Abstract
Wild small rodents are considered the natural reservoirs of Mycobacterium microti, a member of the Mycobacterium tuberculosis complex (MTBC) that can cause tuberculosis (TB) in humans and animals, as well as interfere with current tuberculosis eradication plans in livestock. A cross-sectional study was carried out in the Catalan Pyrenees (Iberian Peninsula) in an area where M. microti was previously isolated from wild boars, to evaluate the role of micromammals in the epidemiology of this outbreak. A total of 350 wild rodents were necropsied (306 Murinae and 44 Arvicolinae) in spring and autumn during two consecutive natural years. Tissues were analyzed by histopathology to look for TB-like lesions and by qPCR and culture to detect MTBC. Sera were analyzed by MTBC-specific ELISA. No evidence of TB infection in wild rodents was confirmed. Results suggest that small rodents did not play a role in the epidemiology of M. microti in the area. The source of this mycobacterium remains unknown, but previous detections of M. microti in various species in southern France suggest the movements of wild boars across the French Pyrenees as the most likely origin of the outbreak detected in the Iberian Peninsula.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Wild small rodents are considered the natural reservoirs of Mycobacterium microti (Wells 1937; Kipar et al. 2014), a member of the Mycobacterium tuberculosis complex (MTBC). Field voles (Microtus agrestis), bank voles (Myodes glareolus), yellow-necked mice (Apodemus flavicollis), wood mice (Apodemus silvaticus) and shrews (Sorex araneus) suffer tuberculosis (TB) after the infection with M. microti and can transmit the disease within their own populations (Cavanagh et al. 2002; Smith et al. 2009; Kipar et al. 2014; Tagliapietra et al. 2021). Besides, M. microti also causes infection in livestock (Michelet et al. 2016, 2017), large wild animals (Boniotti et al. 2014; Michelet et al. 2015a; De Val et al. 2019) and even humans (Frota et al. 2004; Panteix et al. 2010), although it is generally considered low-pathogenic in large mammals. However, since M. microti exposure may induce positive results to tuberculin skin testing, this bacteria interferes with bovine TB eradication programs focused on other members of the MTBC such as M. bovis, M. caprae or M. tuberculosis infections (Michelet et al. 2020).
Studies conducted in England reported relatively high prevalence of vole TB due to M. microti (Kremer et al. 1998; Cavanagh et al. 2002; Smith et al. 2009; Kipar et al. 2014), while in France this pathogen has been isolated in a number of large-mammal species including cattle (Michelet et al. 2017), goats (Michelet et al. 2016), lama (Michelet et al. 2015a), wild boars (Michelet et al. 2015a, b), badgers (Michelet et al. 2020), foxes (Michelet et al. 2021), dogs (Deforges et al. 2004), and cats (Rüfenacht et al. 2011). In addition, closely related strains of M. microti have been recently isolated in both wild boars and wild rodents in Northern Italy strongly suggesting interspecies transmission (Tagliapietra et al. 2021).
Recently, an outbreak of M. microti was detected in the Catalan Pyrenees (north-eastern Iberian Peninsula) involving wild boars during 2017–2019 (De Val et al. 2019). The strain involved presented the same genotype (genogroup D) to that previously isolated in the French side of the Pyrenees (Michelet et al. 2015a). This finding raised the question of whether M. microti was introduced from France via wild boars or was endemic in the outbreak area and whether its natural reservoir, namely wild rodents, played a role in the epidemiology of the outbreak. The aim of this study was to investigate the presence and prevalence of M. microti among wild rodents in different epidemiological scenarios within the outbreak area.
Results
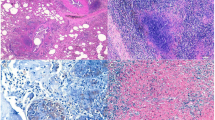
None of the macroscopical lesions observed were subsequently identified microscopically as TB compatible lesions, the most frequent being parasitic granulomatous hepatitis presumably caused by Capillaria spp., and lung adiaspiromycosis presumably caused by Emmonsia crescens. Microscopically, multifocal small granulomatous lesions compatible with TB were identified in 111 animals, mostly in the liver and the lung (Fig. 1). None of them had AFB upon Ziehl Neelsen’s staining. Such lesions were observed in 101 murids (33%, 101/302) and 10 arvicolines (21%, 10/48).
None of the mycobacterial cultures showed growth identified as MTBC, and qPCR tests were all negative. The 347 analyzed sera were negative to MTBC-specific IgG.
Therefore, infection by TB was not confirmed in any of the 302 murines and 48 arvicolines tested. The estimated TB prevalence was 0% [95% Confidence Interval (CI95%): 0 – 1.3] in murines and 0% [CI95%: 0 – 7.4] in arvicolines, while the overall prevalence was 0% [CI95%: 0 – 1.1]. The CI95% of these prevalence were calculated in VassarStats website (Lowry 1998).
Discussion
In the present study, M. microti infection was not detected in the community of wild rodents (302 murines and 48 arvicolines) inhabiting a presumptive endemic area where infected wild boars were previously identified. Similarly, no evidence of M. microti in over 300 trapped wild mice was found in a region of Switzerland with several cases of feline TB due to M. microti in domestic cats (Peterhans et al. 2020). On the contrary, M. microti was confirmed in wild rodents in a region of Northern Italy where infected wild boars were previously found (Tagliapietra et al. 2021).
These findings highlight different roles of wild rodents in the epidemiology of M. microti and point to their apparent negligible role in the area studied in the present work. Given that the maximum possible prevalence in micromammals in the study area was estimated to be 0% [CI95%: 0 – 1.1], TB is most likely not endemic in the micromammal population of the outbreak area, and they cannot be considered as the natural reservoir (De Val et al. 2019). However, the maximum possible prevalence in arvicolines was higher (7.4%) since less animals were captured, and thus the prevalence in this subfamily may have been underestimated. Field voles and bank voles, the main described reservoirs of M. microti (Cavanagh et al. 2002), are members of this subfamily. Therefore, the 44 analyzed animals might fall short to properly detect the infection in their populations.
In endemic regions, the prevalence of M. microti in wild rodents can fluctuate depending on the sampling season or other ecological factors (Cavanagh et al. 2002). However, to avoid this factor, the present study was designed including samples in two different seasons (spring and autumn) of two consecutive years in two different valleys and in areas with two different ecological conditions (farm surroundings and areas distant from human influence).
None of the captured wild rodents presented the TB pathological phenotype described in field M. microti infections which includes skin granulomatous lesions as well as granulomatous lesions of viscera (Kipar et al. 2014). Even though numerous microscopical granulomatous lesions were observed in liver and lung that showed similar features to those observed in the field (Kipar et al. 2014) and in experimental infections (Vidal et al. 2022), these lesions were not confirmed by Ziehl Neelsen’s stain, PCR, nor microbiological culture in any instance.
The M. microti previously isolated in wild boars in the studied area presented the spoligotype pattern SB0423 (De Val et al. 2019), phylogenomically associated with the SB0112, commonly called “llama-type” (Deforges et al. 2004), and both of them characterized by the presence of the spacers 4, 5, 6, 7, 23 and 24 (www.Mbovis.org). This subtype has been previously isolated in a wide range of large-mammals such as badgers, wild boars, cats, dogs and llamas (Michelet et al. 2015a). Nevertheless, to our knowledge, it has never been reported to be isolated in wild rodents, in which the most frequently described are the “vole type” strains (e.g. SB0118, SB2277), characterized by the absence of the spacers mentioned above (Van Soolingen et al. 1998; Tagliapietra et al. 2021). This suggest that micromammals could be less susceptible to the infection by “llama type” strains compared to other mammals. However, a recent experimental infection of bank voles with M. microti SB0423 (“llama-type”) induced similar pathological features (Vidal et al. 2022) to those described in voles naturally infected with “vole-type” strains in the field (Kipar et al. 2014), indicating that the infection is potentially feasible regardless of the natural susceptibility.
In any case, wild boars are newly identified susceptible hosts to both M. microti subtypes that cause TB pathology indistinguishable to that observed in M. bovis or M. caprae infections (De Val et al. 2019), i.e. granulomatous lymphadenopathy, particularly in the submandibular lymph nodes (the sample that is most frequently obtained through the surveillance program). In addition, recent studies indicated that they can play a role in the maintenance and transmission of M. microti (Chiari et al. 2016; De Val et al. 2019; Michelet et al. 2020) and eventually participate, together with wild rodents, in a maintenance community of this pathogen (Tagliapietra et al. 2021). Indeed, TB due to M. bovis and M. caprae is considered a multi-host disease and different susceptible species may play a role in the epidemiology constituting a maintenance community of the infection (Santos et al. 2020) or acting as spillovers depending on the local epidemiological conditions.
Conclusion
During the hunting seasons (2020–2022) following the detection of the positive wild boar, even though the sampling has been irregular or scant in some areas, no further M. microti cases have been detected in this species through the Catalan wildlife health surveillance program. This suggests that the most likely origin of the outbreak are wild boar movements form across the French border where M. microti is endemic in wild boar populations (Michelet et al. 2015a, b, 2020), indeed, in the Ariège region, isolates with the same spoligotype (SB0423 and its closely related SB0112) have been found in wild boars and badgers (Source: ANSES unpublished findings). However, if further cases were diagnosed, the hypothesis of the ability of wild boars to maintain the infection without the involvement of wild rodents will need to be considered.
Therefore, wild boar TB must be carefully monitored in areas where a case is detected in the species to prevent it from spreading to domestic species or interfering with livestock TB control measures. Likewise, the role of micromammals should be assessed in areas with wild boar M. microti cases, such as the Central French Pyrenees, to investigate their involvement in the disease maintenance community.
Methods
Area of study, animal trapping and sampling
Four capture campaigns were planned over a 2-year period in spring and autumn, from autumn 2020 to spring 2022 in the Central Pyrenees, Catalonia (Spain). Each campaign was composed of two weeks of sampling, with one week in Bagergue Valley (Naut Aran, N42.73 E0.91), and the other in Isil Valley (Alt Àneu, N42.68 E1.08), corresponding to the areas where the positive wild boars were previously detected (De Val et al. 2019). In both valleys, 40 baited Heslinga™ traps (Groningen, The Netherlands) were placed around the farms where livestock spend the colder months, as well as in the fields where they spend the summer months.
During these campaigns, wild rodents belonging to the subfamilies Arvicolinae (n = 48, including Microtus arvalis, Myodes glareolus, Microtus gerbei and Chiononys nivalis) and Murinae (n = 302, including Apodemus sylvaticus and Mus musculus) were captured alive (See Table 1). Species were identified, based on their morphological characteristics using Gosàlbez and Noguera(Gosàlbez i Noguera 1988) as reference guide. After capture, mice and voles were humanely euthanized in situ using an isoflurane overdose, following the American Veterinary Medical Association (AVMA) guidelines for the euthanasia of animals (AVMA 2020). Immediately after, blood was collected from all animals (except two arvicolines and one murine) by intracardiac puncture and were stored in a cooler with frozen ice packs until return to the base camp (maximum two hours later). At base camp, necropsies were performed on the animals, and the presence of macroscopic changes was recorded. A fragment of spleen, liver, and lungs were collected and stored at -20 °C. Submandibular and inguinal lymph nodes, as well as all thoracic and abdominal viscera, were removed from the carcasses, placed in cassettes, and immersed in a 4% formaldehyde solution for histopathological examination. Blood was centrifuged and sera was collected and stored at -20 °C until analyses.
Pathological assessment
Formalin fixed tissues were sectioned embedded in paraffin wax. Four micrometer thick sections were obtained and routinely stained with Hematoxylin and eosin for microscopical observation. When TB compatible lesions were observed, additional sections were stained with Ziehl–Neelsen’s stain to visualize acid fast bacilli (AFB).
Mycobacterial culture
After gross pathology examination, tissue samples (~ 0.1 g) from spleen, liver, and lungs were pooled and sliced with sterile scissors and mechanically homogenized in 1.5 ml of sterile distilled water. An aliquot of 0.5 ml of each homogenate was processed for mycobacterial culture in Löwenstein-Jensen with pyruvate and Coletsos solid media (BD diagnostics Sparks, MD, USA) and BACTEC MGIT 320 system (BD diagnostics) as previously described (Vidal et al. 2022).
DNA extraction from one milliliter of tissue homogenates was performed by using the ID Gene™ spin universal extraction kit (ID.vet, Grabels, France) according to the manufacturer procedure. Afterwards, DNA samples were amplified in a 7500 fast real-time PCR system (Applied Biosystems, Walham, MA, USA) using a MTBC-specific real time PCR (ID Gene™ Mycobacterium tuberculosis complex Duplex, ID.vet), following the manufacturer instructions. DNA samples were additionally tested with IS6110 and IS1081 PCR system to detect MTBC (Lesellier et al. 2019). Reactions were carried out in a 25 μl reaction mix containing TaqMan™ Fast Advanced Master Mix (ThermoFisher Scientific, Villebon sur Yvette, France), 300 nM forward and reverse primers, 250 nM probes, sterile water, and 5 μl of DNA template. Thermocycling conditions were 50 °C for 2 min (1 cycle), followed by one cycle of 20 s at 95 °C and 40 cycles of 3 s at 95 °C and 30 s at 60 °C.
IgG to MTBC detection by ELISA
Sera from 347 captured animals (48 of arvicolines and 302 of murines) were analyzed for MTBC-specific antibodies as previously described (Pérez de Val et al. 2017). Briefly, serum samples were thawed and analyzed by a multispecies indirect IgG ELISA to the MPB83 antigen (Lionex, Braunschweig, Germany), using a Protein A/G mixture conjugated with peroxidase (Merck, Darmstadt, Germany) for IgG detection. A sample was considered positive when ΔOD (mean Optical Density -OD- at 450 nm of wells coated with MBP83 minus the OD of the uncoated well) ≥ 0.2.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AFB:
-
Acid fast bacilli
- AVMA:
-
American Veterinary Medical Association
- CI:
-
Confidence interval
- MTBC:
-
Mycobacterium tuberculosis Complex
- OD:
-
Optical Density
- TB:
-
Tuberculosis
References
AVMA (2020) AVMA guidelines for the euthanasia of animals: 2020 edition. American Veterinary Medical Association, Schaumburg, IL
Boniotti MB, Gaffuri A, Gelmetti D et al (2014) Detection and molecular characterization of Mycobacterium microti isolates in wild boar from northern Italy. J Clin Microbiol 52:2834–2843. https://doi.org/10.1128/JCM.00440-14
Cavanagh R, Begon M, Bennett M et al (2002) Mycobacterium microti infection (vole tuberculosis) in wild rodent populations. J Clin Microbiol 40:3281–3285. https://doi.org/10.1128/JCM.40.9.3281-3285.2002
Chiari M, Ferrari N, Giardiello D et al (2016) Spatiotemporal and Ecological Patterns of Mycobacterium microti Infection in Wild Boar (Sus scrofa). Transbound Emerg Dis 63:e381–e388. https://doi.org/10.1111/TBED.12313
De Val BP, Sanz A, Soler M et al (2019) Mycobacterium microti infection in Free-Ranging Wild Boar, Spain, 2017–2019. Emerg Infect Dis 25:2152–2154
Deforges L, Boulouis HJ, Thibaud JL et al (2004) First isolation of Mycobacterium microti (Llama-type) from a dog. Vet Microbiol 103:249–253. https://doi.org/10.1016/j.vetmic.2004.06.016
Frota CC, Hunt DM, Buxton RS et al (2004) Genome structure in the vole bacillus, Mycobacterium microti, a member of the Mycobacterium tuberculosis complex with a low virulence for humans. Microbiology 150:1519–1527. https://doi.org/10.1099/MIC.0.26660-0
Gosàlbez i Noguera J (1988) Insectívors i rosegadors de Catalunya: metodologia d’estudi i catàleg faunístic
Kipar A, Burthe SJ, Hetzel U et al (2014) Mycobacterium microti Tuberculosis in Its Maintenance Host, the Field Vole (Microtus agrestis): Characterization of the Disease and Possible Routes of Transmission. Vet Pathol 51:903–914. https://doi.org/10.1177/0300985813513040
Kremer K, Van Soolingen D, Van Embden J et al (1998) Mycobacterium microti: more widespread than previously thought. J Clin Microbiol 36:2793–2794. https://doi.org/10.1128/JCM.36.9.2793-2794.1998
Lesellier S, Boschiroli ML, Barrat J et al (2019) Detection of live M. bovis BCG in tissues and IFN-γresponses in European badgers (Meles meles) vaccinated by oropharyngeal instillation or directly in the ileum. BMC Vet Res 15:1–15. https://doi.org/10.1186/S12917-019-2166-4/FIGURES/3
Lowry R, (1998) VassarStats: Website for Statistical Computation. http://vassarstats.net/. Accessed 13 Oct 2022
Michelet L, De CK, Zanella G et al (2015a) Infection with Mycobacterium microti in animals in France. J Clin Microbiol 53:981–985. https://doi.org/10.1128/JCM.02713-14
Michelet L, De Cruz K, Karoui C et al (2017) Mycobacterium microti infection in a cow in France. Vet Rec 180:429
Michelet L, De Cruz K, Phalente Y et al (2015b) Letters: Mycobacteria: Mycobacterium microti detection in French wildlife. Vet Rec 177:446. https://doi.org/10.1136/VR.H5754
Michelet L, de Cruz K, Phalente Y et al (2016) Mycobacterium microti infection in dairy goats, France. Emerg Infect Dis 22:569–570. https://doi.org/10.3201/EID2203.151870
Michelet L, de Cruz K, Tambosco J et al (2020) Mycobacterium microti interferes with bovine tuberculosis surveillance. Microorganisms 8:1–7. https://doi.org/10.3390/microorganisms8121850
Michelet L, Richomme C, Réveillaud E et al (2021) Mycobacterium microti infection in red foxes in France. Microorganisms. https://doi.org/10.3390/microorganisms9061257
Panteix G, Gutierrez MC, Boschiroli ML et al (2010) Pulmonary tuberculosis due to Mycobacterium microti: A study of six recent cases in France. J Med Microbiol 59:984–989. https://doi.org/10.1099/JMM.0.019372-0
Pérez de Val B, Napp S, Velarde R et al (2017) Serological follow-up of tuberculosis in a wild boar population in contact with infected cattle. Transbound Emerg Dis 64:275–283. https://doi.org/10.1111/tbed.12368
Peterhans S, Landolt P, Friedel U et al (2020) Mycobacterium microti: not just a coincidental pathogen for cats. Front Vet Sci. https://doi.org/10.3389/FVETS.2020.590037
Rüfenacht S, Bögli-Stuber K, Bodmer T et al (2011) Mycobacterium microti infection in the cat. A case report, literature review and recent clinical experience. J Feline Med Surg 13:195–204. https://doi.org/10.1016/J.JFMS.2011.01.012
Santos N, Richomme C, Nunes T et al (2020) Quantification of the animal tuberculosis multi-host community offers insights for control. Pathog (basel, Switzerland). https://doi.org/10.3390/PATHOGENS9060421
Smith NH, Crawshaw T, Parry J, Birtles RJ (2009) Mycobacterium microti: More diverse than previously thought. J Clin Microbiol 47:2551–2559. https://doi.org/10.1128/JCM.00638-09
Tagliapietra V, Boniotti MB, Mangeli A et al (2021) Mycobacterium microti at the environment and wildlife interface. Microorganisms. https://doi.org/10.3390/MICROORGANISMS9102084/S1
Van Soolingen D, Van Der Zanden AGM, De Haas PEW et al (1998) Diagnosis of Mycobacterium microti infections among humans by using novel genetic markers. J Clin Microbiol 36:1840–1845. https://doi.org/10.1128/JCM.36.7.1840-1845.1998
Vidal E, Burgaya J, Michelet L et al (2022) Experimental Mycobacterium microti infection in bank voles ( Myodes glareolus). Microorganisms. https://doi.org/10.3390/MICROORGANISMS10010135
Wells AQ (1937) Tuberculosis in wild voles. Lancet 229:1221. https://doi.org/10.1016/S0140-6736(00)83505-9
Acknowledgements
We are grateful to Judit Burgaya, and the teams of the pathology laboratory (Mònica Pérez, Rosa López and Anna Barceló) and mycobacteria laboratory (Zoraida Cervera, Abel Muñoz and Maite Martín) of IRTA-CReSA, the French National reference laboratory for bovine TB (Anses) and WildCom Research Group (UAB) for their excellent technical assistance.
Funding
Open Access Funding provided by Universitat Autonoma de Barcelona. This work was supported by the Grant EFA357/INNOTUB (Program Interreg POCTEFA 2004–2020) and the Department of Climate Action, Food, and Rural Agenda (DACC) of the Government of Catalonia and. IRTA is supported by Centres de Recerca de Catalunya (CERCA) Programme / Generalitat de Catalunya (www.cerca.cat). M. P. R. was funded through the 2021 FI Scholarship, Departament de Recerca i Universitats, Generalitat de Catalunya, Spain (FI_B 00171). C. M. is recipient of a pre-doctoral grant of the program “Don Carlos Antonio López” of the Republic of Paraguay (Ref. 88/2020).
Author information
Authors and Affiliations
Contributions
EV, JE, MP, OC and BP conceived and planned the study. EV, JE, MP, CM, LaM, LoM and MB performed the experiments and contributed to data collection. EV, JE, AA and BP analyzed and interpreted the data. BP and AA acquired funding and were in charge of project administration. AS provided resources. EV and BP wrote the original draft. JE, MP and OC contributed to writing, reviewing, and editing the final version of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
The capture, euthanasia, and sampling procedures of wild rodents for the present study were reviewed and approved by the institutional review board of the Generalitat de Catalunya (Government of Catalonia) authorization number SF/0032/21 and SF/0063/22 and the Conselh Generau d’Aran: authorization number 036/2021 and 001/2022. All methods were carried out in accordance with European Union laws for protection of animals used for scientific purposes (2010/63/EU).
Consent for publication
Not applicable.
Competing interests
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Vidal, E., Espunyes, J., Ribas, M.P. et al. Lack of detection of Mycobacterium microti infection in wild rodents from a free-ranging wild boar outbreak area. Eur J Wildl Res 69, 111 (2023). https://doi.org/10.1007/s10344-023-01738-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10344-023-01738-3