Abstract
The Cantabrian brown bear (Ursus arctos) population is threatened although in a constant process of recovery during the last 20 years. Since data on the parasitological status of this bear is still limited, the objective of the present study was to assess the diversity and prevalence of parasites in this population. Thus, 111 bear faecal samples were collected in north-western Spain and analysed for estimating the occurrence of gastrointestinal and bronchopulmonary parasites. Samples were processed by flotation in saline and sucrose solution, sedimentation and Baermann-Wetzel techniques. In addition, a commercial immunofluorescent assay was performed for detecting Giardia duodenalis and Cryptosporidium spp. Dicrocoelium dendriticum was the most prevalent parasite (58.6%), followed by Baylisascaris transfuga (43.2%) and nematodes of the Suborder Strongylida (18.9%) and Spirurida (2.7%). Mixed infections were detected in the 41.4% of the samples. The presence of D. dendriticum was significantly highest in bears from the autonomous region of Castile and León as well as in those in which grass or nuts/acorns were the predominant food item. Moreover, the risk of being positive to B. transfuga was significantly higher during autumn–winter, and in those, faecal samples were mainly composed of fleshy fruit. Some of the parasites detected could infect other wildlife and even humans, and therefore, the risk of pathogen transmission deserves further investigation. Since the impact of endoparasites in the health status of bears is poorly understood, the establishment of a disease surveillance protocol is strongly recommended in order to assess the potential risk of these infections for bears.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Brown bear (Ursus arctos) populations in Europe have been showing a positive trend in recent decades (Chapron et al. 2014; Linnell and Cretois 2018; Cimatti et al. 2021). Based on the last data compiled from all European countries (period 2012–2016), between 15,000 and 16,000, brown bears are estimated in continental Europe (excluding Russia and Belarus) (Linnell and Cretois 2018). Different factors are behind this recovery process, such as conservation and political commitments, law enforcement, institutional arrangements, context-specific management practises favouring coexistence between humans and large carnivores, land abandonment and shifts in land uses, or changes in public acceptance (Chapron et al. 2014; Dressell et al. 2015; Eklund et al. 2017; Cimatti et al. 2021). This is the case of, for example, the endangered brown bear population from the Cantabrian Mountains (northern Spain), which has been showing a positive trend in recent decades (López-Bao et al. 2021). Compared with the six females with cubs detected in 1989, available data from 2017 and 2018 show a minimum annual estimate of 41 and 38 females with cubs, respectively, and it is estimated that more than 320 bears are roaming in these mountains (López-Bao et al. 2021). Among other ecological processes that can be influenced by a recovery in bear populations (e.g., seed dispersal, Lalleroni et al. 2017), the risk of pathogen transmission within bear populations, and among bears, other wildlife species, livestock and humans, may also be influenced (Costa et al. 2022). It has been reported that bears can act as spreaders and reservoirs of a number of bacterial and parasitic pathogens of zoonotic and veterinary concern (Sheikh et al. 2018; Ravaszova et al. 2012; Westmoreland et al. 2017; Sasmal et al. 2019; Di Salvo and Chomel 2020; Masatani et al. 2021). Although information on the parasitofauna of some European bear populations is relatively abundant, especially those from northern Europe (De Ambrogi et al. 2011; Ravaszova et al. 2012; Borecka et al. 2013; Aghazadeh et al. 2015; Orosová et al. 2016; Gawor et al. 2017; Reljic et al. 2017; Strkolcova et al. 2018; Molnar et al. 2020), available data about other less numerous populations are limited, such as the case of Mediterranean and isolated bear populations (Paoletti et al. 2017; Mariacher et al. 2018; Costa et al. 2022). In the case of Cantabrian bears, only one study reported the presence of gastrointestinal parasites (Costa et al. 2022). However, the number of samples included in the study was low.
The objectives of the present study were to complement previous studies on gastrointestinal and bronchopulmonary parasites in Cantabrian brown bears from the western area of the Cantabrian Mountains. Since data on the presence of zoonotic protozoans such as Cryptosporidium spp. and Giardia duodenalis in brown bears are currently limited, the presence of these parasites was studied for the first time in this bear population. In addition, the possible influence of some variables (season, autonomous region, predominant food found in faeces and co-infection with other parasites) on the presence of the detected pathogens was assessed.
Methods
Collection of bear faeces
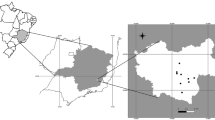
This investigation was carried out in north-western Spain, covering an area of approximately 2200 km2. The study area is mainly covered by mixed temperate deciduous forest and other natural and semi-natural habitats, all with a long history of human use.
Transects were carried out in areas with a high probability of the presence of bears to collect bear faeces. Faecal samples were collected by specialised rangers from the Brown Bear Foundation, as well as a researcher from the INVESAGA Group from the Faculty of Veterinary Sciences of the University of Santiago de Compostela, a native of the bear zone of the Cantabrian Mountains and which was trained by members of the Brown Bear Foundation in the recognition and evaluation of the degree of freshness of the faeces found in the field. The evaluation of the degree of freshness of the faeces was made according to the distinctive colour and appearance of a fresh excrement due to the experience of the Brown Bear Foundation rangers. Only faeces with a high degree of freshness were collected.
A total of 111 fresh faecal samples were collected in the field in three autonomous regions (Asturias n = 69, Galicia n = 8 and Castile and León n = 34) between September 2021 and September 2022 (Fig. 1). Bear scats were introduced in 100 ml sterile containers, and only the portion of faeces not in contact with the soil was collected in order to decrease the probability of contamination by free-living nematodes which make the subsequent identification of parasitic nematodes more difficult during the microscopic examination. To reduce the likelihood of sampling the same individual in a single sampling day, only fresh faeces were taken from locations separated by at least 2 km2. This distance was estimated based on the mean daily movement of bears in Europe (Mertzanis et al. 2005; Pop et al. 2018). Information on the location of the samples (coordinates) and the date of collection was also gathered. All samples were stored at 4 °C for a maximum period of one week until processed. In the laboratory, faeces were classified into five categories (nuts/acorns, fleshy fruits, grass, meat, or honey) according to the predominant food traces found in the faeces (nuts/acorns, fruit skin and seeds of fleshy fruits, grass, bones or hair of preys or bee hive wax). Due to their low frequency (n = 4), those samples belonging to the categories “meat” and “honey” were included in a single category identified as “others” for subsequent analyses. In addition, a macroscopic examination of the faeces was performed in order to detect the presence of parts of adult nematodes or especially cestode proglottids since the sensitivity of coprological techniques for detecting cestode eggs is low (Deplazes et al. 2016).
Map of north-western Spain (Galicia, Asturias and Castile and León provinces). Bear silhouette indicates the points where bear faecal samples were collected from the environment. Basemap modified from Centro Nacional de Información Geográfica (MINISTERIO DE FOMENTO, https://datos.gob.es/en/catalogo/e00125901-spaignllm) using the software Inkscape (https://inkscape.org).
Coprological analysis
All samples were examined using different copromicroscopic methods. A quantitative sedimentation technique was used for detecting the presence of trematode eggs in 5 g of faeces (Foreyt 2001), and the Baermann-Wetzel technique was used for identifying and quantifying metastrongyloid first stage larvae in 10 g of faeces (MAFF 1986). For detecting the presence of gastrointestinal nematodes, cestodes and protozoans, the flotation technique was carried out using 3 g of faeces and 42 ml of water. After filtering the sample through a mesh with a 150 µm pore diameter, two 15 ml tubes were filled and centrifuged at 680 × g during 5 min; the supernatant was discarded. Parasitic forms were concentrated from the sediment of one tube adding Sheather’s sucrose solution (specific gravity 1.28 g/ml) until forming a convex meniscus; a cover slip was placed onto the meniscus. Finally, the tube was centrifuged at 680 × g during 5 min, and the cover slip was placed on a slide and examined at 100–400 × magnification. Since this flotation method is not quantitative, saturated saline solution (specific gravity 1.19 g/ml) was added to the sediment of the other tube and parasite shedding was assessed using a McMaster chamber. In addition, 3 g of each faecal sample was homogenised, filtered and centrifuged as previously indicated, and 10 µl of the sediment was analysed by a commercial immunofluorescent assay (IFAT) for the detection of G. duodenalis cysts and Cryptosporidium oocysts (Aqua-Glo G/C Direct Comprehensive Kit; Waterborne Inc, New Orleans, LA, USA) following the manufacturers’ instructions. This test has been employed for detecting both protozoans in several wildlife species, allowing finding Cryptosporidium oocysts in Black bears (Ursus americanus) (Li et al. 2020).
Statistical analysis
All statistical analyses were performed using the statistical software R 4.0.3 (R Core Team 2023). Two logistic regressions were used for assessing the influence of different variables on the probability of a sample being positive for Dicrocoelium dendriticum and Baylisascaris transfuga. Other parasites detected were not analysed due to their low occurrence. Factors included in the analysis were season (2 levels: spring–summer and autumn–winter), autonomous region (3 levels: Galicia, Asturias and Castile and León), predominant food traces found in the faeces (4 levels: nuts/acorns, fleshy fruits, grass and others) and the co-infection with other parasites (2 levels: absence and presence). For selecting the most parsimonious models, predictors were sequentially eliminated using a backward and forward conditional taking into account the AIC value (Akaike Information Criterion); all pairwise interactions were evaluated. The glm() and exp(coef())/exp(confint()) functions were used to perform the logistic analyses and to calculate the odds ratio values (R Core Team 2023).
Results
At least one parasitic form was detected in 88 out of the 111 (79.3%) analysed bear faeces (Table 1). Dicrocoelium dendriticum was the predominant parasite detected in brown bears (58.6%; 65/111), followed by B. transfuga (43.2%; 48/111). In addition, eggs morphologically compatible with gastrointestinal nematodes of the Suborder Strongylida were found in 21 samples (18.9%). Finally, eggs of Spirurida were identified in three samples (2.7%). All samples tested negative to G. duodenalis, Cryptosporidium spp. and Metastrongylidae bronchopulmonary nematodes. Regarding the intensity of egg shedding, the mean egg counts were highest for B. transfuga (720.8 ± 1934.9 epg), followed by Strongylida (166.7 ± 262.0 epg) and D. dendriticum (6.40 ± 16.2 epg). No more than two Spirurida eggs were detected in each positive sample; however, the egg shedding could not be estimated since these samples were only positive to flotation in sucrose solution which is not a quantitative technique but more sensitive than the flotation in saturated saline solution. Mixed infections were detected in 46 out of 111 samples, being the most common D. dendriticum + B. transfuga followed by D. dendriticum + Strongylida (Table 1). The co-infection B. transfuga and Strongylida was only found in three specimens, and D. dendriticum + Spirurida was identified in a single sample (Table 1). In addition, a triple infection D. dendriticum + B. transfuga + Strongylida was detected in three samples (Table 1).
The probability of being infected with D. dendriticum was 10.9 and 5.9-fold higher in Castile and León and Asturias than in Galicia, respectively (Tables 1 and 2). In addition, the risk of being positive to this trematode was also significantly higher in those animals in which nuts/acorns or grass was the predominant food traces found in the bear faeces when compared to those in which fleshy fruits were mainly detected (Tables 1 and 2). Bears infected with nematodes also had a significant higher risk for being positive (OR = 2.7) for D. dendriticum (Tables 1 and 2). In contrast, the presence of B. transfuga was significantly highest during autumn–winter (OR = 6.0) (Tables 1 and 2); the risk of being positive to B. transfuga was 5.7-fold higher in samples in which fleshy fruits predominated than in those in which grass was mainly detected (Tables 1 and 2). Taking into account the AIC value, the variables season and sampling area were not included in the final model of the probability of being infected with D. dendriticum and B. transfuga, respectively.
Discussion
Our results revealed that infections with gastrointestinal parasites are common in Cantabrian brown bears, being consistent with previous results showing a 71% of positive samples from the same bear population (Costa et al. 2022). These results are among the highest in European brown bears together with those reported in Slovakia (Orosová et al. 2016) and Croatia (Reljic et al. 2017). Most investigations on parasitic infections carried out in bears from Europe showed percentages of infections ranging from 30 to 60%, such as those performed in Slovakia (Ravaszova et al. 2012; Strkolcova et al. 2018; Molnar et al. 2020), Italy (Paoletti et al. 2017), Croatia (Aghazadeh et al. 2015) or Romania (Borka-Vitalis et al. 2017). It is also worth noting that occurrence rates lower than 20% were detected in a limited number of studies from Poland (Gawor et al. 2017), Finland (Lavikainen et al. 2011) and Croatia (De Ambrogi et al. 2011). The differences observed between investigations could be due to the diagnostic techniques and the type of sample used. In this regard, percentages of infection were usually higher in those studies using post-mortem examination (Reljic et al. 2017; Strkolcova et al. 2018) than in those using faeces collected from the environment (Ravaszova et al. 2012; De Ambrogi et al. 2011; Aghazadeh et al. 2015; Molnar et al. 2020). In addition, differences could be also associated to different rates of infection across bear populations (Aghazadeh et al. 2015) as well as to environmental variables such as temperature, humidity, soil alkalinity and vegetation cover which have a marked influence in the transmission of parasites since they determine the presence of both definitive and intermediate hosts and the survival of environmental stages (Manga-Gonzalez et al. 2001; Johnston et al. 2003; Shaw et al. 2021; Khan et al. 2023).
Four types of parasites were found in Cantabrian brown bears, namely D. dendriticum, B. transfuga, Strongylida and Spirurida nematodes. All these parasites have been previously detected in brown bears from Europe. In fact, B. transfuga is one of the most common parasites in ursids worldwide (Crum et al. 1978; Manville 1978; Conti et al. 1983; Gau et al. 1999; Foster et al. 2004; Orosová et al. 2016; Bugmyrin et al. 2017; Strkolcova et al. 2018; Hwang et al. 2021; Haynes et al. 2023). Baylisascaris transfuga has a direct lifecycle; bears get infected after ingesting embryonated eggs from the environment (Bauer 2013). The ingestion of paratenic hosts containing infective larvae is considered a valid transmission route for some Baylisascaris species, although it was not currently demonstrated in B. transfuga (Bauer 2013). This nematode has been detected in European brown bears with positivity rates ranging from 11.7 to 58.0% (De Ambrogi et al. 2011; Aghazadeh et al. 2015; Borka-Vitalis et al. 2017; Gawor et al. 2017; Paoletti et al. 2017; Molnar et al. 2020), agreeing with the present study. This nematode was not detected previously in Cantabrian brown bears from the Somiedo Natural Park (Costa et al. 2022). In contrast, our data showed that B. transfuga was detected in four of the seven samples collected in the same Natural Park; these marked differences could be related to the collection period since most of our samples were collected during autumn–winter whereas those included in the previous investigation (Costa et al. 2022) were taken during May and August, a period when the prevalence of this ascarid is usually low. Baylisascaris transfuga has a special interest from an ecological point of view since clinical baylisascariosis has been previously reported in bears. Thus, Baylisascaris spp. was identified as the cause of a fatal granulomatous peritonitis in a brown bear from Poland (Szczepaniak et al. 2012), and it was identified as one of the most important cause of deaths in pandas (Ailuropoda melanoleuca) (Zhang et al. 2008; Qin et al. 2021). In addition, this nematode can affect other animal species causing larva migrans (Sato et al. 2004; Hoberg et al. 2018; Jurankova et al. 2022), and its zoonotic potential has been discussed (Sheikh et al. 2018).
Our results also show that both the percentage of B. transfuga-positive samples and egg-shedding were lowest during spring–summer. In this regard, some investigations reported the highest prevalence and intensity of infection of B. transfuga in bears prior to hibernation during winter (Gau et al. 1999; Molnar et al. 2020). Orosová et al. (2016) also observed the highest percentage of samples positive to endoparasites during autumn–winter, suggesting that bears eliminate adult nematodes before hibernation because of reducing their food intake and evacuating their bowels shortly before this period. In addition, it has been suggested that hibernation of bears could negatively affect nematode metabolism (Finnegan 2009). The second risk factor for B. transfuga infection was the predominant food traces found in faeces. Thus, the percentage of positive samples to B. transfuga was lower in faeces in which grass was predominant. This may be related to the seasonal distribution of these samples since most of the faecal samples containing mainly grass were collected during spring–summer (91%; 31/34) when a low number of positive samples was detected.
Dicrocoelium dendriticum was the most prevalent parasite detected in Cantabrian brown bears. However, the egg counts were low, agreeing with the previous study carried out in Spain (Costa et al. 2022). This trematode has an indirect lifecycle; ruminants act as definitive hosts harbouring adult stages in the bile ducts and gall bladder. The external cycle includes two intermediate hosts: land snails and ants. The definitive host gets infected after ingesting ants infected with metacercariae. The prepatent period in ruminants is about seven weeks (Deplazes et al. 2016). It is worth noting that D. dendriticum adults have never been found in bears, and consequently, some authors suggest that, after the consumption of a prey infected with this parasite, lancet fluke eggs just pass through the bear intestinal tract (Borka-Vitalis et al. 2017). Data on the presence of this trematode in brown bears from Europe is currently limited; it has been previously identified in Romania (Borka-Vitalis et al. 2017), Russia (Bugmyrin et al. 2017) and Croatia (Reljic et al. 2017) with percentages of infection ranging from 0.5 to 60%. The absence of information in other European countries may be because the sedimentation technique was not performed in most of the studies. Dicrocoelium dendriticum has a worldwide distribution (Otranto and Traversa 2002) with remarkable differences in its prevalence among areas and hosts (Manga-Gonzalez et al. 2001; Khan et al. 2023). In the present study, the percentage of positive samples to this trematode was significantly higher in Castile and León than in Galicia, which is consistent with the prevalences observed in livestock from the same area. Thus, prevalences of 0.8–8.4% and 37.6–100% were reported in livestock from Galicia and Castile and León, respectively (Manga-Gonzalez et al. 1991; Gonzalez-Lanza et al. 1993; Díaz et al. 2007; Painceira, 2012; Garcia-Dios et al. 2021). These differences could be explained by the presence of alkaline soils in Castile and Leon which are more suitable for the development of D. dendriticum intermediate hosts (Manga-Gonzalez et al. 2001). Moreover, there is evidence of higher populations of D. dendriticum definitive hosts such as sheep and goats in this region compared to Galicia (MAPA 2023).
The positivity rate of this trematode in bears was also higher when grass and nuts/acorns were the predominant food traces in faeces. This could be related to the period when this type of food was detected in the faeces (end of autumn–winter), period when also the highest prevalences and egg shedding were observed in sheep from Spain (Manga-Gonzalez et al. 1991).
In the present study, Strongylida eggs were detected in 18.9% of the samples. Ancylostomatidae eggs were detected in European brown bears from Croatia, Romania, Slovakia and Italy (Aghazadeh et al. 2015; Orosová et al. 2016; Borka-Vitalis et al. 2017; Paoletti et al. 2017) with prevalences ranging from 10.6 to 75.7%. In addition, in a study carried out in Russia two morphological different types of Strongylidae eggs were detected in 11.8% and 5.3% of the studied animals (Bugmyrin et al. 2017). No eggs of Strongylida were previously detected in bears from Spain (Costa et al. 2022) which could be related to their low prevalence and the low number of samples included in that study. Finally, Spirurida nematodes were detected in three bears. To the best of the authors’ knowledge, only a single report of Spirurida nematodes is available in brown bears from Europe, and the percentage of positive samples was also very low (0.5%) (Borka-Vitalis et al. 2017).
No samples positive to Cryptosporidium spp., G. duodenalis nor respiratory nematodes were found. Data on the presence of Cryptosporidium spp. in brown bears is currently limited, showing positivity rates ranging from 8.5 to 55.6% in Croatia (Aghazadeh et al. 2015) and Slovakia (Ravaszova et al. 2012; Orosová et al. 2016). In addition, G. duodenalis cysts were identified in a single study carried out in brown bears from Croatia, reporting a prevalence of 4.2% (Aghazadeh et al. 2015). However, it is important to point out that both protozoa showed an intermittent shedding (Johnston et al. 2003; Shaw et al. 2021), and the prevalence, at least for other host species, is age-dependent, being higher in young animals (Jäger et al. 2005).
No bronchopulmonary nematodes were previously detected in European brown bears except for two studies performed in Romania (Gal 2013; Borka-Vitalis et al. 2017) reporting prevalences of Crenosoma vulpis ranging from 2 to 9.1%. It is important to point out that the technique for detecting Metastrongylidae nematodes requires the existence of motile larvae (MAFF 1986). For this reason, the number of studies including the Baermann–Wetzel technique is low, and it also could justify the low prevalence detected in bears compared to other wild carnivores, such as wolves or foxes (Martinez-Rondan et al. 2019; Estevez-Sanchez et al. 2022).
Other parasites not detected in the present study have also been sporadically found (less than 15%) in brown bears from Europe. In this way, Trichuris spp. was detected in Romania and Spain (Borka-Vitalis et al. 2017; Costa et al. 2022), and coccidia oocysts were found in Croatia, Romania and Slovakia (Aghazadeh et al. 2015; Orosová et al. 2016; Borka-Vitalis et al. 2017). In addition, Syngamus spp. was detected in Croatia (Aghazadeh et al. 2015), Sarcocystis spp. in Slovakia (Orosová et al. 2016), Capillariidae nematodes in Italy (Paoletti et al. 2017; Mariacher et al. 2018) and Taenia spp. in Finland and Romania (Gal 2013; Lavikainen et al. 2011; Borka-Vitalis et al. 2017). It is important to point out that the detection of several of these parasites in faecal samples could be secondary to the intestinal passage and digestion of infected preys as it has been previously suggested (Aghazadeh et al. 2015; Costa et al. 2022). The scarce reports of bears acting as definitive host of Taenia and Sarcocystis species could be due to their omnivore diet; these parasites may be more prevalent in those areas and periods in which meat consumption is higher because other type of food is scarce (Lavikainen et al. 2011; Catalano et al. 2014).
Finally, it is important to point out that this investigation has some limitations. In this way, although an area of 2 km2 was excluded after collecting each sample, it is possible that different fresh samples were collected from the same animal at different times. In addition, the direct collection of the samples from the environment could lead to false negatives since some events may destroy the parasitic forms, such as desiccation, UV radiation or growing of bacteria (Deplazes et al. 2016). However, collecting samples from the environment is one of the best ways for obtaining information from wild individuals without disturbing the animals, being one of the most widely used non-invasive sampling methods for the monitoring of pathogens in wild animals (Schilling et al. 2022). In addition, this kind of sample collection is not restricted to sick or weak animals that are found death or are derived from wildlife rehabilitation centres which could also bias the results.
Our results reveal that infections with gastrointestinal parasites are common in Cantabrian brown bears. Some of the detected parasites could be transmitted to other animal species or have a zoonotic potential. Therefore, since the impact of endoparasites in the health status of Cantabrian bears is poorly understood, the establishment of a disease surveillance protocol is strongly recommended in order to assess the potential risk of these infections for bears.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Aghazadeh M, Elson-Riggins J, Reljic S, De Ambrogi M, Huber D, Majnaric D, Hermosilla C (2015) Gastrointestinal parasites and the first report of Giardia spp. in a wild population of European brown bears (Ursus arctos) in Croatia. Veterinarski Arhiv 85:201–210
Bauer C (2013) Baylisascariosis-infections of animals and humans with unusual’ roundworms. Vet Parasitol 193:404–412. https://doi.org/10.1016/j.vetpar.2012.12.036
Borecka A, Gawor J, Zieba F (2013) A survey of intestinal helminths in wild carnivores from the Tatra National Park, southern Poland. Ann Parasitol 59(4):169–172
Borka-Vitalis L, Domokos C, Foldvari G, Majoros G (2017) Endoparasites of brown bears in Eastern Transylvania, Romania. Ursus 28:20–30. https://doi.org/10.2192/ursu-d-16-00015.1
Bugmyrin SV, Tirronen KF, Panchenko DV, Kopatz A, Hagen SB, Eiken HG, Kuznetsova AS (2017) Helminths of brown bears (Ursus arctos) in the Kola Peninsula. Parasitol Res 116:1755–1760. https://doi.org/10.1007/s00436-017-5456-4
Catalano S, Lejeune M, Verocai GG, Duignan PJ (2014) First report of Taenia arctos (Cestoda: Taeniidae) from grizzly (Ursus arctos horribilis) and black bears (Ursus americanus) in North America. Parasitol Int 63:389–391. https://doi.org/10.1016/j.parint.2013.12.012
Chapron G, Kaczensky P, Linnell JD, von Arx M, Huber D, Andrén H, López-Bao JV, Adamec M, Álvares F, Anders O, Balčiauskas L, Balys V, Bedő P, Bego F, Blanco JC, Breitenmoser U, Brøseth H, Bufka L, Bunikyte R, Ciucci P, Dutsov A, Engleder T, Fuxjäger C, Groff C, Holmala K, Hoxha B, Iliopoulos Y, Ionescu O, Jeremić J, Jerina K, Kluth G, Knauer F, Kojola I, Kos I, Krofel M, Kubala J, Kunovac S, Kusak J, Kutal M, Liberg O, Majić A, Männil P, Manz R, Marboutin E, Marucco F, Melovski D, Mersini K, Mertzanis Y, Mysłajek RW, Nowak S, Odden J, Ozolins J, Palomero G, Paunović M, Persson J, Potočnik H, Quenette PY, Rauer G, Reinhardt I, Rigg R, Ryser A, Salvatori V, Skrbinšek T, Stojanov A, Swenson JE, Szemethy L, Trajçe A, Tsingarska-Sedefcheva E, Váňa M, Veeroja R, Wabakken P, Wölfl M, Wölfl S, Zimmermann F, Zlatanova D, Boitani L (2014) Recovery of large carnivores in Europe’s modern human-dominated landscapes. Sci 346(6216):1517–1519. https://doi.org/10.1126/science.1257553
Cimatti M, Ranc N, Benítez-López A, Maiorano L, Boitani L, Cagnacci F, Čengić M, Ciucci P, Huijbregts MAJ, Krofel M, López-Bao JV, Selva N, Andren H, Bautista C, Ćirović D, Hemmingmoore H, Reinhardt I, Marenče M, Mertzanis Y, Pedrotti L, Trbojević I, Zetterberg A, Zwijacz-Kozica T, Santini L (2021) Large carnivore expansion in Europe is associated with human population density and land cover changes. Divers Distrib 27(4):602–617. https://doi.org/10.1111/ddi.13219
Conti JA, Forrester DJ, Brady JR (1983) Helminths of black bears in Florida. Proc Helminthol Soc Wash 50:252–256
Costa H, Hartasanchez R, Santos AR, Camarao A, Cruz L, Nascimento M, Gomes L, de Carvalho LMM (2022) Preliminary findings on the gastrointestinal parasites of the brown bear (Ursus arctos) in the Cantabrian mountains, Spain. Vet Parasitol Reg Stud Rep 28. https://doi.org/10.1016/j.vprsr.2021.100681
Crum JM, Nettles VF, Davidson WR (1978) Studies on endoparasites of black bear (Ursus americanus) in southeastern United States. J Wildl Dis 14:178–186. https://doi.org/10.7589/0090-3558-14.2.178
De Ambrogi M, Aghazadeh M, Hermosilla C, Huber D, Majnaric D, Reljic S, Elson-Riggins J (2011) Occurrence of Baylisascaris transfuga in wild populations of European brown bears (Ursus arctos) as identified by a new PCR method. Vet Parasitol 179:272–276. https://doi.org/10.1016/j.vetpar.2011.02.025
Deplazes P, Eckert J, Mathis A, von Samson-Himmelstjerna G, Zahner H (2016) Parasitology in veterinary medicine. Wageningen Academic Publishers, Enke, Germany
Di Salvo AR, Chomel BB (2020) Zoonoses and potential zoonoses of bears. Zoonoses Public Health 67:3–13. https://doi.org/10.1111/zph.12674
Díaz P, Paz-Silva A, Sánchez-Andrade R, Suárez JL, Pedreira J, Arias M, Díez-Baños P, Morrondo P (2007) Assessment of climatic and orographic conditions on the infection by Calicophoron daubneyi and Dicrocoelium dendriticum in grazing beef cattle (NW Spain). Vet Parasitol 149:285–289. https://doi.org/10.1016/j.vetpar.2007.08.002
Dressel S, Sandström C, Ericsson G (2015) A meta-analysis of studies on attitudes toward bears and wolves across Europe 1976–2012. Conserv Biol 29(2):565–574. https://doi.org/10.1111/cobi.12420
Eklund A, López-Bao JV, Tourani M, Chapron G, Frank J (2017) Limited evidence on the effectiveness of interventions to reduce livestock predation by large carnivores. Sci Rep 7(1):1–9. https://doi.org/10.1038/s41598-017-02323-w
Estevez-Sanchez E, Checa R, Montoya A, Barrera JP, Lopez-Beceiro AM, Fidalgo LE, Miro G (2022) A high prevalence of cardiopulmonary worms detected in the Iberian wolf (Canis lupus): a threat for wild and domestic canids. Animals 12. https://doi.org/10.3390/ani12172289
Finnegan S (2009) Seasonal dynamics in the prevalence of Baylisascaris transfuga ova in the faeces of the brown bear (Ursus arctos) in Slovakia. University of Veterinary Medicine and Pharmacy in Košice, Slovakia, Thesis
Foreyt WJ (2001) Veterinary parasitology reference manual. Iowa State University Press, Ames, IA, USA
Foster GW, Cunningham MW, Kinsella JM, Forrester DJ (2004) Parasitic helminths of black bear cubs (Ursus americanus) from Florida. J Parasitol 90:173–175. https://doi.org/10.1645/ge-127r
Gal G (2013) Epidemiological aspects of the parasite fauna of brown bears (Ursus arctos) in Romania. Thesis, University of Agricultural Sciences and Veterinary Medicine, Cluj, Napoca, Romania
Garcia-Dios D, Panadero R, Diaz P, Vina M, Remesar S, Prieto A, Lopez-Lorenzo G, Martinez-Calabuig N, Díez-Baños P, Morrondo P, López C (2021) The goat as a risk factor for parasitic infections in ovine flocks. Animals 11. https://doi.org/10.3390/ani11072077
Gau RJ, Kutz S, Elkin BT (1999) Parasites in grizzly bears from the central Canadian arctic. J Wildl Dis 35:618–621. https://doi.org/10.7589/0090-3558-35.3.618
Gawor J, Gromadka R, Zwijacz-Kozica T, Zieba F (2017) A modified method for molecular identification of Baylisascaris transfuga in European brown bears (Ursus arctos). Parasitol Res 116:3447–3452. https://doi.org/10.1007/s00436-017-5660-2
Gonzalez-Lanza C, Manga-Gonzalez MY, Del Pozo-Carnero P (1993) Coprological study of the Dicrocoelium dendriticum (Digenea) egg elimination by cattle in highland areas in Leon province, Northwest Spain. Parasitol Res 79:488–491. https://doi.org/10.1007/bf00931589
Haynes E, Coker S, Yabsley MJ, Niedrighaus KD, Ramey AM, Verocai GG, Hilderbrand GV, Joly K, Gustine D, Mangipane B, Leacock W, Crupi AP, Cleveland CA (2023) Survey for selected parasites in Alaska brown bears (Ursus arctos). J Wildl Dis 59:186–191. https://doi.org/10.7589/jwd-d-22-00070
Hoberg EP, Burek-Huntington K, Beckmen K, Camp LE, Nadler SA (2018) Transuterine infection by Baylisascaris transfuga: neurological migration and fatal debilitation in sibling moose calves (Alces alces gigas) from Alaska. J Parasitol Parasites Wildl 7:280–288. https://doi.org/10.1016/j.ijppaw.2018.07.005
Hwang MH, Chin TW, Yu PH (2021) Endoparasites of formosan black bears (Ursus thibetanus formosanus) during acorn season in Yushan National Park, Taiwan. J Wildl Dis 57:345–356. https://doi.org/10.7589/jwd-d-20-00067
Jäger M, Gauly M, Bauer C, Failing K, Erhardt G, Zahner H (2005) Endoparasites in calves of beef cattle herds: management systems dependent and genetic influences. Vet Parasitol 131:173–191. https://doi.org/10.1016/j.vetpar.2005.05.014
Johnston SP, Ballard MM, Beach MJ, Causer L, Wilkins PP (2003) Evaluation of three commercial assays for detection of Giardia and Cryptosporidium organisms in fecal specimens. J Clin Microbiol 41:623–626. https://doi.org/10.1128/jcm.41.2.623-626.2003
Jurankova J, Hofmannova L, Frgelecova L, Danek O, Modry D (2022) Baylisascaris transfuga (Ascaridoidea, Nematoda) from European brown bear (Ursus arctos) causing larva migrans in laboratory mice with clinical manifestation. Parasitol Res 121:645–651. https://doi.org/10.1007/s00436-021-07417-z
Khan MA, Afshan K, Sargison ND, Betson M, Firasat S, Chaudhry U (2023) Spatial distribution of Dicrocoelium in the Himalayan Ranges: potential impacts of ecological niches and climatic variables. Acta Parasitol 68:91–102. https://doi.org/10.1007/s11686-022-00634-1
Lalleroni A, Quenette PY, Daufresne T, Pellerin M, Baltzinger C (2017) Exploring the potential of brown bear (Ursus arctos arctos) as a long-distance seed disperser: a pilot study in South-Western Europe. Mammalia 81(1):1–9. https://doi.org/10.1515/mammalia-2015-0092
Lavikainen A, Laaksonen S, Beckmen K, Oksanen A, Isomursu M, Meri S (2011) Molecular identification of Taenia spp. in wolves (Canis lupus), brown bears (Ursus arctos) and cervids from North Europe and Alaska. Parasitol Int 60:289–295. https://doi.org/10.1016/j.parint.2011.04.004
Li X, Nguyen T, Xiao C, Levy A, Akagi Y, Silkie S, Atwill ER (2020) Prevalence and genotypes of Cryptosporidium in wildlife populations co-located in a protected watershed in the Pacific Northwest, 2013 to 2016. Microorganisms 8:914. https://doi.org/10.3390/microorganisms8060914
Linnell JD, Cretois B (2018) Research for AGRI Committee. The revival of wolves and other large predators and its impact on farmers and their livelihood in rural regions of Europe. European Parliament, Policy Department for Structural and Cohesion Policies, Brussels
López-Bao JV, Planella A, Ballesteros F, Blanco JC, Palomero G, Nores C (2021) Females with cubs and the demography of the brown bear in the Cantabrian Mountains. in: Palomero G, Ballesteros F, Blanco JC, Lopez-Bao JV (eds.). Cantabrian Bears. Demography, coexistence and conservation challenges. Brown Bear Foundation. Lynx Editions, Spain
MAFF (1986) Manual of veterinary parasitological laboratory techniques. Her Majesty's Stationery Office. London, United Kingdom
Manga-Gonzalez MY, Gonzalez-Lanza C, Del-Pozo-Carnero P (1991) Dynamics of the elimination of Dicrocoelium dendriticum (Trematoda, Digenea) eggs in the faeces of lambs and ewes in the Porma basin (León, NW Spain). Ann Parasitol Hum Comp 66(2):57–61. https://doi.org/10.1051/parasite/199166257
Manga-Gonzalez MY, Gonzalez-Lanza C, Cabanas E, Campo R (2001) Contributions to and review of dicrocoeliosis, with special reference to the intermediate hosts of Dicrocoelium dendriticum. Parasitol 123:S91–S114. https://doi.org/10.1017/s0031182001008204
Manville AM (1978) Ecto-endoparasite and endoparasite of black bear in Northern Wisconsin. J Wildl Dis 14:97–101. https://doi.org/10.7589/0090-3558-14.1.97
MAPA (2023) Ministerio de Agricultura, Pesca y Alimentación. Encuestas Ganaderas, análisis del número de animales por tipos. https://www.mapa.gob.es/es/estadistica/temas/estadisticas-agrarias/ganaderia/encuestas-ganaderas/
Mariacher A, Eleni C, Fico R, Perrucci S (2018) Urinary capillariosis in a free-ranging Marsican brown bear (Ursus arctos marsicanus). Int J Parasitol Parasites Wildl 7:429–431. https://doi.org/10.1016/j.ijppaw.2018.11.002
Martinez-Rondan FJ, de Ybanez MRR, Lopez-Beceiro AM, Fidalgo LE, Berriatua E, Lahat L, Sacristan I, Oleaga A, Martínez-Carrasco C (2019) Cardiopulmonary nematode infections in wild canids: does the key lie on host-prey-parasite evolution? Res Vet Sci 126:51–58. https://doi.org/10.1016/j.rvsc.2019.08.008
Masatani T, Kojima I, Tashiro M, Yamauchi K, Fukui D, Ichikawa-Seki M, Harasawa R (2021) Molecular detection of filarial nematode parasites in Japanese black bears (Ursus thibetanus japonicus) from Iwate Prefecture, Japan. J Vet Med Sci 83:208–213. https://doi.org/10.1292/jvms.20-0466
Mertzanis Y, Ioannis I, Mavridis A, Nikolaou O, Riegler S, Riegler A, Tragos A (2005) Movements, activity patterns and home range of a female brown bear (Ursus arctos, L.) in the Rodopi Mountain Range, Greece. Belg J Zool 135:217–221
Molnar L, Konigova A, Major P, Vasilkova Z, Tomkova M, Varady M (2020) Seasonal pattern of prevalence and excretion of eggs of Baylisascaris transfuga in the brown bear (Ursus arctos). Animals 10. https://doi.org/10.3390/ani10122428
Orosová T, Goldová M, Ciberej J, Štrkolcová G (2016) Parasitofauna of brown bear (Ursus arctos) in the protected landscape area CHKO - Poľana. Folia Vet 60(4):20–24. https://doi.org/10.1515/fv-2016-0033
Otranto D, Traversa D (2002) A review of dicrocoeliosis of ruminants including recent advances in the diagnosis and treatment. Vet Parasitol 107:317–335. https://doi.org/10.1016/s0304-4017(02)00121-8
Panceira A (2012) Prevalencia y factores de riesgo asociados a la infección por endoparásitos en rumiantes domésticos y silvestres de la provincia de Lugo. Universidade de Santiago de Compostela, España, Thesis
Paoletti B, Iorio R, Traversa D, Di Francesco CE, Gentile L, Angelucci S, Amicucci C, Bartolini R, Marangi M, Di Cesare A (2017) Helminth infections in faecal samples of Apennine wolf (Canis lupus italicus) and Marsican brown bear (Ursus arctos marsicanus) in two protected national parks of central Italy. Ann Parasitol 63(3):205–212. https://doi.org/10.17420/ap6303.107
Pop IM, Bereczky L, Chiriac S, Iosif R, Nita A, Popescu VD, Rozylowicz L (2018) Movement ecology of brown bears (Ursus arctos) in the Romanian Eastern Carpathians. Nat Cons 26:15–31. https://doi.org/10.3897/natureconservation.26.22955
Qin ZY, Liu SR, Bai MH, Geng Y, Miller DL, Zhao RX, Hou R, Huang WJ, Zhang D, Su X (2021) First report of fatal baylisascariasis-induced acute pancreatitis in a giant panda. Parasitol Int 84. https://doi.org/10.1016/j.parint.2021.102380
R Core Team (2023) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
Ravaszova P, Halanova M, Goldova M, Valencakova A, Malcekova B, Hurnikova Z, Halan M (2012) Occurrence of Cryptosporidium spp. in red foxes and brown bear in the Slovak Republic. Parasitol Res 110:469–471. https://doi.org/10.1007/s00436-011-2523-0
Reljic S, Moñino L, Meijer T, Beck A, Huber D, Sanja B, Renata B, Jurković DB, Huber D, Beck R (2017) Unique Dicrocoelium dendriticum genotypes from Croatian brown bears. [Oral communication] 25th International Conference on Bear Research and Management. Quito, Ecuador
Sasmal I, Gould NP, Schuler KL, Chang YF, Thachil A, Strules J, Olfenbuttel C, Datta S, DePerno CS (2019) Leptospirosis in urban and suburban American black bears (Ursus americanus) in western north Carolina, USA. J Wildl Dis 55:74–83. https://doi.org/10.7589/2017-10-263
Sato H, Matsuo K, Osanai A, Kamiya H, Akao N, Owaki S, Furuoka H (2004) Larva migrans by Baylisascaris transfuga: fatal neurological diseases in Mongolian jirds, but not in mice. J Parasitol 90:774–781. https://doi.org/10.1645/ge-3330
Schilling AK, Mazzamuto MV, Romeo C (2022) A review of non-invasive sampling in wildlife disease and health research: what’s new? Animals 12. https://doi.org/10.3390/ani12131719
Shaw HJ, Armstrong C, Uttley K, Morrison LJ, Innes EA, Katzer F (2021) Genetic diversity and shedding profiles for Cryptosporidium parvum in adult cattle and their calves. Curr Res Parasitol Vector Borne Dis 1:100027. https://doi.org/10.1016/j.crpvbd.2021.100027
Sheikh MM, Tak H, Mustahson F, Fazili MF, Bhat AB, Wani IN (2018) Baylisascaris transfuga: a parasite with zoonotic potential. Int J Adv Sci Res Manag 3(12):174–180
Strkolcova G, Goldova M, Snabel V, Spakulova M, Orosova T, Halan M, Mojzisova J (2018) A frequent roundworm Baylisascaris transfuga in overpopulated brown bears (Ursus arctos) in Slovakia: a problem worthy of attention. Acta Parasitol 63:167–174. https://doi.org/10.1515/ap-2018-0019
Szczepaniak K, Listos P, Lopuszynski W, Skrzypek T, Kazimierczak W (2012) Granulomatous peritonitis in a European brown bear caused by Baylisascaris transfuga. J Wildl Dis 48:517–519. https://doi.org/10.7589/0090-3558-48.2.517
Westmoreland LSH, Stoskopf MK, Maggi RG (2017) Detection and prevalence of four different hemotropic Mycoplasma spp. in Eastern North Carolina American black bears (Ursus americanus). Comp Immunol Microbiol Infect Dis 50:106–109. https://doi.org/10.1016/j.cimid.2016.12.002
Zhang JS, Daszak P, Huang HL, Yang GY, Kilpatrick AM, Zhang S (2008) Parasite Threat to Panda Conservation Ecohealth 5:6–9. https://doi.org/10.1007/s10393-007-0139-8
Acknowledgements
We would like to thank Luis Fernández and Elias Suárez, field staff from the Brown Bear Foundation, who carried out essential work in collecting the samples. We dedicate this article to Fernando Ballesteros, who passed away on August 2023 during the completion of this study.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. This study has received funding from the Ministry of Ecological Transition and Demographic Challenge of Spain. It was also funded by the Program for consolidating and structuring competitive research groups (ED431C2023/16, Xunta de Galicia, Spain). J.V. López-Bao was additionally supported by the Spanish Ministry of Economy, Industry, and Competitiveness (CGL2017-87528-R AEI/FEDER EU) and a GRUPIN grant IDI/2021/000075 (Asturias Government).
Author information
Authors and Affiliations
Contributions
P.D. and C.B. did the conceptualization; C.B., O.R., J.V.L.B. and F.B. collected the samples; J.V.L.B. did the funding acquisition; C.B., P.D. and D.G-D performed the coprological analysis; S.R. performed the statistical analysis; S.R. wrote the original draft; P.D., O.R. and J.V.L.B reviewed and edited the original draft. All authors reviewed and accepted the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
No ethical approval by an Institutional Animal Care and Use Committee was deemed necessary since faecal samples were directly collected from the environment.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Remesar, S., Busto, C., Díaz, P. et al. Presence of gastrointestinal and bronchopulmonary parasites in Cantabrian brown bears. Eur J Wildl Res 70, 23 (2024). https://doi.org/10.1007/s10344-024-01779-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10344-024-01779-2