Abstract
Human-wildlife coexistence is important for a sustainable relationship between humans and the natural environment. However, human activities often act as a disturbance to wild animals, which may show behavioural shifts indicating human avoidance. For large carnivores, which are prone to conflict with many human interests, coexistence with humans can be particularly challenging. We used long-term camera trap data to evaluate seasonal and diel variations in activity of two large carnivores, the brown bear (Ursus arctos) and the grey wolf (Canis lupus), as well as humans in the Cantabrian Mountains, northern Spain. Brown bears were less active in winter than in summer; the opposite was observed for wolves, whereas there was limited seasonal variation in human activity. On a diel scale, both bears and wolves were mostly crepuscular during summer and had less distinct, but generally more nocturnal activity during winter. Humans were strictly diurnal during both seasons. We suggest that the diel activity of bears and wolves was partially caused by human avoidance, but that seasonal variations in both overall and diel activity were mainly caused by ecological and physiological factors. While we suggest that the observed similarity in diel activity of bears and wolves did not have caused strong competition between these two species, it may have influenced interactions with other predators and prey. Since such interactions are likely to be context dependent, we urge for further studies evaluating how humans influence the behaviour of large carnivores across different spatio-temporal scales.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Conservation biology has seen several major paradigm shifts during the last centuries, with the current one being focused on the incorporation of humans within the biological and geophysical environment (Mace 2014). Within this conservation paradigm, sustainable coexistence between humans and wildlife is of obvious importance (Frank and Glikman 2019). However, such coexistence is not without challenges. For instance, there are often intense conflicts between human activities and wildlife (Woodroffe et al. 2005; Leader-Williams et al. 2010; Redpath et al. 2013). Conflicts may arise when wildlife prey on livestock, destroy human properties, eat crops or attack people. These conflicts can also have socio-economic, cultural or political dimensions (Madden 2004; Dalerum 2021). Human-wildlife conflicts often result in persecution, which may have direct demographic effects on wildlife populations (Dalerum and Swanepoel 2017).
Human activities may also impact wildlife indirectly by acting as a disturbance, which may disrupt temporal and spatial patterns of activity (Frid and Dill 2002; Sutherland 2007). Avoidance of humans, in both space and time, is well documented across a wide range of taxa (Stankowich 2008; Larson et al. 2016; Pirotta et al. 2018; Suraci et al. 2019). In part, the strong behavioural responses to human disturbance may be attributed to humans’ unique properties as predators (Darimont et al. 2015). Human persecution has driven directed and rapid selection of various phenotypic traits, including behaviour (Darimont et al. 2009). Disturbance may cause a displacement of animals into nocturnal temporal niches or into protected or inaccessible areas (Rode et al. 2006; Gaynor et al. 2018), but may also alter other aspects of animal behaviour, such as vigilance behaviour and movements (Jayakody et al. 2008; Doherty et al. 2021).
Large carnivores are particularly susceptible to causing conflicts with humans (Hovardas 2018). This is partly due to their predatory behaviour, which puts them in conflict with livestock owners and hunters (van Eeden et al. 2018), as well as other cultural and socio-economic factors (Dalerum 2021). In addition, the large area requirements of many large carnivores may lead to spatial overlaps with human activities, and also make them exposed to habitat degradation (Sunquist and Sunquist 2001; Finnegan et al. 2021). Furthermore, their relatively low natural mortality, low reproductive rates and low population densities make them demographically sensitive to human persecution and disturbance (Purvis et al. 2000). Hence, their biological properties make large carnivores both exposed to potential conflicts and also more sensitive to its possible consequences. However, despite the large amount of studies on the direct effects of human persecution and habitat degradation on carnivore population persistence (reviewed in Gittleman et al. 2001; Hovardas 2018), there are still limited studies on the effects of human activity on large carnivore behaviour.
Brown bears (Ursus arctos, hereafter referred to as “bear” or “bears”) and grey wolves (Canis lupus, hereafter referred to as “wolf” or “wolves”) are two large carnivores that are frequently in conflict with humans (Breitenmoser 1998; Graham et al. 2005). In Europe, they both inhabit human-dominated landscapes, with the southernmost European populations occurring in northern and central Spain (Chapron et al. 2014). Both bears and wolves have historically been persecuted in Spain, mainly due to the damages caused to livestock, crops and beehives (Blanco et al. 1992; Fernández-Gil et al. 2016). Currently, the brown bear is nationally listed as endangered, whereas the Spanish wolf is listed as a species requiring special protection (Ordiz et al. 2022). Spain is a relatively densely populated country, with approximately 20% of its close to 50 million inhabitants living in rural areas (World Bank 2022). Furthermore, livestock husbandry is common across the landscape (Delgado-Serrano and Hurtado-Matos 2018), and outdoor recreation is becoming increasingly popular (Rivera 2015). Hence, Spanish bears and wolves are likely to face frequent encounters with humans which may influence their behaviour in addition to the effects caused by direct persecution.
In this study, we used a long-term dataset of automated camera traps to assess the temporal activity patterns of brown bears, wolves and humans at seasonal and diel scales in a rural area of the Autonomous Region of Cantabria (hereafter referred to as “Cantabria”), northern Spain. Although brown bears in our study area may not necessarily hibernate (Nores et al. 2010; González-Bernardo et al. 2020), we anticipate them to display more seasonal variation in activity compared to wolves. Wolves, being primarily active hunters, are expected to maintain consistent activity levels throughout the seasons (Mech 1970). Since wolves have experienced the hardest persecution (Fernández-Gil et al. 2016; Quevedo et al. 2019), we expect lower overlap in diel activity between wolves and humans than between bears and humans. In addition, we expect the brown bear to show the biggest seasonal variation, both overall and in diel activity.
Material and methods
Study area
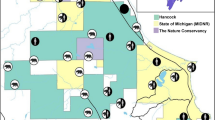
The study area covers approximately 1125 km2 of the southwestern parts of the Autonomous Region of Cantabria, which extends approximately 130 km east to west and 70 km north to south along the south shore of the Bay of Biscaya in northern Spain (Fig. 1). The topography along the coast includes undulating hills and valleys, with the relief of the terrain becoming more pronounced inland closer to the Cantabrian Mountains. The elevation of the study area is highly variable, ranging from 400 to over 2000 m above sea level. The climate is atlantic, with a relatively small seasonal temperature oscillation (Peel et al. 2007). Winters are generally mild except for high alpine areas, with average temperatures of 9 °C, and summers only reaching moderate temperatures averaging 20 °C (Ancell Trueba and Célis Diaz 2012). Precipitation is abundant around the year, with an annual precipitation of approximately 1000 mm (Ancell Trueba and Célis Diaz 2012). Cantabria has a human population of 600,0000, which gives an average human density of 109 humans/km2 (Instituto Nacional de Estadística, https://www.ine.es). Most people are distributed in and around the two main cities of Santander (approximately 180,000 inhabitants) and Torrelavega (approximately 60,000 inhabitants), and human population density generally declines from the coast towards the inland. Important economic activities in rural areas include livestock farming, mostly cattle, but mining, tourism and mountain sports, hunting, agriculture and timber harvesting are also of local importance.
Much of former forest has been transformed into pasture and brushwood, but vegetation and land use vary with altitude. The most common vegetation types in the study area are different types of deciduous forests, comprised of species such as beech (Fagus sylvatica), oak (Quercus sp.), holly (Ilex aquifolium) and birch (Betula sp.), as well as chestnut (Castanea sativa) and hazel (Corylus avellana) (Durán-Gómez 2014). The mammal community consists of two large carnivores, the bear and the wolf, a number of mesocarnivores, large herbivores such as red deer (Cervus elaphus), roe deer (Capreolus capreolus), wild boar (Sus scrofa) and chamoix (Rupicabra rupicabra), as well as smaller mammals (Palomo et al. 2007).
Camera trapping
Camera trapping took place between 2016 and 2022 using a total of 85 camera stations (Fig. 1). To maintain distance between the stations, a grid with a cell size of 5 × 5 km was superimposed across the study area. The Cantabrian Mountains are highly heterogeneuous and complex both topographically and in land cover (Grilo et al. 2019; Ávlarez García 2007). This grid size therefore represents spatially independent sample units. From 2016 to 2018, one station was placed within each of 36 cells, with a minimum distance of 3.5 km between two stations. From 2019 to 2022, the survey was expanded so that one station was placed within each of 45 cells, with the same minimum distance. Of these 45 stations, 15 were kept in the same locations as the earlier period. In addition, from 2018 to 2022, an additional 2–9 extra cameras were placed within the grid every year. The number of active stations at any given time therefore varied from 36 to 54. The number of active days for each station varied from 21 to 660 (mean of 325 days ± SD 171 days).
Each station consisted of a single motion triggered digital camera (Bushnell Trophy Cam Aggressor No Glow, Bushnell, Corp., Overland Park, Kansas, USA) placed on a tree trunk approximately 1 m above the ground. Each station was baited with a fish scent placed approximately 7 m in front of the camera. This bait was placed to attract animals into an appropriate position in front of the camera. Since the bait was used in a consistent manner during the full duration of the survey and for all staions, we argue that it did not influence our estimates of either seasonal or diel variations in animal activity. The angle of detection of each camera was 43.9°, and the angle of view was 35°. Each camera had a trigger speed of 0.6 s and was configured to take one photo as well as one 30-s video in daylight and one photo and one 15-s video at night. After each set of a photo and video, there was a period of 6 s until the camera could be triggered again. Maximum distance of detection was 25 m in daylight and 20 m at night. Each station was visited on a monthly basis to change batteries, download images and reapply the bait (Government of Cantabria 2021).
Although the camera trapping program was ongoing during the whole period, we focused only on data recorded during June and July, reflecting late spring and early summer (referred to as “summer”) and December and January, reflecting late fall and early winter (referred to as “winter”), except for the summer period in 2020. These two periods represent the months with most (summer) and least (winter) daylight hours in our study area (https://gml.noaa.gov/grad/solcalc/). We opted to define our sample periods based on light regimes due to their strong effects on animal activity across different temporal scales (Wetterberg 1994). We excluded observations from June and July in 2020 due to possibly confounding effects of Covid-19-related restrictions of human movements (Rutz et al. 2020). We used all observations of bears, wolves, humans and domestic dogs, except shepherd dogs, made at different stations or at the same stations at least 30 min apart for our data analyses. Since there are no feral dogs in our area, we assumed that all dogs except shepherd dogs were accompanied by a person and hence reflected human activity. Shepherd dogs are frequently left unattended for days in the area, and can thus not be used as indicators of human activity.
Data analyses
We estimated seasonal levels of activity from the single season occupancy model initially proposed by MacKenzie et al. (2003). For each species, we fitted separate models for winter and summer. Each model was fitted on data pooled across all years, using a whole sampling eriod (i.e. December and January for winter and June and July for summer) as our smallest independent observation unit. We fitted number of days active as a station-level covariate for each sampling period. Fitting the models on data pooled in this way prevented the fitting of models on zero-inflated data, which may hamper the model-fitting process (MacKenzie et al. 2006). We recognize that there may not be much seasonal difference in the occupancy of neither bears, wolves nor humans in our study area, i.e. we do not expect that either of these species would leave or enter the study area on a seasonal basis. However, we believe that occupancy estimates provide a useful heuristic measurement of overall activity that accounts for imperfect detection.
We estimated diel activity patterns using a kernel-based density estimator based on the time stamp of camera trap observations, converted to radians (Ridout and Linkie 2009). We made separate activity estimations for each species and each season. We used a non-parametric estimation of the common area under the estimated probability density curves as an index of temporal overlap both between seasons for bears, wolves and humans separately, as well as between each pair of species within each season. The estimator ranges from 0 (no overlap) to 1 (overlap), and was calculated using the following equation:
where T is a large number of equally spaced values between 0 and 2π, which in our case was set to 128, and f(ti) and ĝ(ti) are the two estimated density distributions reflecting activity at time t (Schmid and Schmidt 2006). Since we were interested in describing differences in activity rather than overlap, we have presented the results as the additive inverse of the overlap index (Greco et al. 2021). We used permutation tests to evaluate if the observed values of overlap deviated from random expectations for each contrast, i.e. winter versus summer for each species and each species pair within each season. These tests were based on 1000 permutated datasets where the time stamps were randomly re-assigned to each observation. We evaluated the likelihood of the observed overlap using Z score conversion but adjusted the corresponding two-tailed p values for multiple comparisons using a method controlling the false discovery rate (Benjamini and Hochberg 1995).
Results
Our observations were based on 27,731 individual camera trap nights, 15,185 during winter and 12,546 during summer. We obtained 44 independent observations of bears during winter and 79 during summer, 215 and 82 observations of wolves during winter and summer, respectively, and 238 observations of humans during winter and 108 during summer. These observations were recorded at 16 and 38 stations for bears during winter and summer, 61 and 29 stations for wolves and 60 and 41 stations for humans.
Bears had substantially lower activity in winter (mean occupancy ± SE = 0.33 ± 0.10) than in summer (0.84 ± 0.14), whereas the opposite was observed for wolves (winter 0.99 ± 0.08; summer 0.71 ± 0.16, Fig. 2). Humans, in contrasts, showed limited seasonal variation in activity (winter 0.89 ± 0.06; summer 0.85 ± 0.12) (Fig. 2).
Occupancy estimates for winter (December and January) and summer (June and July) for bears, wolves and humans in the Autonomous Region of Cantabria, northern Spain. Occupancy estimates were derived from occupancy models and based on camera trap data collected between 2016 and 2022, and should be interpreted as a relative index of seasonal activity within each species
Brown bears and wolves had less pronounced diel variation in activity in winter than in summer, with both species showing distinct crepuscular activity during summer (Fig. 3a, c) and mainly nocturnal activity in winter (Fig. 3b, d). Humans were strictly diurnal during both summer (Fig. 3e) and winter (Fig. 3f). There were significant differences in diel activity between summer and winter for both bears (Z = 3.45, padj = 0.001) and wolves (Z = 4.74, padj < 0.001), but not for humans (Z = 0.50, padj = 0.615). There were also significant differences in diel activity between both bears and wolves in winter (Z = 2.41, padj = 0.020), but not in summer (Z = 1.97; padj = 0.06). Diel activity of humans differed from both bears and wolves during winter (bears: Z = 10.54 padj < 0.001; wolves: Z = 21.31, padj < 0.001) as well as during summer (bears: Z = 9.59, padj < 0.001; wolves: Z = 8.00; padj < 0.001) (Table 1).
Probability distributions describing temporal activity of bears in winter (a) and summer (b), wolves in winter (c) and summer (d) and humans in winter (e) and summer (f). The probability distributions were estimated from camera trap observations made from 2016 to 2022 in the Autonomous Region of Cantabria, northern Spain
Discussion
We noted clear distinctions in the diel activity patterns of both bears and wolves when compared to humans. The two carnivores exhibited predominantly crepuscular or nocturnal activity, whereas humans displayed strictly diurnal activity. We suggest that these results indicate temporal avoidance of humans on a diel timescale. Such avoidance is likely a consequence of past or present persecution (Blanco et al. 1992; Fernández-Gil et al. 2016). The observed bear and wolf diel activity in Cantabria generally agrees with previous observations of these species (Theuerkauf et al. 2003; Ordiz et al. 2011; Støen et al. 2015), and our interpretation of these results lends further support to general suggestions of an increased nocturnality in animals due to human disturbance (Gaynor et al. 2018; Nix et al. 2018). However, the observed seasonal variation in activity of bears and wolves did not seem to be linked to similar seasonal variation in human activity. Therefore, the activity patterns in these two large carnivores appear to be dictated by a combination of human avoidance as well as other factors linked to energetic constraints and prey activity (e.g. Capellini et al. 2008; Vallejo-Vargas et al. 2022). Our study therefore points to the complexities involved with the regulation of animal activity across different timescales (Halsey et al. 2018).
We suggest several possible ecological processes that could have been influenced by the observed patterns of temporal activity in bears and wolves. First, a similar diel activity of bears and wolves might lead to an intensification of intra- and inter-specific competition. However, even if carnivore competition can be severe (Polis et al. 1989; Palomares and Caro 1999; Donadio and Burskik 2006), we suggest that competition is unlikely in our area. We base this suggestion on the different resource utilization of the two species, with Cantabrian bears generally being omnivores and scavengers (Clevenger et al. 1992; Naves et al. 2006; Rodríguez et al. 2007) whereas wolves are hyper-predatory (Lagos and Bárcena 2018; Janeiro-Otero et al. 2022). While both bears and wolves were primarily crepuscular or nocturnal, they did separate their diel temporal niches, especially during winter. There is also the possibility of spatial rather than temporal avoidance among the two species (e.g. Ordiz et al. 2015). However, if the diel activity patterns were caused by human avoidance, so that animals were forced into similar temporal niches, it could influence intra-specific competition. This may be particularly relevant for the bears, which may suffer from human-induced increased infanticide risk in our study area (Penteriani et al. 2020). Altered activity patterns in response to humans could also cause changes in predation patterns, mainly in the hyper-predatory wolf.
Bears showed a very marked seasonal pattern in overall activity, with lower activity in winter than in summer. While this observation agrees with the ecology of this species, which is largely dormant during winter across the majority of its range (Pasitschniak-Arts 1993), it provides further evidence for a lack of extended dormancy in this southern population (Nores et al. 2010; González-Bernardo et al. 2020). However, we suggest that the lower activity of bears during winter still highlights the energetic constraints experienced during colder periods (Capellini et al. 2008). We observed a largely crepuscular diel pattern in bear activity during summer, and a more nocturnal activity during winter. In northern Europe, where bears are hunted, bears become even more nocturnal when hunting season starts (Ordiz et al. 2012), as well as after encounters with people in the forest (Ordiz et al. 2013). Since there were less overall human activity during winter than during summer, and strict diurnal activity of humans during both seasons, bear seasonal shifts in diel activity might be prevalently caused by factors other than human avoidance.
Similar to the bears, the wolves exhibited mainly crepuscular diel activity during the summer, but this variation was less pronounced during winter, though they remained primarily active at night. Interestingly, the wolves showed increased activity in winter compared to summer. This lower summer activity could be attributed, in part, to restricted movements near breeding sites and broader territorial movements during non-breeding seasons to avoid prey depletion and conduct territorial surveillance (Jędrzejewski et al. 2001). On a diel timescale, lack of daytime activity in wolves has been mainly explained by temperature regulation, particularly during summer (Mech 1970), the need to hunt during times when prey are active (Torretta et al. 2017) and human avoidance (Ciucci et al. 1997; Frey et al. 2022). While we do not regard temperature regulation to be a particularly likely explanation in the Cantabrian Mountains, we recognize that we cannot distinguish between the effects of prey activity and direct human avoidance on wolf diel activity. However, as humans may influence the activity of many of the main prey species of wolves as well, we suggest that humans may influence wolf activity both directly, through avoidance behaviour, and indirectly by shifting the diel activity of prey (Monk et al. 2018). Such a shift in diel activity may influence wolf predation success, with possible demographic consequences both for the wolves and their prey (Wilson et al. 2020).
Humans, in contrast to bears and wolves, had a strictly diurnal diel activity but limited seasonal variation, both in overall activity and at a diel scale. A strictly diurnal human activity agrees with previous findings across the globe, and has frequently been suggested to cause increased nocturnality in wildlife as a way of avoiding disturbance (Gaynor et al. 2018; Shamoon et al. 2018; Gallo et al. 2022), although animals already occupying nocturnal niches may be less affected by human activity (Reilly et al. 2017; Khatiwada et al. 2023). The lack of seasonal variation in human activity was slightly surprising, considering the strong pulse of tourist into the region in summer (Insituto Nacional de Estadísitica 2023), as well as a documented shift in human diel activity when people are not working (Green et al. 2023). The observed lack of seasonal variations point suggests that the observed activity primarily consisted of local and regional residents, and also to some extent of professional activities such as animal husbandry or forestry.
Despite our study being based on a robust set of data collected during 6 consecutive years, we do recognize some limitations with our analyses. First, we have also only evaluated the activity of bears, wolves and humans along temporal scales. We recognise that behavioural responses to human activity might also include context-dependent spatio-temporal responses that were not captured in our analyses (e.g. Catford et al. 2022; Palmer et al. 2022), for instance related to the effects of density and distribution of human infrastructure or roads (e.g. González-Bernardo et al. 2023). Second, to achieve appropriate sample sizes for each season, we ran our analyses on pooled data across the whole study period. This was necessary, as the number of observations within individual seasons was too low for meaningful analyses, particularly for bears. Hence, we cannot rule out that our temporally pooled analyses could have masked changes in seasonal or diel variation in activity during the study period. Third, we based our study periods strictly on light regimes, and not on climate-based seasonal definitions or on biologically important periods for bears and wolves, e.g. during reproduction. While we recognize that other definitions of the study periods might have caused slight variations in the results, we justify our choice by the strong effects light regimes have on animal activity. Hence, we believe that our choice was appropriate for our analyses since it should generate strong seasonal contrast in activity. Finally, we asumed that seasonal differences in occupancy reflected seasonal differences in activity. Under the assumption that neither species differed in abundance across seasons, seasonal differences in occupancy could only be attributed to seasonal changes in activity or to seasonally altered space use. Altered activity would result in different number of observations whereas altered space use, given equal activity, would result in different number of camera stations with observations. Since we observed both, i.e. both different number of observations as well as different number of stations with observations, we believe that the observed seasonal differences in occupancy reflected actual variation in activity.
To conclude, we have highlighted important differences in the activity of two large carnivores, the brown bear and the wolf, and humans, during both summer and winter in a human-modified landscape. We suggest that these results were at least partly caused by a temporal avoidance of humans on a diel scale, which in the case of wolves may also have been a consequence of human avoidance by wolf prey. However, we also observed seasonal variation in overall activity and diel activity patterns of both bears and wolves that appeared to have been less linked to human presence, but rather to energetic constraints on movements for bears and to reproductive events for wolves. The complexity of the interactions of factors regulating large carnivore activity asks for further studies evaluating how humans influence the behaviour of large carnivores across different spatial and temporal scales.
Availability of data
The datasets analysed during the current study are available from the corresponding author on reasonable request.
References
Álvarez García MA (2007) La cartografía temática ambiental del Principado de Asturias. Conservación Vegetal 11:25–26
Ancell Trueba R, Célis Díaz R (2012) Termopluviometría de Cantabria durante el periodo 1981–2010 [Thermopluviometry of Cantabria during the period 1981–2010]. State Meteorological Agency, Madrid
Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Roy Stat Soc Ser B (methodol) 57:289–300. https://doi.org/10.1111/J.25176161.1995.TB02031.X
Blanco JC, Reig S, de la Cuesta L (1992) Distribution, status and conservation problems of the wolf Canis lupus in Spain. Biol Cons 60:73–80. https://doi.org/10.1016/0006-3207(92)91157-N
Breitenmoser U (1998) Large predators in the Alps: the fall and rise of man’s competitors. Biol Cons 83:279–289. https://doi.org/10.1016/S0006-3207(97)00084-0
Capellini I, Nunn CL, MacNamara P, Preston BT (2008) Energetic constraints, not predation, influence the evolution of sleep patterning in mammals. Funct Ecol 22:847–853. https://doi.org/10.1111/j.1365-2435.2008.01449.x
Catford JA, Wilson JRU, Pysek P, Hulme PE, Duncan RP (2022) Addredding context dependence in ecology. Trends Ecol Evol 37:158–170. https://doi.org/10.1016/j.tree.2021.09.007
Chapron G, Kaczensky P, Linnell JD, von Arx M, Huber D et al (2014) Recovery of large carnivores in Europe’s modern human-dominated landscapes. Science 346:1517–1519. https://doi.org/10.1126/science.1257553
Clevenger AP, Purroy FJ, Pelton MR (1992) Food habits of brown bears (Ursus arctos) in the Cantabrian Mountains, Spain. J Mammal 73:415–421. https://doi.org/10.2307/1382077
Ciucci P, Boitani L, Francisci F, Andreoli G (1997) Home range, activity and movements of a wolf pack in central Italy. J Zool 243:803–819. https://doi.org/10.1111/J.1469-7998.1997.TB01977.X
Dalerum F (2021) Socioeconomic characteristics of suitable wolf habitat in Sweden. Ambio 50:1259–1268. https://doi.org/10.1007/s13280-021-01524-y
Dalerum F, Swanepoel LH (2017) Humans as predators: an overview of predation strategies of hunters with contrasting motivational drivers. Arbor 193:a419. https://doi.org/10.3989/arbor.2017.786n4008
Darimont CT, Carlson SM, Kinnison MT, Paquet PC, Reimchen TE, Wilmers CC (2009) Human predators outpace other agents of trait change in the wild. Proc Natl Acad Sci 106:952–954. https://doi.org/10.1073/pnas.0809235106
Darimont CT, Fox CH, Bryan HM, Reimchen TE (2015) The unique ecology of human predators. Science 349:858–860. https://doi.org/10.1126/science.aac4249
Delgado-Serrano MM, Hurtado-Martos JÁ (2018) Land use changes in Spain. Drivers and trends in agricultural land use. EU Agrarian Law 7:1–8. https://doi.org/10.2478/eual-2018-0006
Doherty TS, Hays GC, Driscoll DA (2021) Human disturbance causes widespread disruption of animal movement. Nat Ecol Evol 5:513–519. https://doi.org/10.1038/s41559-020-01380-1
Donadio E, Buskirk SW (2006) Diet, morphology, and interspecific killing in Carnivora. Am Nat 167:524–536. https://doi.org/10.1086/501033
Durán-Gómez JA (2014) Catálogo de la Flora Vascular de Cantabria [Catalogue of the Vascular Flora of Cantabria]. Monografías e Botánicas Iberica No 13. Jolube Consultor Botánico y Editor, Jaca
Fernández-Gil A, Naves J, Ordiz A, Quevedo M, Revilla E, Delibes M (2016) Conflict misleads large carnivore management and conservation: brown bears and wolves in Spain. PLoS One 11:e0151541. https://doi.org/10.1371/journal.pone.0151541
Finnegan SP, Galvez-Bravo L, Silveira L, Tôrres NM, Jácomo ATA, Alves GB, Dalerum F (2021) Reserve size, dispersal and population viability in wide ranging carnivores: the case of jaguars in Emas National Park, Brazil. Anim Conserv 24:3–14. https://doi.org/10.1111/acv.12608
Frank B, Glikman JA (2019) Human-wildlife conflicts and the need to include coexistence. In: Frank B, Gilkman JA, Marchini S (ed) Human-wildlife interactions: turning conflict into coexistence. Cambridge University Press, Cambridge, pp 1–19. https://doi.org/10.1017/9781108235730.004
Frey S, Tejero D, Baillie-David K, Burton C, Fisher JT (2022) Predator control alters wolf interactions with prey and competitor species over the diel cycle. Oikos 2022:e08821. https://doi.org/10.1111/oik.08821
Frid A, Dill L (2002) Human-caused disturbance stimuli as a form of predation risk. Conserv Ecol 6:1. https://doi.org/10.5751/ES-00404-060111
Gallo T, Fidino M, Gerber B, Ahlers AA, Angstmann JL, Amaya M, Concilio AL, Drake D, Gay D, Lehrer EW, Murray MH, Ryan TJ, St Clair CC, Salsbury CM, Sander HA, Stankowich T, Williamson J, Belaire JA, Simon K, Magle SB (2022) Mammals adjust diel activity across gradients of urbanization. eLife 11:e74756. https://doi.org/10.7554/eLife.74756
Gaynor KM, Hojnowski CE, Carter NH, Brashares JS (2018) The influence of human disturbance on wildlife nocturnality. Science 360:1232–1235. https://doi.org/10.1126/science.aar7121
Gittleman JL, Funk SM, MacDonald DW, Wayne RK (2001) Carnivore conservation. Cambridge University Press
Grilo C, Lucas PM, Fernández-Gil A, Seara M, Costa G, Roque S, Rio-Maior H, Nakamura M, Álvares F, Petrucci-Fonseca F, Revilla E (2019) Refuge as major habitat driver for wolf presence in human-modified landscapes. Anim Conserv 22:59–71. https://doi.org/10.1111/acv.12435
González-Bernardo E, Delgado MDM, Matos DGG, Zarzo-Arias A, Morales-González A, Ruiz-Villar H, Skuban M, Maiorano CP, Balbontín J, Penteriani V (2023) The influence of road networks on brown bear spatial distribution and habitat suitability in a human-modified landscape. J Zool 319:76–90. https://doi.org/10.1111/jzo.13023
González-Bernardo E, Russo LF, Valderrábano-Cano E, Férnandez González A, Penteriani V (2020) The denning period in brown bears. Ecol Evol 10:6844–6862. https://doi.org/10.1002/ece3.6372
Government of Cantabria (2021) Red de seguimiento de fauna silvestre de Cantabria [Wildlife monitoring network of Cantabria]. Government of Cantabria, Santander
Graham K, Beckerman AP, Thirgood S (2005) Human–predator–prey conflicts: ecological correlates, prey losses and patterns of management. Biol Cons 122:159–171. https://doi.org/10.1016/J.BIOCON.2004.06.006
Greco I, Chizzola M, Meloro C, Swanepoel L, Tamagnini D, Dalerum F (2021) Similarities in diel activity, size and morphology between lions and sympatric carnivores. Hystrix 32:122–129. https://doi.org/10.4404/HYSTRIX-00434-2021
Green AM, Young E, Keller H, Grace T, Pendergast ME, Şekercioğlu CH (2023) Variation in human diel activity patterns mediates periodic increases in recreational activity on mammal behavioural response: investigating the presence of a temporal ‘weekend effect.’ Anim Behav 198:117–129. https://doi.org/10.1016/j.anbehav.2023.02.002
Halsey LG, Green JA, Twiss SD, Arnold W, Burthe SJ, Butler PJ, Cooke SJ, Grémillet D, Ruf T, Hicks O, Minta KJ, Prysta TS, Wascher CAF, Careau V (2018) Flexibility, variability and constraint in energy management patterns across vertebrate taxa revealed by long-term heart rate measurements. Funct Ecol 33:260–272. https://doi.org/10.1111/1365-2435.13264
Hovardas T (2018) Large carnivore conservation and management. Routeledge, London
Insituto Nacional de Estadísitica (2023) Movimientos turísticos de los españoles. https://www.ine.es/
Janeiro-Otero A, Álvarez X, Fernández Crespo C, Valero E, Dormann CF (2022) Grey wolf feeding habits and their geographical variation in Northwest Spain. Food Webs 32:e00248. https://doi.org/10.1016/j.fooweb.2022.e00248
Jayakody S, Sibbald AM, Gordon IJ, Lambin X (2008) Red deer Cervus elephus vigilance behaviour differs with habitat and type of human disturbance. Wildl Biol 14:81–91. https://doi.org/10.2981/0909-6396(2008)14[81:RDCEVB]2.0.CO;2
Jędrzejewski W, Schmidt K, Theuerkauf J, Jędrzejewski B, Okarma H (2001) Daily movements and territory use by radio-collared wolves (Canis lupus) in Bialowieza Primeval Forest in Poland. Can J Zool 79:1993–2004. https://doi.org/10.1139/Z01-147
Khatiwada AP, Wright W, Kunkel K, Khatiwada MP, Waterman C, Bhattarai S, Baral HS, Pokheral CP, Dalerum F (2023) Human influence on burrow activity of the Chinese pangolin in Nepal. Wildl Res 50:76–83. https://doi.org/10.1071/WR21024
Lagos L, Bárcena F (2018) Spatial variability in wolf diet and prey selection in Galicia (NW Spain). Mammal Res 63:125–139. https://doi.org/10.1007/s13364-018-0352-6
Larson CL, Reed SE, Merenlender AM, Crooks KR (2016) Effects of recreation on animals revealed as widespread through a global systematic review. PLoS One 11:e0167259. https://doi.org/10.1371/journal.pone.0167259
Leader-Williams N, Adams WM, Smith RJ (2010) Deciding what to save: trade-offs in conservation. In: Leader-Williams N, Adams W, Smith RJ (ed) Trade-offs in conservation: deciding what to save. Wiley-Blackwell, Chichester, pp 3–13. https://doi.org/10.1017/S0030605311001256
Mace GM (2014) Whose conservation? Science 345:1558–1560. https://doi.org/10.1126/science.1254704
MacKenzie DI, Nichols JD, Hines JE, Knutson MG, Franklin AB (2003) Estimating site occupancy, colonization, and local extinction when a species is detected imperfectly. Ecology 84:2200–2207. https://doi.org/10.1890/02-3090
MacKenzie DI, Nichols JD, Royle JA, Pollock KH, Bailey LA, Hines JE (2006) Occupancy modeling and estimation. Academic Press, New York
Madden F (2004) Creating coexistence between humans and wildlife: global perspectives on local efforts to address human–wildlife conflict. Hum Dimens Wildl 9:247–257. https://doi.org/10.1080/10871200490505675
Mech LD (1970) The Wolf: The Ecology and Behavior of an Endangered Species. The Natural History Press. https://doi.org/10.2307/1378616
Monk CT, Barbier M, Romanczuk P, Watson JR, Alos J, Nakayama S, Rubenstein DI, Levin SA, Arlinghaus R (2018) How ecology shapes exploitation: a framework to predict the behavioural response of human and animal foragers along exploration-exploitation trade-offs. Ecol Lett 21:779–793. https://doi.org/10.1111/ele.12949
Naves J, Fernández-Gil A, Rodríguez C, Delibes M (2006) Brown bear food habits at the border of its range: a long-term study. J Mammal 87:899–908. https://doi.org/10.1644/2F05-MAMM-A-318R2.1
Nix JH, Howell RG, Hall LK, McMillan BR (2018) The influence of periodic increases of human activity on crepuscular and nocturnal mammals: testing the weekend effect. Behav Proc 146:16–21. https://doi.org/10.1016/j.beproc.2017.11.002
Nores C, Ballesteros F, Blanco JC, García-Serrano A, Herrero J, Palomero G (2010) Evidence of non-hibernation in Cantabrian brown bears. Acta Theriol 55:203–209. https://doi.org/10.4098/j.at.0001-7051.085.2008
Ordiz A, Canestrari D, Echegaray J (2022) Wolf conservation and management in Spain, an open debate. Front Environ Sci 10:781169. https://doi.org/10.3389/fenvs.2022.781169
Ordiz A, Milleret C, Kindberg J, Månsson J, Wabakken P, Swenson JE, Sand H (2015) Wolves, people, and brown bears influence the expansion of the recolonizing wolf population in Scandinavia. Ecosphere 6:1–14. https://doi.org/10.1890/ES15-00243.1
Ordiz A, Støen OG, Delibes M, Swenson JE (2011) Predators or prey? Spatio-temporal discrimination of human-derived risk by brown bears. Oecologia 166:59–67. https://doi.org/10.1007/s00442-011-1920-5
Ordiz A, Støen OG, Sæbø S, Kindberg J, Delibes M, Swenson JE (2012) Do bears know they are being hunted? Biol Cons 152:21–28. https://doi.org/10.1016/J.BIOCON.2012.04.006
Ordiz A, Støen OG, Sæbø S, Sahlén V, Pedersen BE, Kindberg J, Swenson JE (2013) Lasting behavioural responses of brown bears to experimental encounters with humans. J Appl Ecol 50:306–314. https://doi.org/10.1111/1365-2664.12047
Palmer MS, Gaynor KM, Becker JA, Mumma MA, Pringle RM (2022) Dynamic landscapes of fear: understanding spatiotemporal risk. Trends Ecol Evol 37:911–925. https://doi.org/10.1016/j.tree.2022.06.007
Palomares F, Caro TM (1999) Interspecific killing among mammalian carnivores. Am Nat 153:492–508. https://doi.org/10.1086/303189
Palomo J, Gisbert J, Blanco JC (2007) Atlas y Libro Rojo de los Mamíferos Terrestres de España. Dirección General para la Biodiversidad-SECEM-SECEMU, Madrid
Pasitschniak-Arts M (1993) Ursus Arctos Mammalian Species 439:1–10. https://doi.org/10.2307/3504138
Peel MC, Finlayson BL, Mcmahon TA (2007) Updated world map of the Köppen-Geiger climate classification. Hydrol Earth Syst Sci 11:1633–1644. https://doi.org/10.5194/hess-11-1633-2007
Penteriani V, Zarzo-Arias A, del Mar Delgado M, Dalerum F, Gurarie E, Torre PP, Corominas TS, Vázquez VM, García PV, Ordiz A (2020) Female brown bears use areas with infanticide risk in a spatially confined population. Ursus 31:e2. https://doi.org/10.2192/URSUS-D-18-00019R4
Pirotta E, Booth CG, Costa DP, Fleishman E, Kraus SD, Lusseau D, Moretti D, New LF, Schick RS, Schwarz LK, Simmons SE, Thomas L, Tyack PL, Weise MJ, Wells RS, Harwood J (2018) Understanding the population consequences of disturbance. Ecol Evol 8:9934–9946. https://doi.org/10.1002/ece3.4458
Polis GA, Myers CA, Holt RD (1989) The ecology and evolution of intraguild predation—potential competitors that eat each other. Ann Rev Ecol Syst 20:297–330. https://doi.org/10.1146/ANNUREV.ES.20.110189.001501
Purvis A, Gittleman JL, Cowlishaw G, Mace GM (2000) Predicting extinction risk in declining species. Proc R Soc B Biol Sci 267:1947–1952. https://doi.org/10.1098/rspb.2000.1234
Quevedo M, Echegaray J, Fernández-Gil A, Leonard JA, Naves J, Ordiz A, Revilla E, Vilà C (2019) Lethal management may hinder population recovery in Iberian wolves. Biodivers Conserv 28:415–432. https://doi.org/10.1007/S10531-018-1668-X
Redpath SM, Young J, Evely A, Adams WM, Sutherland WJ, Whitehouse A, Amar A, Lambert RA, Linnell JDC, Watt A, Gutiérrez RJ (2013) Understanding and managing conservation conflicts. Trends Ecol Evol 28:100–109. https://doi.org/10.1016/j.tree.2012.08.021
Reilly ML, Tobler MW, Sonderegger DL, Beier P (2017) Spatial and temporal response of wildlife to recreational activities in the San Francisco Bay ecoregion. Biol Cons 207:117–126. https://doi.org/10.1016/j.biocon.2016.11.003
Ridout MS, Linkie M (2009) Estimating overlap of daily activity patterns from camera trap data. J Agric Biol Environ Stat 14:322–337. https://www.jstor.org/stable/20696577
Rivera M (2015) La oferta comercial de turismo activo de naturaleza en España: estructuración, tendencias recientes y contextualización territorial. Turismo y Sociedad 16:85–108. https://doi.org/10.18601/01207555.n16.06
Rode KD, Farley SD, Robbins CT (2006) Sexual dimorphism, reproductive strategy, and human activities determine resource use by brown bears. Ecology 87:2636–2646. https://doi.org/10.1890/0012-9658(2006)87[2636:sdrsah]2.0.co;2
Rodríguez C, Naves J, Fernández-Gil A, Obeso JR, Delibes M (2007) Long-term trends in food habits of a relict brown bear population in northern Spain: the influence of climate and local factors. Environ Conserv 34:36–44. https://www.jstor.org/stable/44520999
Rutz C, Loretto MC, Bates AE, Davidson SC, Duarte CM, Jetz W, Johnson M, Kato A, Kays MT, Primack RB, Ropert-Coudert Y, Tucker MA, Wikelski M, Cagnacci F (2020) COVID-19 lockdown allows researchers to quantify the effects of human activity on wildlife. Nature Ecology and Evolution 4:1156–1159. https://doi.org/10.1038/s41559-020-1237-z
Schmid F, Schmidt A (2006) Nonparametric estimation of the coefficient of overlapping—theory and empirical application. Comput Stat Data Anal 50:1583–1596. https://doi.org/10.1016/j.csda.2005.01.014
Shamoon H, Maor R, Saltz D, Dayan T (2018) Increased mammal nocturnality in agricultural landscapes results in fragmentation due to cascading effects. Biol Cons 226:32–41. https://doi.org/10.1016/j.biocon.2018.07.028
Stankowich T (2008) Ungulate flight responses to human disturbance: a review and meta-analysis. Biol Cons 141:2159–2173. https://doi.org/10.1016/j.biocon.2008.06.026
Støen OG, Ordiz A, Evans AL, Laske TG, Kindberg J, Fröbert O, Swenson JE, Arnemo JM (2015) Physiological evidence for a human-induced landscape of fear in brown bears (Ursus arctos). Physiol Behav 152:244–248. https://doi.org/10.1016/j.physbeh.2015.09.030
Sunquist ME, Sunquist F (2001) Changing landscapes: consequences for carnivores. In: Wayne RK, Gittleman JL, Funk SM, Macdonald D (ed) Carnivore conservation. Cambridge University Press, Cambridge, pp 399–418
Suraci JP, Clinchy M, Zanette LY, Wilmers CC (2019) Fear of humans as apex predators has landscape-scale impacts from mountain lions to mice. Ecol Lett 22:1578–1586. https://doi.org/10.1111/ele.13344
Sutherland WJ (2007) Future directions in disturbance research. Ibis 149:120–124. https://doi.org/10.1111/j.1474-919X.2007.00673.x
Theuerkauf J, Jȩdrzejewski W, Schmidt K, Okarma H, Ruczyński I, Śniezko S, Gula R (2003) Daily patterns and duration of wolf activity in the Białowieza Forest, Poland. J Mammal 84:243–253. https://doi.org/10.1644/1545-1542(2003)084%3C0243:DPADOW%3E2.0.CO;2
Torretta E, Serafini M, Imbert C, Milanesi P, Meriggi A (2017) Wolves and wild ungulates in the Ligurian Alps (Western Italy): prey selection and spatial-temporal interactions. Mammalia 81:537–551. https://doi.org/10.1515/mammalia-2016-0066
Vallejo-Vargas AF, Sheil D, Semper-Pascual A, Beaudrot L, Ahumada JA, Akampurira E, Bitariho R, Espinosa S, Estienne V, Jansen PA, Kayijamahe C, Martin EH, Guimarães M, Lima M, Mugerwa B, Rovero F, Salvador J, Santos F, Spironello WR, Uzabaho E, Bischof R (2022) Consistent diel activity patterns of forest mammals among tropical regions. Nat Commun 13:7102. https://doi.org/10.1038/s41467-022-34825-1
van Eeden LM, Crowthe MS, Dickman CR, Macdonald DW, Ripple WJ, Ritchie EG, Newsome TM (2018) Managing conflict between large carnivores and livestock. Conserv Biol 32:26–34. https://doi.org/10.1111/cobi.12959
Wetterberg L (1994) Light and biological rhythms. J Int Med 235:5–19. https://doi.org/10.1111/j.1365-2796.1994.tb01027.x
Woodroffe R, Thirgood S, Rabinowitz A (2005) People and wildlife: conflict or co-existence? Cambridge University Press, Cambridge
Wilson MW, Ridlon AD, Gaynor KM, Gaines SD, Stier AC, Halpern BS (2020) Ecological impacts of human-induced animal behaviour change. Ecol Lett 23:1522–1536. https://doi.org/10.1111/ele.13571
World Bank (2022) World Development Indicators: Population total-Spain 2021. World Bank. http://www.worldbank.org
Acknowledgements
Maria Miranda kindly reviewed a draft of the manuscript.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. Funding was provided by the Yo Investigo program by the Spanish National Research Council.
Author information
Authors and Affiliations
Contributions
TV and FD conceptualized the study, conducted data analyses and wrote the first draft of the manuscript, CM and VP assisted with data interpretation and manuscript preparation, and JG, MÁL, EM, PG, AC, BC, MJV and EÁ contributed with field data designed and carried out the field data collection. All authors commented on previous versions of the manuscript and have read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Vicedo, T., Meloro, C., Penteriani, V. et al. Temporal activity patterns of bears, wolves and humans in the Cantabrian Mountains, northern Spain. Eur J Wildl Res 69, 100 (2023). https://doi.org/10.1007/s10344-023-01728-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10344-023-01728-5