential management actions to support roan populations include the creation of grassland habitat. Although there are existing grasslands in the MNP, additional grasslands may be beneficial to increase roan population size. Grassland creation should take into account the habitat requirements of other herbivores, such as sable, which were more abundant in sites with less grass cover in this study. Current management of both species and other ungulates in the MNP includes dry season prescribed fires by management, which have been shown to result in reduction of moribund material, recycle nutrients, regenerate palatable grasses, provide wildlife habitat by controlling bush encroachment, and prevent severe fire hazards by reducing fuel loads such as dead trees and shrubs (Scholes and Walker 1993; Du Plessis 1997; Fabiano et al. 2020). We recommend that MNP’s fire management plan incorporate the habitat requirements identified in this study for roan and sable. For example, Park managers could use existing roads in the Park as firebreaks to control which areas are burned and introduce an annual rotation system of burning areas in the park (Du Plessis 1997). This could allow roan and sable to thrive in the Park by not burning all the habitats in the Park at once. Other active fire management could include rangers burning vegetation ahead of the fire to create a buffer and control which areas are burned, and extinguishing or containing lightning fires to the smallest area possible (Turner et al. 2022). Park management should work with MNP's neighboring community-managed conservancies to ensure that fires do not cross jurisdictional boundaries. Public education is important to prevent fires being started by people, either deliberately or accidentally, and if the smoke becomes too extreme and affects Park visitors and nearby communities, rangers can cool areas of fire with water and extinguish them as quickly as possible (Du Plessis 1997).
Data availability
No datasets were generated or analysed during the current study.
References
Alfeus M (2022) An assessment of trends in population abundance and spatial distribution of roan antelope (Hippotragus equinus) and sable antelope (Hippotragus niger) in the Greater Waterberg Plateau Complex, north-central. Thesis, University of Namibia, Namibia
Allsopp R (1979) Roan antelope population in the Lambwe Valley. Kenya J Appl Ecol 16:109–115
Ampoorter E, Barbaro L, Jactel H et al (2019) Tree diversity is key for promoting the diversity and abundance of forest-associated taxa in Europe. Oikos 129:33–146
Anderson TM, White S, Davis B et al (2016) The spatial distribution of African savannah herbivores: species associations and habitat occupancy in a landscape context. Philos Trans R Soc Lond B Biol Sci 371:20150314
Ansell WHF (1971) Order Actilodactyla. In: Meester J, Setzer HW (editors); The mammals of Africa: an identification manual. Smithsonian Institution Press, Washington
Becker M, Huggard DJ, Dickie M et al (2022) Applying and testing a novel method to estimate animal density from motion-triggered cameras. Ecosphere 13:e4005
Bianchi MC (1991) The ecology of the sable antelope Hippotragus niger niger (Harris 1838) in the Masebe Nature Reserve. Thesis, University of Pretoria, Lebowa
Bowyer RT, Bleich VC, Stewart KM et al (2014) Density dependence in ungulates: a review of causes, and concepts with some clarifications. CFWJ 100:550–572
Brennan A, Beytell P, Aschenborn O et al (2020) Characterizing multispecies connectivity across a transfrontier conservation landscape. J Appl Ecol 57:1700–1710
Burnham KP, Anderson DR (2002) Model selection and multi-model inference: a practical information-theoretic approach. Springer, Berlin
Burton AC, Neilson E, Moreira D et al (2015) Wildlife camera trapping: a review and recommendations for linking surveys to ecological processes. J Appl Ecol 52:675–685
Campos-Cerqueira M, Robinson WD, Leite GA et al (2021) Bird occupancy of a neotropical forest fragment is mostly stable over 17 years but influenced by forest age. Diversity 13:50
Camera Trap V7 User Guide (2020) Camera Trap V7. https://panthera.org/. Accessed 11 September 2020
Chaudhuri S, Rajaraman R, Kalyanasundaram S et al (2022) N-mixture model-based estimate of relative abundance of sloth bear (Melursus ursinus) in response to biotic and abiotic factors in a human-dominated landscape of central India. PeerJ 10:e13649
Couturier T, Cheylan M, Bertolero A et al (2013) Estimating abundance and population trends when detection is low and highly variable: a comparison of three methods for the Hermann’s tortoise. J Wildl Manage 77:454–462
Dail D, Madsen L (2011) Models for estimating abundance from repeated counts of an open metapopulation. Biom J 67:57–587
Davis KL, Silverman ED, Sussman AL et al (2022) Errors in aerial survey count data: identifying pitfalls and solutions. Ecol Evol 12:e8733
Du Plessis W (1997) Refinements to the burning strategy in the Etosha National Park, Namibia. Koedoe 40:63–76
Estes RD (1991) Horse Antelopes: tribe Hippotragini. In: Estes RD (ed) The behavior guide to African mammals. University of California Press, California, pp 115–122
Fabiano CE, Klingelhoeffer E, Simon A (2020) Current status of key biodiversity and research gaps in the Mudumu National Park. University of Namibia, Windhoek
Finn C, Grattarola F, Pincheira-Donoso D (2023) More losers than winners: investigating Anthropocene defaunation through the diversity of population trends. Biol Rev 98:1732–1748
Fiske I, Chandler R (2011) Unmarked: an R package for fitting hierarchical models of wildlife occurrence and abundance. J Stat Softw 43:1–23
Gaillard C, Langan L, Pfeiffer M et al (2018) African shrub distribution emerges via a trade-off between height and sawood conductivity. J Biogeogr 45:2815–2826
García-Marmolejo G, Chapa-Vargas L, Weber M et al (2015) Landscape composition influences abundance patterns and habitat use of three ungulate species in fragmented secondary deciduous tropical forests. Mexico Glob Ecol Conserv 3:744–755
Gilbert NA, Clare JDJ, Stenglein JL et al (2021) Abundance estimation of unmarked animals based on camera-trap data. Conserv Biol 35:88–100
Grant CC, Davidson T, Funston PJ et al (2002) Challenges faced in the conservation of rare antelope: a case study on the northern basalt plains of the Kruger National Park. Koedoe 45:45–66
Gray TNE (2017) Monitoring tropical forest ungulates using camera-trap data. J Zool 305:173–179
Harrington R, Owen-Smith N, Viljoen PC et al (1999) Establishing the causes of the roan antelope decline in the Kruger National Park. South Africa Biol Conserv 90:69–78
Harris GM, Butler MJ, Stewart DR et al (2020) Accurate population estimation of Caprinae using camera traps and distance sampling. Sci Rep 10:17729
Havemann CP, Retief TA, Tosh CA et al (2016) Roan antelope Hippotragus equinus in Africa: a review of abundance, threats and ecology. Mamm Rev 46:144–158
IUCN Species Survival Commission Antelope Specialist Group (2017) Hippotragus niger. The IUCN Red List of Threatened Species. https://doi.org/10.2305/IUCN.UK.2017-2.RLTS.T10170A50188654.en.Accessed11September2020
Josling GC, Lepori AA, Neser FWC et al (2019) Evaluating horn traits of economic importance in sable antelope (Hippotragus niger niger). S Afr J Anim Sci 49:41–49
Joubert SCJ (1976) The population ecology of the roan antelope, Hippotragus equinus equinus (Desmarest, 1804), in the Kruger National Park. Thesis, University of Pretoria, D.Sc
Kéry M (2018) Identifiability in N-mixture models: a large-scale screening test with bird data. Ecol 99:281–288
Kimanzi JK (2011) Mapping and modelling the population and habitat of the roan antelope (Hippotragus equinus langheldi) in Ruma National Park, Kenya. Dissertation, Newcastle University
Lamichhane S, Khanal G, Karki JB et al (2020) Natural and anthropogenic correlates of habitat use by wild ungulates in Shuklaphanta National Park. Nepal Glob Ecol Conserv 24:e01338
Le Roux P, Müller M, Mannheimer C et al (2018) Trees and shrubs of Namibia. Namibia Publishing House (Pty) Ltd, Windhoek
Leggett KE (2006) Home range and seasonal movement of elephants in the Kunene Region, northwestern Namibia. J Afr Zool 41:17–36
Mace GM, Collar NJ, Gaston KJ et al (2008) Quantification of extinction risk: IUCN’s system for classifying threatened species. Conserv Biol 22:1424–1442
MacKenzie DI, Nichols JD (2004) Occupancy as a surrogate for abundance estimation. Anim Biodivers Conserv 27:461–467
Marshal CP, Rankin C, Nel HP et al (2016) Drivers of population dynamics in sable antelope: forage, habitat or competition? Eur J Wildl Res 62:549–556
Martin RB (2003) Species report for roan, sable and tsessebe. Ministry of Environment and Tourism, Windhoek
Mayengo G, Piel AK, Treydte AC (2020) The importance of nutrient hotspots for grazing ungulates in a Miombo ecosystem. Tanzania Plos One 15:e0230192
McCollum KR, Belinfonte E, Conway AL et al (2018) Occupancy and habitat use by six species of forest ungulates on Tiwai Island. Sierra Leone Koedoe 60:a1484
McCullough DR (1999) Density dependence and life-history strategies of ungulates. J Mammal 80:1130–1146
Mendelsohn J, Roberts C (1997) An Environmental profile and atlas of Caprivi. Gamsberg Macmillan, Windhoek
Milligan K, Ajayi SS, Hall JB (1982) Density and biomass of the large herbivore community in Kainji Lake National Park. Nigeria Afr J Ecol 20:1–12
Mishra C, Van Wieren SE, Ketner P et al (2004) Competition between domestic livestock and wild bharal Pseudois nayaur in the Indian Trans-Himalaya. J Appl Ecol 41:344–354
Mobæk R, Narmo AK, Moe SR (2005) Termitaria are focal feeding sites for large ungulates in Lake Mburo National Park. Uganda J Zool 267:97–102
Murray MG, Illius AW (2000) Vegetation modification and resource competition in grazing ungulates. Oikos 89:501–508
Naidoo R, Beytell P, Brennan A et al (2022) Challenges to elephant connectivity from border fences in the world’s largest transfrontier conservation area. Front Conserv Sci 3:788133
Nakashima Y (2019) Potentiality and limitations of N-mixture and Royle-Nichols models to estimate animal abundance based on noninstantaneous point surveys. Popul Ecol 62:151–157
Nakashima Y, Fukasawa K, Samejima H (2017) Estimating animal density without individual recognition using information deliverable exclusively from camera traps. J Appl Ecol 55:735–744
Namibian Association of CBNRM Support Organisations (NACSO) (2023) Game Count Posters. https://www.nacso.org.na/resources/game-count-poster
Nauyoma LT (2023) Assessment of the conservation status of roan (Hippotragus equinus) and sable (Hippotragus niger) using ecological and genetic parameters in the Mudumu National Park, Namibia. Dissertation, University of Namibia
O’Connell AF, Nichols JD, Karanth KU (2011) Camera traps in animal ecology: methods and analyses. Springer, New York
Oladipo OS, Folorunso AA, Lewiska LF et al (2019) Population density, diversity and abundance of antelope species in Kainji Lake National Park. Nigeria J Ecol 9:92208
Owen-Smith N (2019) Ramifying effects of the risk of predation on African multi-predator, multi-prey large-mammal assemblages and the conservation implications. Biol Conserv 232:51–58
Pal R, Bhattacharya T, Qureshi Q et al (2021) Using distance sampling with camera traps to estimate the density of group-living and solitary mountain ungulates. Oryx 55:668–676
Perrin MR, Taolo C (1998) Home range, activity pattern and social structure of an introduced herd of roan antelope in KwaZulu-Natal. South Africa s Afr J Wildl 28:27–32
Phumanee W, Steinmetz R, Phoonjampa R et al (2020) Occupancy-based monitoring of ungulate prey species in Thailand indicates population stability, but limited recovery. Ecosphere 11:e03208
R Development Core Team (2022) R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing. http://www.Rproject.org/. Accessed 11 September 2022
Rich LN, Miller DAW, Muñoz DJ et al (2019) Sampling design and analytical advances allow for simultaneous density estimation of seven sympatric carnivore species from camera trap data. Biol Conserv 233:12–20
Rowcliffe JM, Field J, Turvey ST et al (2008) Estimating animal density using camera traps without the need for individual recognition. J Appl Ecol 45:1228–1236
Royle JA (2004) N-mixture models for estimating population size from spatially replicated counts. Biom J 60:108–115
Schlettwein CH, Simmons RE, McDonald A et al (1991) Flora, fauna and conservation of East Caprivi wetlands. Modoqua 17:67–76
Schlossberg S, Chase MJ, Griffin CR (2016) Testing the accuracy of aerial surveys for large mammals: an experiment with African savanna elephants (Loxodonta africana). PLoS ONE 11:1–19
Scholes RJ, Walker BH (1993) An African savanna: synthesis of the Nylsvley study. Cambridge University Press
Schuêtte JR, Leslie DM Jr, Lochmiller RL et al (1998) Diets of hartebeest and roan antelope in Burkina Faso: support of the long-faced hypothesis. J Mammal 79:426–436
Sinclair ARE (1985) Does interspecific competition or predation shape the African ungulate community? J Anim Ecol 54:899–918
Singh PB, Buckingham DL (2015) Population status and habitat ecology of Bristled Grassbird Chaetornis striata in Chitwan National Park, central Nepal. Forktail 31:87–91
Skogland T (1985) The effects of density-dependent resource limitations on the demography of wild reindeer. J Anim Ecol 54:359–374
Stohlgren TJ, Falkner M, Schell LD (1995) A modified-Whittaker nested vegetation sampling method. Plant Ecol 117:113–121
Stoldt M, Göttert T, Mann C et al (2020) Transfrontier conservation areas and human-wildlife conflict: the case of the Namibian component of the Kavango-Zambezi (KAZA) TFCA. Sci Rep 10:7964
Turner WC, P´eriquet S, Goelst CE, et al (2022) Africa’s drylands in a changing world: challenges for wildlife conservation under climate and land-use changes in the Greater Etosha Landscape. Glob Ecol Conserv 38:e02221
Van Lavieren LP, Esser JD (1980) Numbers, distribution and habitat preference of large mammals in Bouba Ndjida National Park. Cameroon Afr J Ecol 18:141–153
Warbington CH, Boyce MS (2020) Population density of sitatunga in riverine wetland habitats. Glob Ecol Conserv 24:e01212
Western D, Mose VN (2021) The changing role of natural and human agencies shaping the ecology of an African savanna ecosystem. Ecosphere 12:e03536
Williams BK, Nichols JD, Conroy MJ (2002) Estimating abundance for closed populations with mark-capture methods. Pp. 289 – 332 in Analysis and management of animal populations. Academic Press
Acknowledgements
We thank the Namibian Directorate of Wildlife and National Parks for permission to conduct this study and for logistical support. Many thanks to the staff of Mudumu National Park and members of the Namibian Police and Defence Forces for their support during the fieldwork. We thank the National Commission on Research Science and Technology for granting us permission to conduct field research in Namibia (certificate number: RCIV00022018). We acknowledge the contributions of Prof. M. Hipondoka, Prof. A. Peters, Prof. Y. Nakashima, Mr. W. Tjipueja, Ms. S. Shihwandu, Ms. E. Shilumbu, Mr. B. Ndana, Mr. B. Zingolo, Mashi Conservancy, Idea Wild, National Botanical Research Institute, and Cheetah Conservation Fund.
Funding
Open access funding provided by University of Namibia. PhD student L. T. Nauyoma received financial support from the Partnership between the Universities of Namibia and of Bonn and the Namibia Students Financial Assistance Fund.
Author information
Authors and Affiliations
Contributions
L.T.N, E.C.F, F.G.L, F.C.A, F.S conceived the idea. E.C.F, F.G.L, F.S supervised the research; L.T.N, E.C.F collected data; L.T.N, E.C.F, C.H.W analyzed data; L.T.N wrote the manuscript; E.C.F, F.G.L, F.S, F.C.A, C.H.W edited the manuscript. All authors contributed critically to the drafts and gave final approval for publication.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nauyoma, L.T., Warbington, C.H., Azevedo, F.C. et al. Density and abundance estimation of unmarked ungulates using camera traps in the Mudumu National Park, Namibia. Eur J Wildl Res 70, 28 (2024). https://doi.org/10.1007/s10344-024-01783-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10344-024-01783-6