Abstract
Agricultural landscapes had been inhabited by a vast number of bird species in the past; however, especially in the last decades, agricultural intensification had negatively affected habitat composition. Habitat heterogeneity decreased and the number of many species inhabiting farmland has severely declined. These landscapes still offer a home for species, but with decreasing environmental variability, less suitable habitat might be available and interspecific competition might have been altered. Agricultural fields under organic farming are often assumed to provide adequate habitats for farmland birds, thus competition for these areas might be high and affect species’ habitat selection. We compared habitat selection of two typical farmland bird species, Grey Partridges (Perdix perdix) and Common Pheasants (Phasianus colchicus), to determine the extent of habitat overlap in agricultural landscapes under organic and conventional farming in spring. Our study showed that both species preferred study squares with high habitat heterogeneity. In addition, squares with agricultural fields (e.g. without culture, winter cereals and fallow land) under organic farming were preferred by Grey Partridges, while Common Pheasants were mainly found on study squares containing agricultural fields under conventional farming. A broad habitat width in respect to food selection might have driven habitat choice of Common Pheasants; however, occupation of agricultural fields under organic farming by Grey Partridge males might also explain habitat selection of Common Pheasants. Awareness should be raised when releasing captive-rearing pheasants because interspecific competition between Grey Partridges and Common Pheasants could also affect fecundity and survival of both species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Populations of many common farmland birds faced a rapid decline throughout the last decades in Europe (Gregory et al. 2019). Several changes in the agricultural landscape are assumed to account for this development. The intensification of agriculture — arable cropland as well as grassland — is characterized by homogenization and might have negatively affected farmland bird populations (Chamberlain et al. 2000; Donald et al. 2001; Geiger et al. 2010b). Rearrangement of parcels leads to increased field sizes and is thereby causing reduced availability of complex habitat structures accompanying fields, e.g. hedgerows and field margins (Benton et al. 2003). In addition, increased use of mineral fertilizers and pesticides may have negatively affected invertebrates (Geiger et al. 2010a), serving as food for many bird species during the reproductive period (Taylor et al. 2006). Ongoing changes in agricultural practices, thus, will also pose a threat to biodiversity of natural flora and fauna in future (Green et al. 2005).
Agricultural landscapes under organic farming seem to provide adequate habitats for many farmland birds in spring and summer during breeding seasons (Chamberlain et al. 1999; Fischer et al. 2011), in autumn during migration (Danhardt et al. 2010) and in winter (Chamberlain et al. 2010; Goded et al. 2018). Organic farming is characterized by high and wide hedgerows accompanying fields, small field sizes and rotational practices (Chamberlain et al. 1999). Thereby, organic farming provides more heterogeneous landscape types, finally leading to increased habitat diversity (Belfrage et al. 2005; Norton et al. 2009). The lack of chemical pesticides and inorganic fertilizers in organic farming is also positively affecting farmland birds (McKenzie and Whittingham 2009; McKenzie et al. 2011). And indeed, the agricultural landscape is still home to farmland birds.
The Grey Partridge (Perdix perdix) is one of the most important indicator species for biodiversity in agricultural areas due to its complex and large habitat requirements (Potts 2012). Another typical inhabitant of agricultural landscape is the Common Pheasant (Phasianus colchicus) (Burger 1988). These two small game species have benefited from traditional agriculture characterized by small farms and yield sizes (Guerrero et al. 2011). The European Red List Assessments showed that Grey Partridge (BirdLife International 2015a) and Common Pheasant (BirdLife International 2015b) are still widespread in Europe. Both species are evaluated as ‘Least Concern’ in Europe; however, while the population trend of Common Pheasants in Europe is increasing (BirdLife International 2015b), the Grey Partridge population trend appears to be decreasing (BirdLife International 2015a). When taking a closer look at the population development of these two bird species within Austria, it is striking that over the past 23 years (1998–2020), there has been a sharp decline in Grey Partridge populations (− 81%), while the Common Pheasant population has decreased less (− 27%) (Teufelbauer and Seaman 2021), raising the question, whether decreasing population trends might be related to the availability of suitable habitats in agricultural landscapes (see Harmange et al. 2019). Moreover, it is unclear, whether the remaining habitats are used by both species (habitat overlap) or exclusively used by either one or the other species. Species-specific knowledge is necessary to gain insight into habitat selection (Harmange et al. 2019); however, interspecific competition can shape habitat choice and thereby the species’ realized niches (Wang et al. 2020). Until now, only a few studies have focussed on the effects of interspecific competition on habitat selection of bird species having a strong association to agricultural areas (Robillard et al. 2012; Rinaud et al. 2020).
Grey Partridges and Common Pheasants prefer agricultural landscapes with a high proportion of cereals (Robertson et al. 1993; Harmange et al. 2019) and diverse crop cultivation (Henderson et al. 2009; Ronnenberg et al. 2016). Both species overwinter in groups, show breeding behaviour in spring, breed on the ground within dense vegetation (Anderson 2002; Potts 2012), and their chicks are insectivorous (Green 1984; Hill 1985; Browne et al. 2006). During breeding season, Grey Partridges seem to prefer wild vegetation (Panek 2002) and Common Pheasants are often found in areas with meadows and tree plantations (Chiatante and Meriggi 2022). However, while Grey Partridges are socially and genetically monogamous bird species (Vaněčková 2011), Common Pheasants are mostly polygynous (Ridley and Hill 1987). In addition, male Common Pheasants display very noticeable by following a ritual consisting of several phases (Czyżowski et al. 2020), compared to male Grey Partridges (Glutz von Blotzheim et al. 1973) in spring. Pheasant cocks look for open areas when calling (Lachlan and Bray 1976), as the display behaviour is often accompanied by visual signals like posture or fluttering of the wings (Czyżowski et al. 2020). In contrast, male Grey Partridges are calling and standing upright in front of a female (Glutz von Blotzheim et al. 1973).
Similarities in the choice of habitat used for breeding and foraging might lead to competition for resources available within the agricultural landscape (see Potts 1970; McCrow 1982). It is assumed that interspecific competition increases once habitat overlap of two species extends above a tolerable degree (Pianka 1984), thereby probably affecting individual fitness (Martin and Martin 2001). To overcome this issue, species usually adapt their habitat selection, but it is unclear whether the used habitats of Grey Partridges and Common Pheasants overlap in agricultural landscapes under conventional and organic farming.
To gain insight into interspecific habitat selection of farmland bird species, we collected data on environmental habitat use of Grey Partridge and Common Pheasant. We analysed habitat selection of both species in spring by calculating Manly’s selectivity ratios, focussing on agricultural management systems (organic vs. conventional) and small-scale habitat parameters (agricultural fields). In addition, habitat overlap was studied using Broennimann’s framework of implementing a principal component analysis to originally study niche selection (Broennimann et al. 2012), describing the environmental parameters in the agricultural landscape. We assume that study plots with high habitat heterogeneity and high surface area covered by fields under organic farming are preferably used by Grey Partridges and Common Pheasants. In addition, we assume that Common Pheasants prefer open areas (e.g. agricultural areas without mature crops) in spring, since these sites provide an ideal habitat for cocks’ display behaviour.
Material and methods
Study area
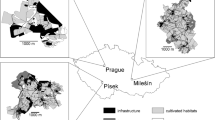
Habitat use of Grey Partridges and Common Pheasants was examined in our study area (1064 ha) located in Lower Austria (48°25′51.35″N, 16°30′51.58″E), northeast of the Austrian capital Vienna in the district of Mistelbach. Within the district, 10–20% of the livestock farms were managed according to organic farming principles in 2017 (Rech et al. 2020). The study area itself is characterized by a flat to slightly hilly landscape and is strongly dominated by arable farmland. The northern region of our study area is dominated by conventional farming, while organic agriculture predominates in the southern part (Fig. 1). The climate within the study area is characterized by a conspicuous lack of precipitation (mean annual precipitation ranges between 400 and 600 mm), hot summers (mean monthly temperature June–August: 20.9 °C) and moderately cold winters (mean monthly temperature December–February: 1.6 °C, Höfler et al. 2020). Two adjacent hunting grounds existed within the study area (see also Cybulska et al. 2020). Predator control (e.g. Red Fox Vulpes vulpes, Carrion Crow Corvus corone) took place in both hunting grounds based on the legal foundation. The hunting season for Grey Partridges and Common Pheasants in the study area starts on 21st September and 1st October and ended on 30th November and 31st December, respectively. Within the whole study area, annual harvest rates of Common Pheasants range between 5 and 6 individuals per 100/ha, while Grey Partridges are not shot in the study site. Neither Grey Partridges, nor Common Pheasants are released in the hunting estates within the study region.
Map of the study area located in Lower Austria. Occurrence of Grey Partridges and Common Pheasants was assessed within 29 study squares in spring 2018
(Source: INVEKOS Schläge Österreich 2018: © Agrarmarkt Austria, CC BY 4.0; Administrative boundaries: © EuroGeographics, Eurostat/GISCO: https://ec.europa.eu/eurostat/web/gisco/geodata/reference-data/administrative-units-statistical-units/countries#countries20, access date: 17-Aug-2022)
A grid was created, placed over the study area and occurrence of Grey Partridges and Common Pheasants was determined (see Fig. 1). A grid size of 300 × 300 m (9 ha) was small enough to have an overview of the terrain within each individual square. The open and semi-open landscape allowed for an easy detection of both species, especially because there was no to little vegetation on the agricultural fields at this time of year. In addition, the grid size approximately reflects the home range of Grey Partridges (winter: 14.4 ha, spring: 6.8 ha, Buner et al. 2005) and Common Pheasants (11.1 ha, Draycott et al. 2009) during breeding season. Grid squares were regularly spaced and a total of 29 squares were systematically selected. Unsuitable habitats for both species (e.g. settlements and forests) were avoided. Each square touched other squares only at corner points to minimize spatial autocorrelation. The distance between squares dominated by conventional farming in the northern part of the study site and squares dominated by organic farming in the southern part of the study site varied between a minimum of 424 and a maximum of 4460 m (mean ± SD, 2266 ± 847 m).
Occurrence of Grey Partridges and Common Pheasants
Grey Partridge and Common Pheasant occurrences were assessed within two field trips, which were performed by a single field observer. The two field trips were conducted in spring between 5th March and 6th April 2018 (see Online Resource, Table S1). The order of monitoring squares was random, but the minimum time interval between the two repeated observations of a study square was at least 7 days (max: 30 days, mean ± SD, 15.1 ± 5.7 days). Sampling effort within and between the two field trips was equal. In case a square was visited in the early morning in the first field trip, the second field trip was made in the evening or vice versa. Thus, both species were counted once in the early morning (starting 30 min before sunrise) and once in the evening (starting 30 min before sunset), respectively. Survey points were located in the centre of every grid square. Thereby, distance between neighbouring survey points was at least 424 m, reducing probability of double counting. When the field observer approached the survey point, birds had 3 min to settle, before the count itself started. Within each square, the landscape was visually observed and listened for spontaneously calling males. In addition, the observer used playbacks of Grey Partridges for 1 min and counted the answering males during the next 3 min after playback (Panek 1998), regardless of whether Grey Partridges were seen or heard before. We are aware that playback calls affect detection probability of Grey Partridges (Kasprzykowski and Goławski 2009; Warren et al. 2018), but since playback was used in all squares, once in the morning and once in the evening, we assume that sampling effort and detection probability of Grey Partridges was uniform. There are no indications that Grey Partridge calls affect Common Pheasant behaviour. Surveys were only conducted during good weather conditions (no rainfall, fog or strong winds). Location and movement of each male Grey Partridge or male Common Pheasant was mapped in a field map to avoid false double counts. The count period lasted for 8 min at each count point.
Land use
We digitally analysed land cover within our 29 study squares by using the software ArcGIS version 10.7 (ESRI) and open government data (INVEKOS Schläge Österreich 2018, © Agrarmarkt Austria, CC BY 4.0) to quantify the surface area (hectare, ha) covered by different habitat types within each square (Table 1). Within each field, the habitat type and its area were determined during fieldwork. Moreover, two local hunters provided information on local farming practices (organic vs. conventional). In Austria, organic farming follows the EU Regulations No 834/2007 and 889/2008, including amendments (Rech et al. 2020). Organic farmers are certificated through a control body and controlled by independent inspection bodies (Rech et al. 2020).
To study differences between fields under organic or conventional farming, ground vegetation characteristics at three random points within each agricultural field were determined. Within three 1 × 1-m plots delimited in a habitat type-specific field, ground vegetation density (%) in five height classes (classified as 0, 1–5, 6–25, 26–50, and > 50 cm) covering the soil was measured. We also collected data on additional “border” habitat structures, not linked to organic or conventional farming, that Grey Partridges and Common Pheasants might use, such as hedgerows or field paths (including linear green strips alongside paths). Paved roads, settlements and forests were assumed to be unsuitable habitats for both species. Serving as a proxy of study square land use type heterogeneity, we calculated Shannon–Wiener-indices using the R package “vegan” (Oksanen et al. 2022).
Habitat selection
Habitat selection was studied by calculating Manly’s selectivity ratios (wi) (Manly et al. 2002). Values of wi < 1 indicate avoidance of habitat types, values of wi > 1 indicate a preference of habitat types and values of wi = 1 indicate that the habitat type is used in proportion to the availability in the study site. For easier interpretation, selection ratios are standardized (Bi) and add to 1. With a value ranging from 0 to 1, Bi gives the estimated probability that a habitat type would be the next one selected, if all habitat types were equally available. We assume that habitat types within the 29 squares were available for all animals within the study site because average distance between squares dominated by conventional and organic farming (mean ± SD, 2266 ± 847 m) was similar to dispersal distances found for Grey Partridges (see Šálek and Marhoul 2008; Rymešová et al. 2013) and Common Pheasants (Felley 1996). Therefore, analyses were made at the population level (Manly design I). The relative amount of all different available habitat types within the 29 squares was compared to the proportion of habitat types which were used by Grey Partridges or Common Pheasants. We therefore used the location of each male Grey Partridge and male Common Pheasant detected within the first and the second field trips, which were mapped throughout the field trips in a field map, to assess the used habitat type. Calculations were done by using the R package “adehabitatHS” (Calenge 2006) and estimated significance of selection of a particular habitat type by conducting log-likelihood chi-squared tests, using a corrected Bonferroni level. Frequencies of detected Grey Partridges or Common Pheasants for each habitat type should be five or more for the chi-squared test to be valid; otherwise, results have to be interpreted with caution (Manly et al. 2002). Therefore, we calculated Manly selectivity indices (i) on a coarse level for the two farming practices (organic vs. conventional) and all other remaining habitat types (others) combined (habitat categories, Bonferroni level 0.017, see Table 2) and (ii) on a fine level focussing on the habitat types without culture, winter cereals, lucerne and fallow land separately, and combined for the remaining rare farming cultures (vineyard, green manure, fennel, oilseed rape, poppy, orchard), the remaining rare grasslands (meadow, grazing area) and all other remaining habitat types (hedgerow, field path, roads, forests, water bodies and settlements) (habitat types, Bonferroni level 0.007, Table 2).
Habitat overlap, equivalency and similarity
More specific habitat analyses on the effect of farming practices in agricultural fields (organic vs. conventional) were then performed using the methodology proposed by Broennimann et al. (2012) and the R package “ecospat” (Di Cola et al. 2017). To avoid duplicate occurrences, pseudo-replication or effects of imperfect detection when analysing the data, we combined the datasets of the two field trips. Therefore, we determined occurrence (presence vs. absence) of Grey Partridges or Common Pheasants for each study square by defining “presence” of the species, when at least one individual of the corresponding species had been recorded once, either throughout the first or throughout the second field trip (see Online Resource, Table S1).
We then analysed habitat overlap of both species across the grid squares. Highly correlated environmental variables were transformed into two uncorrelated linear combinations (axes) by using a principal component analysis (PCA-env, see Broennimann et al. 2012, for detailed information on the procedure). The habitat types included in the PCA were without culture, winter cereals, lucerne and fallow land (Table 1). In addition, the Shannon–Wiener Index of each square was included in the PCA to account for habitat heterogeneity within squares. The PCA scores of the first two axes for both species were projected onto a grid of cells (100 × 100 cells), where each axis summarizes all the selected environmental variables and each cell corresponds to a unique set of environmental conditions (Broennimann et al. 2012). Then, smoothed density of occurrence for each species was estimated using a Gaussian kernel density function and overlap between species was visualized (Di Cola et al. 2017).
Habitat overlap between species and within species over time was quantified by calculating the metric Schoener´s D (Schoener 1970; Broennimann et al. 2012), which ranges from 0 (no overlap) to 1 (total overlap). Afterwards, habitat equivalency and habitat similarity were tested, following a statistical framework proposed by Warren et al. (2008), originally focussing on niche selection and adapted for our purpose to study habitat selection; while the first test predicts that used habitats between species or within species over time are identical, the latter predicts that used habitats are more similar than expected by chance (niche conservatism, Wiens and Graham 2005; Warren et al. 2008). Thereby, habitat equivalency tests only consider the exact space occupied by the species (conservative), whereas habitat similarity tests also account for the surrounding environmental space where the species occur (less conservative) and are performed in both directions (species 1 vs. species 2, species 2 vs. species 1) (for details see Broennimann et al. 2012). For both analyses, observed D values of the species were compared to the distribution of simulated D values, which were based on 1000 iterations. A p-value < 0.05 was used to evaluate significance of habitat equivalency and similarity. Thus, if the observed D values fall within 95% (p > 0.05) of the simulated D values, the hypotheses of habitat equivalency or habitat similarity cannot be rejected (Broennimann et al. 2012). Dynamics of the environmental variables contributing most to the two PC axis and affecting species’ occurrence density were visualized. All analyses were performed using the software R 3.6.2 (R Development Core Team 2019). Mean ± standard deviation (SD) are shown where appropriate.
Results
Occurrence of Grey Partridges and Common Pheasants
Within the first field trip, Grey Partridges and Common Pheasants were detected in 41.4% (n = 12) and 65.5% (n = 19) of all study squares, respectively (see Online Resource, Table S1). The number of individual Grey Partridges detected within a square of 300 × 300 m ranged from 0 to 5 (in total: 31 individuals), with a Grey Partridge density of 11.9 individuals/km2 in the 29 study squares. The number of Common Pheasants detected within a square ranged from 0 to 3 (in total: 27 individuals; density, 10.3 individuals/km2). Both species were detected within less study squares during the second field trip. Grey Partridges and Common Pheasants were detected on 31.0% (n = 9) and 58.6% (n = 17) squares (see Online Resource, Table S1). While the number of Grey Partridges counted within the 29 study square decreased (in total: 12 individuals, range: 0 to 3 individuals per square; density: 6.5 individuals/km2), the number of Common Pheasants increased (in total: 36 individuals, range: 0–4 individuals per square; density: 13.8 individuals/km2) from the first to the second field trips. When combining the data of the two field trips, a total of 43 Grey Partridges and 63 Common Pheasants were detected within 13 and 24 study squares, respectively.
Land use and habitat heterogeneity
Almost half of the surface of all 29 study squares (mean ± SD, 4.4 ± 3.1 ha per square) was covered by agricultural land under conventional farming (see Table 1). Slightly less surface (mean ± SD, 3.6 ± 3.4 ha per square) was covered by agricultural land under organic farming. The remaining surface area of the 29 study squares (mean ± SD, 1.0 ± 0.9 ha per square) was covered by other land use categories (e.g. hedgerows: mean ± SD, 0.6 ± 0.9 ha per square, field paths: mean ± SD, 0.3 ± 0.2 ha per square of total surface area). Roads, forests, water bodies and settlements covered each less than 1% of the total 29 study square surface area (together 1.7%). In total, only 5 study squares did not contain any arable field under organic farming at all. In contrast, all 29 study squares did contain arable fields under conventional farming. Habitat heterogeneity within study squares varied (Shannon–Wiener-indices: range: 0.9–2.2, mean ± SD, 1.4 ± 0.4).
Almost one third of the surface of all 29 study squares was covered by arable land, which had been prepared for cultivation, but crops had not yet started to grow when field work was conducted (without culture: mean ± SD, organic: 1.3 ± 1.8 ha per square; conventional: 1.3 ± 1.7 ha per square, Fig. 2). Winter cereals (e.g. winter wheat Triticum aestivum) were cultivated on 28.8% of the total surface area (mean ± SD, organic: 1.2 ± 1.7 ha per square; conventional: 1.4 ± 1.4 ha per square). Winter cereals had just started to germinate and grow in spring. Hence, vegetation density measured at different height classes in fields under organic and conventional management was similar (Fig. 2). About 10.5% of the total surface area was covered by lucerne (Medicago sativa) (mean ± SD, organic: 0.5 ± 1.1 ha per square; conventional: 0.4 ± 1.2 ha per square). Agricultural fields with lucerne under organic farming had a high vegetation density at height class 1–5 cm, whereas a high proportion of agricultural fields with lucerne under conventional farming were barely covered with any vegetation (Fig. 2).
The remaining arable area was covered by the farming cultures vineyards (mean ± SD, organic: 0.1 ± 0.3 ha per square; conventional: 0.5 ± 1.0 ha per square, see Online Resource, Fig. S1), green manure (mainly field been Vicia faba and phacelia Phacelia tanacetifolia) (mean ± SD, organic: 178.5 ± 755.3 m² per square; conventional: 1983.8 ± 10,497.4 m² per square, Fig. S1), fennel (Foeniculum vulgare) (mean ± SD, organic: 0.2 ± 0.5 ha per square, Fig. S1), oilseed rape (Brassica napus) (mean ± SD, conventional: 0.1 ± 0.3 ha per square, Fig. S1), poppy (Papaver somniferum) (mean ± SD: conventional, 0.1 ± 0.4 ha per square, Fig. S1) and orchard (mean ± SD, conventional: 132.5 ± 498.9 m² per square, Fig. S1). Fallow land occurred within 17 study squares and covered 5.1% of the whole study surface area (mean ± SD: organic, 0.1 ± 0.3 ha per square; conventional: 0.3 ± 0.7 ha per square). Vegetation density of different height classes of fallow land under organic and conventional farming was similar (Fig. 2). The other rare grassland types occurring within our study site were meadows (mean ± SD: organic, 0.1 ± 0.3 ha per square; conventional: 0.1 ± 0.2 ha per square, Fig. S1) and grazing areas (mean ± SD: organic, 0.1 ± 0.3 ha per square, Fig. S1).
Habitat selection
Grey Partridges preferred habitats under organic farming and avoided habitat under conventional farming (Table 2). There is also an indication that Grey Partridges avoid other habitat, but as only a single Grey Partridge was detected within this habitat category, the result has to be interpreted with caution. Habitats under organic farming (Bi = 0.709) were more than two times as likely to be selected by Grey Partridges as habitats under conventional farming (Bi = 0.207, Table 2). In contrast, Common Pheasants seemed not to select specific habitat categories; indicating a proportional use to their availability (Table 2). Most of the Grey Partridges and Common Pheasants were detected within fields covered by winter cereals, followed by fields without culture, fields covered by lucerne and fallow land (in descending order, Table 2). However, neither Grey Partridges nor Common Pheasants seemed to prefer specific habitat types per se.
Overlap and similarity in habitat selection
The first two axes of the PCA captured 28.5% and 19.7% of the variation within the environmental variables (Fig. 3). The land cover variable “winter cereals.org” and “without culture.con” contributed most to PC1, whereas “Shannon-diversity index”, “fallow land.org” and “without culture.org” contributed most to PC2 (Online Resource, Table S2).
Habitat types used by Grey Partridges showed a relative high overlap with habitats used by Common Pheasants (D = 64.2%, see Fig. 4). The observed D value of Grey Partridges and Common Pheasants fell within the density of 95% of simulated D values, thus, the hypothesis of retained habitat equivalency could not be rejected (p = 0.484, Online Resource, Fig. S2). Habitats used by Grey Partridges were not more similar to habitats used by Common Pheasants than expected by chance (p = 0.067, Fig. S2), but used habitats of Common Pheasants were more similar to habitats of Grey Partridges than expected by chance (p = 0.002, Fig. S2).
Occupancy of the two-dimensional environmental space used by a Grey Partridges, b Common Pheasants and c habitat overlap between species (Grey Partridge in light grey and Common Pheasant in grey, overlap in dark grey) monitored in spring (5th March until 6th April 2018). The grey gradient in figure (a) and (b) corresponds to the increase in occurrence density of the species. The solid line in the habitat overlap plot (c) corresponds to the limit of the environmental space, which was available in the 29 study squares. The dashed line corresponds to the 75% most frequently available conditions
Habitat dynamics of Grey Partridges and Common Pheasants were affected by environmental variables (Fig. 5). Density of occurrence of both species shifted towards squares with high habitat heterogeneity, with a more pronounced effect in highly heterogeneous squares being detected in Grey Partridges (Fig. 5). While Grey Partridges seemed to occur on study squares with a high amount of surface area not yet covered by any vegetation, covered by winter cereals and fallow land under organic farming, Common Pheasants seemed to prefer squares without culture under conventional farming (Fig. 5).
Habitat dynamics of the environmental variables contributing most to the two PC axis and affecting species´ occurrence density of Grey Partridge and Common Pheasant. Smoothed and rescaled species densities (ranging between 0 and 1) along environmental variables are shown for Grey Partridge (dashed lines) and Common Pheasant (dotted lines) ranges. The solid black contour line delimits the 100th quantile of the density at the land use category
Discussion
Grey Partridges and Common Pheasants are typical inhabitants of agricultural landscapes (Potts 2012; Ronnenberg et al. 2016), and we showed in our study that the agricultural landscape was used by Grey Partridges and Common Pheasants and that habitats used by both species overlapped to a high extent. Grey Partridges preferred squares with agricultural farmland under organic farming, while Common Pheasants did not show a clear preference for organic or conventional farming per se. Study squares with high habitat heterogeneity seemed to have a higher importance to Grey Partridges in comparison to Common Pheasants. However, results have to be interpreted with caution, as both species were observed only during two field trips in spring. Datasets of the two field trips were combined to avoid that habitat overlap, and similarity analyses were affected by imperfect detection; an issue that can also be accounted for by analysing data using occupancy models (MacKenzie et al. 2006), when an adequate number of sampling replicates is available (MacKenzie and Royle 2005; Paniccia et al. 2018). In addition, the two main axes of the principal component analyses, which described the environmental variables to study habitat similarities of both species explained only a part (48.2%) of the environmental variation in the study squares.
Grey Partridges usually form family groups in late summer and stay together in coveys during winter (Potts 2012). In spring, they start to separate again; males show courtship display behaviour; and finally breeding pairs are formed (Potts 2012; Rinaud et al. 2020). Common Pheasants concentrate on traditional areas, which provide food and shelter to overwinter in groups (Gates and Hale 1974). Cock dispersal from wintering areas, as well as display of territorial behaviour starts in late March (Gates and Hale 1974). Survival of both species is dependent on habitat structures, providing not only shelter from severe weather conditions and predators (Gottschalk and Beeke 2014), but also access to food (Anderson 2002; Bro et al. 2004). Since predation risk close to or along linear or rare habitat structures (e.g. hedgerows and field boundaries) is high (Morris and Gilroy 2008; Černý et al. 2020), extensive usage of such structures might be especially risky for species roosting on the ground. Using telemetry, Rantanen et al. (2010) showed that probability of survival of Grey Partridges in spring increased when breeding pairs stayed in crop fields, whereas survival decreased when they stayed on field margins. Maladaptation, which was proven for newly released Grey Partridge pairs preferring high risk habitats (Rantanen et al. 2010), seemed not to occur in wild birds in our study site, since density of occurrence of Grey Partridges and Common Pheasants was high in squares with high habitat heterogeneity. In contrast, a study performed in peri-urban farmland in the vicinity of the city of Vienna (Austria) showed that Grey Partridges’ selected habitats with low habitat diversity (Hille et al. 2021).
Differences in vegetation density at various height classes in agricultural fields under organic and conventional farming were barely visible (except in lucerne) in our study; thereby indicating that vegetation height might not be the most important factor affecting habitat choice of Grey Partridges and Common Pheasants in spring. Instead, other factors (e.g. food availability) associated with farming practices might have affected habitat suitability. Food of both species mainly consists of grains and leaves of cereals during winter (Gates and Hale 1974; Huss 1983), but insect diet is of high importance during chick rearing (e.g. sawfly larvae, Browne et al. 2006; Potts 2012; Warren et al. 2017). Thus, habitats providing such food items are necessary to successfully raise chicks (Potts 2012).
In organic farming, the usage of chemical inputs (e.g. insecticides, herbicides and fungicides) and fertilizers is restricted. The reduced use of synthetic pesticides is associated with an increase of invertebrate densities and weed cover; thereby positively affecting availability of insects for insectivorous birds and chick survival rates (Chiverton 1999). And indeed, a meta-analysis showed that organic farming has a positive effect on birds, predatory insects (e.g. carabids and spiders) and weed abundance (Bengtsson et al. 2005; Fuller et al. 2005; but see also Gabriel et al. 2010). Grey Partridges, known to be affected by pesticides causing dramatic reductions in food availability (Kuijper et al. 2009), might therefore have occupied study squares mainly consisting of agricultural fields under organic farming in our study site. Nesting sites of Grey Partridges can be located within cereal fields in open landscapes (Bro et al. 2000), permanent vegetation (Panek 2002; Gottschalk and Beeke 2014) and unmanaged habitats (Černý et al. 2020). Mechanical techniques to control weeds in cereal fields, mainly used in organic farming, can however be problematic during breeding seasons, as nests can be destroyed and chicks or incubating females can be killed (Newton 2004). Pesticides can, however, also affect farmland birds directly, if pesticide-treated seeds are consumed by the animals during the sowing season (Fernández-Vizcaíno et al. 2022). Several experimental studies showed that seed-treatment can affect sexual hormones (Fernández-Vizcaíno et al. 2020), offspring development (Gaffard et al. 2022) and reproductive performance (Lopez-Antia et al. 2018). In addition, imidacloprid-treated seeds consumed by granivorous birds (e.g. Grey Partridge) can have lethal effects (Millot et al. 2017). A study of Draycott et al. (2009) has shown that Common Pheasants preferred rotational set-asides planted with seed mixtures of grasses and forbs during brood-rearing, thereby indicating the importance of insect-rich foraging habitats. In addition, Common Pheasant harvest rates seem to be positively related to the proportion of agricultural fields under organic farming (Holá et al. 2015). In our study, Common Pheasants seemed to rather use agricultural fields without culture under conventional than under organic farming. The usage of this habitat type by Common Pheasants cannot be explained by a more pronounced selection of study squares with low vegetation, as vegetation height of agricultural fields without culture under conventional and organic farming was rather similar. However, the detection of Common Pheasants within these sites might be explained by two, non-exclusive phenomena: (i) Common Pheasants might either be less specialized in their habitat and food selection (Potts 1970) and thus have not shown any preference for agricultural sites under organic or conventional farming per se or (ii) interspecific competition for habitats might have affected habitat choice of Common Pheasants (Wang et al. 2018, 2020) and Grey Partridges. To test the mechanisms of interspecific competition between both species in more detail, field experiments (e.g. using telemetry) have to be conducted to compare habitat selection on sites, where both species occur individually or together (see also Rinaud et al. 2020) and subsequent effects on fecundity and survival have to be monitored (Martin and Martin 2001). Common Pheasants can compete with Grey Partridges for nesting habitats (McCrow 1982; Bro et al. 2000; Anderson 2002) and invertebrate food resources (Potts 1970; Gates and Hale 1974; Green 1984), but can also transmit parasites (Tompkins et al. 1999, 2000). Assuming that interspecific competition between Grey Partridges and Common Pheasants could exist and affect population trends (Vrezec 2006; McCrow 1982), awareness should be raised with regard to large-scale captive-reared and released pheasants (Bicknell et al. 2010).
The availability of suitable habitat is of high importance to reduce the sharp decline in farmland bird populations (Harmange et al. 2019). Resources provided within these habitats (e.g. nesting sites and food items) can be used by a variety of bird species and interspecific competition for breeding and foraging habitats can affect a species’ habitat selection, breeding success (Martin and Martin 2001) and thereby population trends (Vrezec 2006). Whether purely promoting organic farming is the key to halt the decline in Grey Partridge populations is, however, under debate, since arable land under “environmentally friendly management” (Marja et al. 2014) seems to provide enough suitable habitat for farmland birds (Kragten and de Snoo 2008). Moreover, some studies found no differences in Grey Partridge abundance between organic and conventional farming (Chamberlain et al. 1999, 2010), thereby indicating that a variety of other factors can also strongly affect abundance in agricultural landscapes. Measures that can be carried out by both, conventional and organic farmers (Bengtsson et al. 2005), are for example, the extent of pesticides and fertilizers used (Chiverton 1999), the choice of crop diversity (Ronnenberg et al. 2016), the crop sequence patterns (Joannon et al. 2008) and the provisioning of additional habitat structures (e.g. hedgerows: Batary et al. 2010; uncultivated habitats: Černý et al. 2020; manure heaps: Šálek et al. 2020). Indeed, recent studies showed that positive effects of organic farming are highlighted especially in homogeneous landscapes (Danhardt et al. 2010) or in regions with intensive agriculture (Kirk et al. 2020). Thus, even agricultural landscapes under conventional farming can provide valuable habitats for farmland birds, if overall landscapes heterogeneity is high (Benton et al. 2003; Smith et al. 2010).
Data availability
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Anderson BC (2002) Habitat use and nesting ecology of ring-necked pheasant (Phasianus colchicus) on a landscape dominated by agriculture in Lower Austria. Master thesis, University of Georgia, Athens, Georgia
Batary P, Matthiesen T, Tscharntke T (2010) Landscape-moderated importance of hedges in conserving farmland bird diversity of organic vs. conventional croplands and grasslands. Biol Conserv 143:2020–2027. https://doi.org/10.1016/j.biocon.2010.05.005
Belfrage K, Bjorklund J, Salomonsson L (2005) The effects of farm size and organic farming on diversity of birds, pollinators, and plants in a Swedish landscape. Ambio 34:582–588. https://doi.org/10.1639/0044-7447(2005)034[0582:Teofsa]2.0.Co;2
Bengtsson J, Ahnstrom J, Weibull AC (2005) The effects of organic agriculture on biodiversity and abundance: a meta-analysis. J Appl Ecol 42:261–269. https://doi.org/10.1111/j.1365-2664.2005.01005.x
Benton TG, Vickery JA, Wilson JD (2003) Farmland biodiversity: is habitat heterogeneity the key? Trends Ecol Evol 18:182–188. https://doi.org/10.1016/s0169-5347(03)00011-9
Bicknell J, Smart J, Hoccom D, Amar A, Evans A, Walton P, Knott J (2010) Impacts of non-native gamebird release in the UK: a review. RSPB Research Report Number 40. ISBN: 978–1–905601–26–4:73.
BirdLife International (2015a) Species factsheet: Perdix perdix. http://datazone.birdlife.org/userfiles/file/Species/erlob/summarypdfs/22678911_perdix_perdix.pdf. Accessed 17 August 2022
BirdLife International (2015b) Species factsheet: Phasianus colchicus. http://datazone.birdlife.org/userfiles/file/Species/erlob/summarypdfs/45100023_phasianus_clchicus.pdf. Accessed 17 August 2022
Bro E, Mayot P, Corda E, Reitz F (2004) Impact of habitat management on grey partridge populations: assessing wildlife cover using a multisite BACI experiment. J Appl Ecol 41:846–857. https://doi.org/10.1111/j.0021-8901.2004.00939.x
Bro E, Reitz F, Clobert J (2000) Nest-site selection of grey partridge (Perdix perdix) on agricultural lands in north-central France. Game Wildl Sci 17:1–16
Broennimann O, Fitzpatrick MC, Pearman PB, Petitpierre B, Pellissier L, Yoccoz NG, Thuiller W, Fortin M-J, Randin C, Zimmermann NE, Graham CH, Guisan A (2012) Measuring ecological niche overlap from occurrence and spatial environmental data. Glob Ecol Biogeogr 21:481–497. https://doi.org/10.1111/j.1466-8238.2011.00698.x
Browne SJ, Aebischer NJ, Moreby SJ, Teague L (2006) The diet and disease susceptibility of grey partridges Perdix perdix on arable farmland in East Anglia, England. Wildl Biol 12:3–10
Buner F, Jenny M, Zbinden N, Naef-Daenzer B (2005) Ecologically enhanced areas – a key habitat structure for re-introduced grey partridges Perdix perdix. Biol Conserv 124:373–381. https://doi.org/10.1016/j.biocon.2005.01.043
Burger GV (1988) 100 years of ringnecks: a historical perspective on pheasants in North America. In: Hallett, DL, WR Edwards, GV Burger Pheasants: symptoms of wildlife problems on agricultural lands. North Central Section of The Wildlife Society, Bloomington pp 1–26
Calenge C (2006) The package “adehabitat” for the R software: a tool for the analysis of space and habitat use by animals. Ecol Model 197:516–519. https://doi.org/10.1016/j.ecolmodel.2006.03.017
Černý M, Rymešová D, Šálek M (2020) Habitat scarcity forms an ecological trap for the grey partridge (Perdix perdix) within a central European agricultural landscape. Eur J Wildl Res 66:83. https://doi.org/10.1007/s10344-020-01422-w
Chamberlain DE, Fuller RJ, Bunce RGH, Duckworth JC, Shrubb M (2000) Changes in the abundance of farmland birds in relation to the timing of agricultural intensification in England. J Appl Ecol 37:771–788. https://doi.org/10.1046/j.1365-2664.2000.00548.x
Chamberlain DE, Joys A, Johnson PJ, Norton L, Feber RE, Fuller RJ (2010) Does organic farming benefit farmland birds in winter? Biol Lett 6:82–84. https://doi.org/10.1098/rsbl.2009.0643
Chamberlain DE, Wilson JD, Fuller RJ (1999) A comparison of bird populations on organic and conventional farm systems in southern Britain. Biol Conserv 88:307–320. https://doi.org/10.1016/s0006-3207(98)00124-4
Chiatante G, Meriggi A (2022) Habitat selection and density of common pheasant (Phasianus colchicus) in Northern Italy: effects of land use cover and landscape configuration. Eur J Wildl Res 68. https://doi.org/10.1007/s10344-022-01575-w
Chiverton PA (1999) The benefits of unsprayed cereal crop margins to grey partridges Perdix perdix and pheasants Phasianus colchicus in Sweden. Wildl Biol 5:83–92. https://doi.org/10.2981/wlb.1999.012
Cybulska N, Hackländer K, Palme R, Frey-Roos A, Schai-Braun SC (2020) Influence of cereal harvest on adrenocortical activity in European hares (Lepus europaeus). Eur J Wildl Res 66:95. https://doi.org/10.1007/s10344-020-01430-w
Czyżowski P, Karpiński M, Beeger S, Zieliński D (2020) Analysis of the territorial vocalization ritual of the common pheasant Phasianus colchicus. Acta Zool Academiae Scientiarum Hungaricae 66. https://doi.org/10.17109/azh.66.3.299.2020
Danhardt J, Green M, Lindstrom A, Rundlof M, Smith HG (2010) Farmland as stopover habitat for migrating birds - effects of organic farming and landscape structure. Oikos 119:1114–1125. https://doi.org/10.1111/j.1600-0706.2009.18106.x
Di Cola V, Broennimann O, Petitpierre B, Breiner FT, D’Amen M, Randin C, Engler R, Pottier J, Pio D, Dubuis A, Pellissier L et al (2017) ecospat: an R package to support spatial analyses and modeling of species niches and distributions. Ecography 40:774–787. https://doi.org/10.1111/ecog.02671
Donald PF, Green RE, Heath MF (2001) Agricultural intensification and the collapse of Europe’s farmland bird populations. Proc R Soc Lond B 268:25–29. https://doi.org/10.1098/rspb.2000.1325
Draycott RAH, Bliss TH, Carroll JP, Pock K (2009) Provision of brood-rearing cover on agricultural land to increase survival of wild ring-necked pheasant Phasianus colchicus broods at Seefeld Estate, Lower Austria, Austria. Conserv Evid 6:6–10
Felley DL (1996) Recruitment and survival of ring-necked pheasants on the Nampa study area in southwestern Idaho. Graduate Student Theses, Dissertations, & Professional Papers 6895. https://scholarworks.umt.edu/etd/6895
Fernández-Vizcaíno E, Fernández de Mera IG, Mougeot F, Mateo R, Ortiz-Santaliestra ME (2020) Multi-level analysis of exposure to triazole fungicides through treated seed ingestion in the red-legged partridge. Environ Res 189:109928. https://doi.org/10.1016/j.envres.2020.109928
Fernández-Vizcaíno E, Ortiz-Santaliestra ME, Fernández-Tizón M, Mateo R, Camarero PR, Mougeot F (2022) Bird exposure to fungicides through the consumption of treated seeds: a study of wild red-legged partridges in central Spain. Environ Pollut 292:118335. https://doi.org/10.1016/j.envpol.2021.118335
Fischer C, Flohre A, Clement LW, Batary P, Weisser WW, Tscharntke T, Thies C (2011) Mixed effects of landscape structure and farming practice on bird diversity. Agric Ecosyst Environ 141:119–125. https://doi.org/10.1016/j.agee.2011.02.021
Fuller RJ, Norton LR, Feber RE, Johnson PJ, Chamberlain DE, Joys AC, Mathews F, Stuart RC, Townsend MC, Manley WJ, Wolfe MS et al (2005) Benefits of organic farming to biodiversity vary among taxa. Biol Lett 1:431–434. https://doi.org/10.1098/rsbl.2005.0357
Gabriel D, Sait SM, Hodgson JA, Schmutz U, Kunin WE, Benton TG (2010) Scale matters: the impact of organic farming on biodiversity at different spatial scales. Ecol Lett 13:858–869. https://doi.org/10.1111/j.1461-0248.2010.01481.x
Gaffard A, Pays O, Monceau K, Teixeira M, Bretagnolle V, Moreau J (2022) Feeding on grains containing pesticide residues is detrimental to offspring development through parental effects in grey partridge. Environ Pollut 312:120005. https://doi.org/10.1016/j.envpol.2022.120005
Gates JM, Hale JB (1974) Seasonal movement, winter habitat use, and population distribution of an east central Wisconsin pheasant population. Wisconsin Department of Natural Resources Technical Bulletin 76:55.
Geiger F, Bengtsson J, Berendse F, Weisser WW, Emmerson M, Morales MB, Ceryngier P, Liira J, Tscharntke T, Winqvist C, Eggers S et al (2010a) Persistent negative effects of pesticides on biodiversity and biological control potential on European farmland. Basic Appl Ecol 11:97–105. https://doi.org/10.1016/j.baae.2009.12.001
Geiger F, de Snoo GR, Berendse F, Guerrero I, Morales MB, Onate JJ, Eggers S, Part T, Bommarco R, Bengtsson J, Clement LW et al (2010b) Landscape composition influences farm management effects on farmland birds in winter: a pan-European approach. Agric Ecosyst Environ 139:571–577. https://doi.org/10.1016/j.agee.2010.09.018
Glutz von Blotzheim UN, Bauer KM, Bezzel E (1973) Handbuch der Vögel Mitteleuropas - Band 5 Galliformes und Gruiformes. Akademische Verlagsgesellschaft, Frankfurt am Main
Goded S, Ekroos J, Dominguez J, Guitian JA, Smith HG (2018) Effects of organic farming on bird diversity in North-West Spain. Agric Ecosyst Environ 257:60–67. https://doi.org/10.1016/j.agee.2018.01.020
Gottschalk E, Beeke W (2014) How can the drastic decline in the Grey Partridge (Perdix perdix) be stopped? Lessons from ten years of the Grey Partridge Conservation Project in the district of Göttingen. Berichte Zum Vogelschutz 51:95–116
Green RE (1984) The feeding ecology and survival of partridge chicks (Alectoris rufa and Perdix perdix) on arable farmland in east Anglia. J Appl Ecol 21:817–830. https://doi.org/10.2307/2405049
Green RE, Cornell SJ, Scharlemann JP, Balmford A (2005) Farming and the fate of wild nature. Science 307:550–555. https://doi.org/10.1126/science.1106049
Gregory RD, Skorpilova J, Vorisek P, Butler S (2019) An analysis of trends, uncertainty and species selection shows contrasting trends of widespread forest and farmland birds in Europe. Ecol Indic 103:676–687. https://doi.org/10.1016/j.ecolind.2019.04.064
Guerrero I, Morales MB, Oñate JJ, Aavik T, Bengtsson J, Berendse F, Clement LW, Dennis C, Eggers S, Emmerson M, Fischer C et al (2011) Taxonomic and functional diversity of farmland bird communities across Europe: effects of biogeography and agricultural intensification. Biodivers Conserv 20:3663–3681. https://doi.org/10.1007/s10531-011-0156-3
Harmange C, Bretagnolle V, Sarasa M, Pays O (2019) Changes in habitat selection patterns of the gray partridge Perdix perdix in relation to agricultural landscape dynamics over the past two decades. Ecol Evol 9:5236–5247. https://doi.org/10.1002/ece3.5114
Henderson IG, Ravenscroft N, Smith G, Holloway S (2009) Effects of crop diversification and low pesticide inputs on bird populations on arable land. Agric Ecosyst Environ 129:149–156. https://doi.org/10.1016/j.agee.2008.08.014
Hill A (1985) The feeding ecology and survival of pheasant chicks on arable farmland. J Appl Ecol 22:645–654. https://doi.org/10.2307/2403218
Hille SM, Schöll EM, Schai-Braun S (2021) Rural landscape dynamics over time and its consequences for habitat preference patterns of the grey partridge Perdix perdix. PLoS One 16:e0255483. https://doi.org/10.1371/journal.pone.0255483
Höfler A, Andre K, Orlik A, Stangl M, Spitzer H, Ressl H, Hiebl J, Hofstätter M (2020) Klimarückblick Niederösterreich 2019, CCCA (Hrsg.) Wien
Holá M, Zíka T, Šálek M, Hanzal V, Kušta T, Ježek M, Hart V (2015) Effect of habitat and game management practices on ring-necked pheasant harvest in the Czech Republic. Eur J Wildl Res 61:73–80. https://doi.org/10.1007/s10344-014-0875-3
Huss H (1983) Zur Ernährung des Rebhuhns (Perdix p. perdix L.) in einem nordburgenländischen Ackerbaugebiet. Egretta 26:1–14
Joannon A, Bro E, Thenail C, Baudry J (2008) Crop patterns and habitat preferences of the grey partridge farmland bird. Agron Sustain Dev 28:379–387. https://doi.org/10.1051/agro:2008011
Kasprzykowski Z, Goławski A (2009) Does the use of playback affect the estimates of numbers of grey partridge Perdix perdix? Wildl Biol 15:123–128. https://doi.org/10.2981/08-001
Kirk DA, Martin AE, Lindsay KE (2020) Organic farming benefits birds most in regions with more intensive agriculture. J Appl Ecol 57:1043–1055. https://doi.org/10.1111/1365-2664.13589
Kragten S, de Snoo GR (2008) Field-breeding birds on organic and conventional arable farms in the Netherlands. Agric Ecosyst Environ 126:270–274. https://doi.org/10.1016/j.agee.2008.02.006
Kuijper DPJ, Oosterveld E, Wymenga E (2009) Decline and potential recovery of the European grey partridge (Perdix perdix) population—a review. Eur J Wildl Res 55:455–463. https://doi.org/10.1007/s10344-009-0311-2
Lachlan C, Bray RP (1976) Habitat selection by cock pheasants in spring. J Appl Ecol 13:691–704. https://doi.org/10.2307/2402247
Lopez-Antia A, Ortiz-Santaliestra ME, Mougeot F, Camarero PR, Mateo R (2018) Brood size is reduced by half in birds feeding on flutriafol-treated seeds below the recommended application rate. Environ Pollut 243:418–426. https://doi.org/10.1016/j.envpol.2018.08.078
MacKenzie DI, Nichols JD, Royle JA, Pollock KH, Bailey LL, Hines JE (2006) Occupancy estimation and modeling: Inferring patterns and dynamics of species occurrence. Academic Press, Burlington, MA
MacKenzie DI, Royle JA (2005) Designing occupancy studies: general advice and allocating survey effort. J Appl Ecol 42:1105–1114. https://doi.org/10.1111/j.1365-2664.2005.01098.x
Manly BFJ, McDonald LL, Thomas DL, McDonald TL, Erickson WP (2002) Resource selection by animals: statistical design and analysis for field studies, 2nd edn. Kluwer Academic Publishers, London
Marja R, Herzon I, Viik E, Elts J, Mand M, Tscharntke T, Batary P (2014) Environmentally friendly management as an intermediate strategy between organic and conventional agriculture to support biodiversity. Biol Conserv 178:146–154. https://doi.org/10.1016/j.biocon.2014.08.005
Martin PR, Martin TE (2001) Ecological and fitness consequences of species coexistence: a removal experiment with wood warblers. Ecology 82:189–206. https://doi.org/10.1890/0012-9658(2001)082[0189:EAFCOS]2.0.CO;2
McCrow VP (1982) Gray partridge habitat use and nesting biology in north-central Iowa. Retrospective Theses and Dissertations 7516. https://lib.dr.iastate.edu/rtd/7516.
McKenzie AJ, Vickery JA, Leifert C, Shotton P, Whittingham MJ (2011) Disentangling the effects of fertilisers and pesticides on winter stubble use by farmland birds. Basic Appl Ecol 12:80–88. https://doi.org/10.1016/j.baae.2010.10.007
McKenzie AJ, Whittingham MJ (2009) Why are birds more abundant on organic farms? J Agric Environ 7:807–814
Millot F, Decors A, Mastain O, Quintaine T, Berny P, Vey D, Lasseur R, Bro E (2017) Field evidence of bird poisonings by imidacloprid-treated seeds: a review of incidents reported by the French SAGIR network from 1995 to 2014. Environ Sci Pollut Res Int 24:5469–5485. https://doi.org/10.1007/s11356-016-8272-y
Morris AJ, Gilroy JJ (2008) Close to the edge: predation risks for two declining farmland passerines. Ibis 150:168–177. https://doi.org/10.1111/j.1474-919X.2008.00857.x
Newton I (2004) The recent declines of farmland bird populations in Britain: an appraisal of causal factors and conservation actions. Ibis 146:579–600. https://doi.org/10.1111/j.1474-919X.2004.00375.x
Norton L, Johnson P, Joys A, Stuart R, Chamberlain D, Feber R, Firbank L, Manley W, Wolfe M, Hart B, Mathews F et al (2009) Consequences of organic and non-organic farming practices for field, farm and landscape complexity. Agric Ecosyst Environ 129:221–227. https://doi.org/10.1016/j.agee.2008.09.002
Oksanen J, Simpson GL, Blanchet FG, Kindt R, Legendre P, Minchin PR, O'Hara RB, Solymos P, Stevens MHH, Szoecs E, Wagner H, et al (2022) vegan: Community ecology package. R package version 2.6–2. Available from: https://CRAN.R-project.org/package=vegan
Panek M (1998) Use of call counts for estimating spring density of Grey Partridge Perdix perdix. Acta Ornithologica 33:143–148
Panek M (2002) Space use, nesting sites and breeding success of grey partridge (Perdix perdix) in two agricultural management systems in western Poland. Game Wildl Sci 19:313–326
Paniccia C, Di Febbraro M, Frate L, Sallustio L, Santopuoli G, Altea T, Posillico M, Marchetti M, Loy A (2018) Effect of imperfect detection on the estimation of niche overlap between two forest dormice. iForest 11:482–490. https://doi.org/10.3832/ifor2738-011
Pianka ER (1984) Niche overlap and diffuse competition. Proc Nat Acad Sci USA 71:2141–2145
Potts GR (1970) Recent changes in the farmland fauna with special reference to the decline of the Grey Partridge. Bird Study 17:145–166. https://doi.org/10.1080/00063657009476266
Potts GR (2012) Partridges. HarperCollins Publishers, London, UK
R Development Core Team (2019) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available from: http://www.R-project.org/.
Rantanen EM, Buner F, Riordan P, Sotherton N, Macdonald DW (2010) Habitat preferences and survival in wildlife reintroductions: an ecological trap in reintroduced grey partridges. J Appl Ecol 47:1357–1364. https://doi.org/10.1111/j.1365-2664.2010.01867.x
Rech T, Tischler K, Rumler D (2020) Organic farming in Austria Federal Ministry of Agriculture, RaT Vienna, Austria, pp 28
Ridley MW, Hill DA (1987) Social organization in the pheasant (Phasianus colchicus): harem formation, mate selection and the role of mate guarding. J Zool 211:619–630. https://doi.org/10.1111/j.1469-7998.1987.tb04475.x
Rinaud T, Harmange C, Pays O, Sarasa M, Saillard M, Bretagnolle V (2020) Interspecific competition between two partridges in farmland landscapes. Anim Behav 165:23–34. https://doi.org/10.1016/j.anbehav.2020.04.018
Robertson PA, Woodburn MIA, Neutel W, Bealey CE (1993) Effects of land use on breeding pheasant density. J Appl Ecol 30:465–477. https://doi.org/10.2307/2404187
Robillard A, Garant D, Bélisle M (2012) The swallow and the sparrow: how agricultural intensification affects abundance, nest site selection and competitive interactions. Landsc Ecol 28:201–215. https://doi.org/10.1007/s10980-012-9828-y
Ronnenberg K, Strauss E, Siebert U (2016) Crop diversity loss as primary cause of grey partridge and common pheasant decline in Lower Saxony. Germany Bmc Ecol 16:39. https://doi.org/10.1186/s12898-016-0093-9
Rymešová D, Tomášek O, Šálek M (2013) Differences in mortality rates, dispersal distances and breeding success of commercially reared and wild grey partridges in the Czech agricultural landscape. Eur J Wildl Res 59:147–158. https://doi.org/10.1007/s10344-012-0659-6
Šálek M, Brlík V, Kadava L, Praus L, Studecký J, Vrána J, Gamero A (2020) Year-round relevance of manure heaps and its conservation potential for declining farmland birds in agricultural landscape. Agric Ecosyst Environ 301:107032. https://doi.org/10.1016/j.agee.2020.107032
Šálek M, Marhoul P (2008) Spatial movements of grey partridge Perdix perdix: male-biased spring dispersal and effect of habitat quality. J Ornithol 149:329–335. https://doi.org/10.1007/s10336-008-0279-9
Schoener TW (1970) Nonsynchronous spatial overlap of lizards in patchy habitats. Ecology 51:408–418. https://doi.org/10.2307/1935376
Smith HG, Danhardt J, Lindstrom A, Rundlof M (2010) Consequences of organic farming and landscape heterogeneity for species richness and abundance of farmland birds. Oecologia 162:1071–1079. https://doi.org/10.1007/s00442-010-1588-2
Taylor RL, Maxwell BD, Boik RJ (2006) Indirect effects of herbicides on bird food resources and beneficial arthropods. Agric Ecosyst Environ 116:157–164. https://doi.org/10.1016/j.agee.2006.01.012
Teufelbauer N, Seaman B (2021) Monitoring der Brutvögel Österreichs - Bericht über die Saison 2020. https://birdlife.at/page/monitoring-der-brutvogel. Accessed 17 June 2021
Tompkins DM, Dickson G, Hudson PJ (1999) Parasite-mediated competition between pheasant and grey partridge: a preliminary investigation. Oecologia 119:378–382. https://doi.org/10.1007/s004420050798
Tompkins DM, Greenman JV, Robertson PA, Hudson PJ (2000) The role of shared parasites in the exclusion of wildlife hosts: Heterakis gallinarum in the ring-necked pheasant and the grey partridge. J Anim Ecol 69:829–840. https://doi.org/10.1046/j.1365-2656.2000.00439.x
Vaněčková D (2011) Development of grey partridge (Perdix perdix) microsatellite markers. Master thesis, Faculty of Science, Charles University, Prague
Vrezec A (2006) Is the Grey Partridge Perdix perdix population decline in Slovenia the result of apparent competition with the pheasant Phasianus colchicus? Acrocephalus 27:73–81
Wang B, Xu Y, Price M, Yang N, Liu W, Zhu B, Zhong X, Ran J (2020) Niche partitioning among three montane ground-dwelling pheasant species along multiple ecological dimensions. Ibis 163:171–182. https://doi.org/10.1111/ibi.12842
Wang B, Xu Y, Zhang B, Wu Y, He X, Ran J, Zeng T (2018) Overlap and selection of dust-bathing sites among three sympatric montane galliform species. Auk 135:1076–1086. https://doi.org/10.1642/auk-18-44.1
Warren DL, Glor RE, Turelli M (2008) Environmental niche equivalency versus conservatism: quantitative approaches to niche evolution. Evolution 62:2868–2883. https://doi.org/10.1111/j.1558-5646.2008.00482.x
Warren P, Hornby T, Baines D (2017) Habitat use, nest-sites and chick diet of Grey Partridge Perdix perdix on hill farms in north east England. Bird Study 64:138–145. https://doi.org/10.1080/00063657.2017.1306485
Warren P, Hornby T, Baines D (2018) Comparing call-playback to an observation-only method to survey Grey Partridge Perdix perdix on hill farms in northern England. Bird Study 65:225–231. https://doi.org/10.1080/00063657.2018.1475466
Wiens JJ, Graham CH (2005) Niche conservatism: integrating evolution, ecology, and conservation biology. Annu Rev Ecol Evol Syst 36:519–539. https://doi.org/10.1146/annurev.ecolsys.36.102803.095431
Acknowledgements
We would like to thank the hunting societies of Traunfeld and Kronberg for cooperation, and especially Herbert and Robert Schütz for providing information about farming practices.
Funding
Open access funding provided by University of Natural Resources and Life Sciences Vienna (BOKU).
Author information
Authors and Affiliations
Contributions
Conceptualization: Eva M. Schöll, Armin Eschberger-Friedl and Alfred Frey-Roos; methodology: Eva M. Schöll, Armin Eschberger-Friedl and Alfred Frey-Roos; data collection: Armin Eschberger-Friedl; formal analysis and investigation: Eva M. Schöll and Stéphanie Schai-Braun; project administration: Alfred Frey-Roos; writing — original draft preparation: Eva M. Schöll; and writing — review and editing: Eva M. Schöll, Armin Eschberger-Friedl, Stéphanie Schai-Braun and Alfred Frey-Roos.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
This was an observational study. Therefore, no approval of research ethics committees was required to accomplish the goals of the study.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Schöll, E.M., Eschberger-Friedl, A., Schai-Braun, S. et al. Habitat preferences and similarities of Grey Partridges and Common Pheasants in agricultural landscapes under organic and conventional farming. Eur J Wildl Res 69, 29 (2023). https://doi.org/10.1007/s10344-023-01659-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10344-023-01659-1