Abstract
Surveys based on indirect signs and camera trapping are two non-invasive methods extensively used for monitoring elusive mammals. Both approaches can be useful to obtain key information on wildlife in remote areas, since they may allow for the logistically viable design of optimal field frameworks. The sand cat (Felis margarita) is a feline that inhabits the Sahara Desert, the Arabian Peninsula, and western Asian deserts. Its basic ecology is poorly known and the status and impacts of threats are difficult to assess. Some local population declines have been detected, and more research is needed. Based on field surveys carried out in the Atlantic Sahara, we have evaluated the applications of both methods to study this species. Our results show that (a) camera trapping provided reliable data on several key aspects of its ecology, (b) walking surveys to collect feces for molecular data failed completely, and (c) for footprints, identification problems and the marked effects of the absence of optimal substrates and the prevalence of wind are relevant handicaps. Beyond this evaluation, we provide for the first time some key aspects of the ecology of sand cats in the Sahara Desert, including habitat selection, density, diel activity, and predator–prey relationships.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Elusive mammal species in remote areas are among the least studied vertebrate taxa; moreover, some may be endangered, but scientific data are frequently very scarce (IUCN 2016). Testing logistically viable survey methods is a crucial goal toward improving the knowledge about this type of fauna, both to assess populations and to gather key ecological information, which is repeatedly requested by specialists for the species assessments of the IUCN Red List (IUCN 2016).
There are two non-invasive methods that are extensively used for surveying and monitoring elusive mammals: surveys of indirect signs and camera trapping. The first consists of looking for footprints and scats usually along walking itineraries (see, e.g., Long et al. 2008 for carnivores). The sampled feces can be subsequently identified at the species and/or individual level through molecular approaches (see, e.g., Waits and Paetkau 2005), whereas some footprints can be visually assigned at the species level with high certainty (see, e.g., Long et al. 2008). Camera trapping consists of deploying remote camera devices, e.g., passive infrared-triggered digital cameras, to take images (photographs and/or videos), to be used for subsequent identification of species and individuals using exclusive morphological characteristics, color patterns, and marks (O’Connell et al. 2011). One key advantage of camera trapping is that this method registers the date and hour of every record, therefore allowing the study of phenology and diel activity patterns (O’Connell et al. 2011). Both methods can be particularly interesting to gather key information on the wildlife inhabiting remote areas, since they allow for the logistically viable design of optimal surveys (see, e.g., Gil-Sánchez et al. 2017, 2020a, for mammals of the Sahara Desert).
The sand cat Felis margarita, one of the smallest wildcats in the world (Sunquist and Sunquist 2009), is a good example of the abovementioned scenario. This nocturnal feline inhabits remote areas of the Sahara Desert, the Arabian Peninsula, and western Asian deserts (Sunquist and Sunquist 2009, Fig. 1a). Only a handful of local studies on its ecology have been carried out (Strauss et al. 2007; Sher Shah and Cunningham 2008; Breton et al. 2016; Ghafaripour et al. 2017, 2018), and most of the remaining available information have been obtained from opportunistic records (see review by Sliwa et al. 2016). The sand cat is not a threatened species according to the updated IUCN Red List assessment (least concern), but it is recognized that its basic ecology is poorly known, and its actual status and the impacts of threats on the species are difficult to assess (Sliwa et al. 2016). In fact, there are areas of apparent population decline, which deserve for more research (Sliwa et al. 2016).
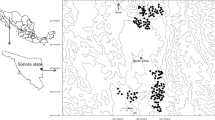
a Sand cat distribution (from Sliwa et al. 2016) and study area location (arrow). b Surveyed areas (Areas #1, #2, #3). c Survey Area #2. d Survey Area #3. e, f Survey Area #1. For c, d, and e, dots represent the surveyed locations of walking surveys (black squares: possible footprints of sand cat; white dots: negative surveys). For f, white dots represent the camera locations without sand cat detection and black squares camera locations with captures of sand cats (numbers: references in Table 1), the framed dots are the intensively surveyed area (ISA), and white squares represent four opportunistic records obtained during the fieldwork
While camera trapping has been previously used to study the sand cat (Ahmed et al. 2016; Al Zaabi et al. 2019; Amin et al. 2021), to our knowledge, indirect sign surveys have not been considered. Fecal sampling combined with molecular analysis is a non-intrusive method that has provided key information for felid species, including surveys of presence at a large spatial scale (e.g., Palomares et al. 2002), estimations of abundance (e.g., Anile et al. 2014), and genetic diversity (e.g., Mengüllüoğlu et al. 2019). Our primary objective was to compare the applications of (A) surveys of indirect signs and (B) camera trapping to obtain reliable data for the sand cat on key aspects of its ecology, particularly presence, abundance, and demographic composition; activity patterns; and interspecific relationships. By doing this, we aim to provide pioneering information on the ecology of sand cats in the main stronghold of this species, the Sahara Desert, where wildlife has suffered a severe collapse in the last century due to overhunting and habitat destruction (Durant et al. 2014; Brito et al. 2018). Hence, this type of research is an urgent action to assess the current status of the sand cat and help establish effective conservation approaches based on scientific knowledge.
Material and methods
Study area
The study area was located in the Atlantic Sahara (Morocco), a low-latitude subtropical desert with an arid hot climate (after Köppen-Geiger classification, Kottek et al. 2006). Within the sand cat distribution range in the Atlantic Sahara (Fig. 1a), we surveyed three areas (Fig. 1b). The first area (area #1) was located between the lower Draa River and the upper basin of the Sequiat Al Hamra, (ca 20,000 km2; altitude range 290–770 m a.s.l.; Fig. 1e, f). This is a hilly and mountain rocky terrain with intercalated regs (plains) and hamadas (elevated plains). The vegetation is scarce except along the dry river basins, where open savannah-like forests of thorn trees (Acacia tortilis) persist, with some Egyptian balsam (Balanites aegyptiaca), Sodom apple (Calotropis procera), and Rhus tripartita bushes. Within area #1, we selected a central section of approximately 100 km2 where we previously detected the presence of sand cats (hereinafter, the “intensively surveyed area,” ISA, Fig. 1f); this zone was a sand and gravel reg at 450 m a.s.l., with vegetation dominated by scattered scrubs of Anabasis articulata and Hammada scoparium and scarce horn trees. The second area (area #2) comprises the Oued Khat Basin (Fig. 1c), a fossil river valley dominated by reg plains with rocky hills and steps at the borders (ca 1200 km2, 110–220 m a.s.l.). The vegetation is formed by scattered horn trees and camephites such as Nucularia perrini. The third area (area #3) includes the Negjyr Mounts, the Aouserd Mounts, and the Oued Jenna (Fig. 1d), which conform to small island mounts (6–80 km2) and an ancient dry river located in the huge Atlantic Saharan plain (ca 5100 km2, 180–350 m a.s.l.). Vegetation is similar to the Oued Khat area, but horn trees and the grass Panicum turgidum are more abundant. Based on scarce local surveys and opportunistic data, the sand cat is assumed to be widespread in the study area (Fig. 1a).
Sampling locations were chosen before the field surveys following a stratified strategy with the aim of sampling most of the habitat variability that characterizes the study areas. Given the remoteness of the study area, the fieldwork was planned recognizing a tradeoff between the logistic limitations and the expected data collection. This was particularly important for the camera trapping designs, which require at least two visits (installation and pick-up). We planned expeditions with 4 × 4 vehicles of 1 to 2 weeks each, during which we combined walking surveys and camera trapping as follows.
Walking surveys
We surveyed the carnivore community of the three study areas during twenty-two expeditions (from April 2011 to April 2019). A total of 68, 4, and 9 locations were respectively sampled within the three areas (Fig. 1), searching for mammalian carnivore signs along 1–4 transects/location (mean transect length: 12.08 ± 0.72 km, total length of transects: 2490 km). For each location, two to nine observers searched for carnivore feces, tracks, and den sites (Barea-Azcón et al. 2007; Long et al. 2008). Twenty-nine locations were “a priori” classified as optimal habitat for sand cats, particularly sandy or gravel flat areas of regs and small ergs (Sunquist and Sunquist 2009; Sliwa et al. 2016), whereas the other locations were mainly hilly and rocky terrains. Two extra walking surveys of 18.5 km each were carried out in December 2019 within the ISA by two observers. We detected sand cats in the ISA by remote cameras during the first week after the walking surveys (see “Results”), and therefore, the presence of sand cats was assumed during the walking surveys. Signs putatively attributed to sand cats were footprints (see a description in Sunquist and Sunquist 2002, and SI Fig. 1) and scats. Most of the collected carnivore feces were confirmed to the species level in the laboratory through genetic analyses (see details in Gil-Sánchez et al. 2020a). All the walking surveys were carried out by experienced observers.
Remote camera surveys
Remote cameras were deployed in area #1 (where the main habitats of the region were well represented), to survey small- to large-sized mammals including sand cats (Table 1). A camera trapping setup consisted of one camera, installed perpendicularly to a natural path used by wild mammals (except for sand cats in the ISA, see below), oriented toward the north, and fixed on large stones, acacia trunks, or wooden sticks at a height above the ground of 25–30 cm. We decided to use Iberian lynx urine as a lure to improve the capture rates, since this had been tested as an optimal attractant for small carnivore species belonging to the families present in our study area, the Felis genus included (Monterroso et al. 2011). All the camera locations were baited with Iberian lynx urine, of which approximately 1 cm3 was deposited 4–6 m in front of the camera on a stone or a piece of wood or on a menstrual tampon that was impregnated by dipping it into a bottle of urine (Table 1). The tampons were tied on a piece of branch and placed between two stones. All devices were installed by a core team of experienced camera survey personnel.
We used three models of passive infrared PIR camera devices: Bushnell Trophy Cam Essential E2™ and Moultrie 880™ equipped with infrared LEDs and BolyGuard SG562-C™ cams equipped with white LEDs. Both illumination types are battery-saving, a mandatory condition for our long-term surveys. Cameras were programmed to take 1–3 pictures per event, with 0–5 s of PIR interval. The BolyGuard cameras take full-color images and were selected for the sand cat surveys carried out in the ISA to improve individual identification, allowing for the application of density estimation methods. This flat area lacks well-defined natural paths, and cameras could not be installed following the protocol; alternatively, they were placed in the nearest scrub (usually A. articulata or H. scoparium, fixed on a wooden stick) to a previously GIS-designed location. In the ISA, the tampon was used as a lure.
Two types of camera surveys were carried out: (1) preliminary training short-term duration surveys to explore the chances of remote devices for wildlife sampling in the study area (ca. 5 days), with four surveys from 2012 to 2016 (ref. 1 to ref. 4 in Table 1), and (2) long-term duration camera surveys (3 months to 1 year) from 2016 to 2020, of two types, (2A) surveys designed for the detection of all species (ten surveys, ref. 5a to ref. 10) and (2B) surveys specifically designed to detect and estimate abundance of sand cats by capture/recapture CR approaches (two surveys, ref. 11a and 11b in Table 1). Type 2B were developed during the last stage of our fieldwork after type 1 and type 2A surveys (Table 1), once we had gathered basic information on presence and habitat used by the sand cats in the study area, for which we selected the ISA. Cameras lasting up to 165 days were only baited during their field installation (i.e., only one occasion) and were not visited until the end of the survey period, as was the case for the two sand cat surveys in the ISA. During the complete year surveys (ref. 5b, 6b, and 8b in Table 1), two checking expeditions were carried out every ca. 3 months to replace memory cards, lures, batteries, and lost or damaged cameras. We installed 224 cameras, of which 203 were operative, and 21 were stolen or damaged, with a global effort of 19,865 camera days (see details of each block in Table 1).
The spatial sampling strategy for type 1 and type 2A surveys consisted of clustering an average number of fourteen cameras in eleven sampling blocks (sense Gil-Sanchez et al. 2020b) with 1–2 km of separation between cameras (range = 3–40 cameras per block, Table 1, Fig. 1). Some blocks were sampled twice, including the ISA (see Table 1), under wet and dry conditions, respectively (SI Fig. 2). The sampling strategy designed specifically for sand cats (type 2B) had two phases: (1) First, we designed a pilot survey (ref. 11a in Table 1) consisting of a block of 20 cameras distributed within a 48 km2 rectangle and a regular grid of 2 km between cameras (Fig. 1f). This size was chosen to include at least one potential territory of our targeted species (Tobler and Powell 2013), following the known home ranges and/or densities of sand cats: 20–50 km2 (n = 7) in hyper-arid areas of Saudi Arabia (Sliwa et al. 2016) and an estimated 2.9 individuals/100 km2 in Israel (Abbadi 1993). (2) Second, we carried out another survey (ref. 11b in Table 1) designed based on the pilot survey results, which basically consisted of increasing the sampling array up to 114 km2 by adding 20 cameras at the south (see Fig. 1f). The pilot survey lasted 3.5 months and the second survey 5.5 months (Table 1). To prevent camera theft, a local field assistant was hired for surveillance and to keep contact with the nomadic herders during the second survey.
Data analyses
Sign surveys did not offer sufficient information, particularly because only footprints were detected (see “Results”), and they can be confused with African wildcat tracks (see Supplementary Material S1). Hence, thereafter, we only deal with the analyses of camera trapping data. An independent record was defined as when > 1 h (for larger species, O’Connell et al. 2011) or > 0.5 h (for small rodents, Monterroso et al. 2013) elapsed between camera captures of the same species or when the presence of different individual could be confirmed. We conducted a visual examination of the response of sand cats to the bait, through the rate of individuals smelling the lynx urine. We also explored the decrease in the effects of the lure as an attractant over time through the plotting of standardized records (captures per 100 camera days, RAI) in 1-month periods since the installation of cameras.
We calculated the optimal minimum effort required to detect sand cats where they are present, through back-step subsampling of all the possible square- or rectangular-shaped camera arrays. This was separately made for the two camera surveys of the ISA. We stopped the subsampling when the average percentage of detection was < 1 for a given array (where for a given array: %detection = arrays with sand cat/total array possibilities). We used the same sampling duration for both surveys taking the shorter one (3.5 months of the pilot survey). Next, we carried out estimations of the probability of presence (\(\psi\)) for the ISA in 2019 and 2020, through occupancy models (Mackenzie et al. 2006); the rest of the blocks in which we detected sand cats had very little effort for this estimation (a few days in ref. 3 and a few cameras in ref. 6b, 9a and 9b of Table 1). We built a capture matrix for the history of each camera by taking a 1-day period as the sampling occasion (O’Connell et al. 2011), assigning the value “1” when sand cats were present at a given camera on a given day and “0” when not. The presence (MacKenzie et al. 2006) single-species, single-station, P-specific approach was used for this occupancy analysis.
Density estimation was performed by spatially explicit capture-recapture. Individuals recorded by the ISA cameras were identified based on the typical striped pattern of the species, which we assumed a priori as individual characteristics (see, e.g., Gil-Sánchez et al. 2015 for the case of the European wildcat F. silvestris, and Fig. 2). We used the random thinning SCR model (Jiménez et al. 2021) to make full use of all collected data (including two non-ID events). This model utilizes encounters of both known and unknown identity samples using a natural mechanistic dependence between samples arising from a single observation model and is useful to deal with scarce data. Additionally, to improve sigma estimates, we used the locations from spatial capture data from 2019 (see SI Text 1). We fit this model in NIMBLE (De Valpine et al. 2017; 2021) integrating this data into the model (Tenan et al. 2017). We ran 3 chains of 5,000,000 iterations, discarding 1,000,000 iterations as burn-in and thinning by 50. We confirmed model convergence by examining trace plots and ensuring that the potential scale reduction factor (R-hat) statistic for each parameter was < 1.1 (Gelman et al. 2013).
The diel activity pattern of sand cats was assessed following Monterroso et al. (2013) and Gil-Sánchez et al. (2020a), using Kernel density estimates (see details in Ridout and Linkie 2009). We explored whether the diel activity was shaped by the activity of its potential prey, basically small rodents (Sunquist and Sunquist 2009; Sliwa et al. 2016), which were represented in the ISA by the lesser jerboa (Jaculus jaculus), the Tarabul’s gergil (Gerbillus tarabuli), and the fat-tailed gerbil (Pachyuromys duprasi). Since the number of sand cat records was < 50 detections, a pairwise comparison of activity patterns between sand cats and the three rodents separately was conducted by estimating the coefficient of overlap ∆1 (Ridout and Linkie 2009; Linkie and Ridout 2011). These coefficients of overlap range from 0 (no overlap) to 1 (complete overlap). The R code used to estimate overlap coefficients was provided by Ridout and Linkie (2009).
Results
Walking surveys
We were unsuccessful in detecting sand cat scats within area #1, including the ISA that resulted in a false negative sampling, while we obtained 92 genetically confirmed scats of African wildcat F. lybica. No scats were found in area #2, and only one putative sand cat scat was found in area #3 (Oued Jenna, Fig. 1d), but it was not ultimately analyzed in lab. We did not find any scat in the ISA. Within area #1, we were able to detect putative sand cat footprints in seven locations (Fig. 1e), representing 10.3% of the 68 total surveys and 43.4% of the 16 surveys carried out in flat areas. No footprints were found in area #2, and two possible tracks were detected in area #3 (Fig. 1d) representing 22.2% of the nine total surveys and 50.0% of the four surveys carried out in flat areas. We failed to detect sand cat footprints in the ISA; therefore, the result was again a false negative. All footprints were exclusively found in sandy terrain during surveys carried out after at least 1 day without wind.
Remote camera surveys
The camera traps registered 7039 independent records of wild mammals: 312 ungulates, 1948 carnivores, 962 hares, 3549 rodents, 175 hedgehogs, and 93 macroscelids and insectivores. Twenty-nine records of sand cats were obtained, two in the type 1 training surveys (50% positive surveys in flat areas, N = 2), six in the type 2A surveys (80% positive surveys in flat areas, N = 5), and twenty-one in the ISA (Table 1 and Fig. 1f). Sand cats were detected in 36.4% of the surveyed blocks (4/11), all of them exclusively within the flat areas (Table 1).
Sand cats were photographed smelling the lure (see Fig. 2) in 22 of the 29 total detections (75.8% of the occasions) and in 19 of the 21 detections for the case of the ISA (90.5% of the occasions); five of the seven occasions without apparent attraction to the lure were during the third or four month after the last baiting. A decrease in RAI was observed throughout the survey period in the ISA, but important differences were observed between the 2019 and the 2020 surveys (Fig. 3), despite the fact that the same two individuals were detected in both years, plus one more cat in 2020 (see below). Comparing the same sample period (January to April), the efficiency reduction was not as clear in 2020 (Rs = 0.4, P = 0.6).
The optimal minimum array required to detect sand cats during a 3.5-month survey was 3 × 3 cameras in 2019 and 3 × 4 cameras in 2020; the 3 × 3 camera array resulted in a detection % = 0.94 in 2020 (17/18 possible arrays). The naïve occupancy (cameras with sand cat/total cameras) in the ISA was 0.29 (5/17) in 2019 and 0.27 (10/37) in 2020. \(\psi\) was 0.40 (SE = 0.18, 95% CI = 0.13–0.75; P = 0.0047, SE = 0.0017) and 0.70 (SE = 0.35, 95% CI = 0.08–0.98; P = 0.0025, SE = 0.0007) for 2019 and 2020, respectively.
Most of the photos taken in the ISA allowed individual identification using the striped pattern of the species (90.5% of captures, Fig. 2). During 2019, only two males were detected at ISA, with 5 records in 4 cameras and 4 records in 3 cameras, respectively (RAI = 0.56); during 2020, the same males were registered 5 times in 4 cameras and 3 times in 3 cameras, respectively, plus a third male with 2 records at two cameras and another two low quality captures that could not be identified (RAI = 0.23). The estimated density in 2020 was 1.12 ind./100 km2 (SD = 0.59), the identification probability was 0.78 (SD = 0.10), the basal detection rate (λ0) was 0.0064 (SD = 0.0068), and the scale parameter for half-normal distribution that described the movement of animals (σ) was 2.68 (SD = 0.39) km.
The data collected allowed estimations of diel activity. Sand cats were strictly nocturnal, showing a bimodal pattern with two peaks of activity, at midnight and 1–2 h before dawn (Fig. 4). The camera traps registered 2791 records of rodents, resulting in 1960 records of lesser jerboa, 753 of Tarabul’s gerbil, 67 of fat-tailed gerbil, 9 of fat sand rat (Psammomys obesus), and 2 of pleasant gerbil (G. amoneus). We detected a high overlap of activity between sand cats and their potential prey (> 78% in all cases), with some differences related to the different activity patterns of three dominant rodent species (Fig. 4).
Discussion
Efficiency of non-intrusive field methods
We found substantial differences in the efficiency of the two methods studied in terms of their applications to obtain reliable distribution and ecological data on the sand cat. Our surveys of indirect signs were not able to detect the species with total certainty, in contrast to camera trapping, which achieved all of the objectives with reasonable efficiency. We were unsuccessful in detecting sand cat feces (with only one possible scat in area #3) despite our huge sampling effort; in contrast, 92 scats of African wildcats were collected and subsequently identified following genetic protocols (see Gil-Sánchez et al. 2020a). Two circumstances could explain this difference in the detection rate of the two felids: first, our camera trapping data shows that African wildcats were apparently much more abundant than sand cats in area #1 (396 versus 29 independent captures by cameras); second, sand cats inhabit flat areas that are very frequently swept by the wind, which probably covers the scats with sand. This would be true for not only the sand cat but also for Ruppell’s fox (V. rueppellii) and fennec fox (V. zerda). Both canids were much more abundant in the ISA than sand cats (355 and 196 independent captures respectively), but we were unable to detect feces during the walking surveys (African wildcats were absent there). For other species of the Felis genus, the efficiency of scat sampling showed important geographical differences, sometimes unrelated to the local abundance (see Gil-Sánchez et al. 2020b for the case of European wildcats). Although footprints offered better results than scats, identification problems related to confusion with African wildcats and the marked effects of the presence of optimal substrates and windy conditions make this a low-efficiency indirect field method, at best. In any case, when and where the conditions are optimal (i.e., substrate and weather), surveying footprints could be a first approach to detect the presence of sand cats (43.4% and 50.0% of detection rates in optimal habitats of Area #1 and Area #2, respectively). The false negative obtained in the ISA after our significant effort was related to the constant wind during the previous night that erased any footprints.
Camera trapping fulfilled our objectives: detection and estimation of presence and density, demographic composition, activity patterns, and relationships with prey. The main limitations for this type of survey were related to the relatively high rate of theft (particularly by nomad herders and poachers), the abrasive effects of sandy wind on the camera lens, and the high number of blind photos due to over-shooting in the strong winds typical of the Sahara, which filled the memory cards of some cameras before the end of the survey. In any case, these problems could be reasonably solved through the following: (1) hiring a local assistant to prevent theft and/or to check memory cards and batteries and (2) covering the camera devices with a wooden cage.
The relatively low number of records of sand cats was probably more related to their low density in the ISA (1.12 ind./100 km2) than to the effectiveness of our camera sampling design. The scarcity of this species has been previously confirmed elsewhere (2.9 ind./100 km2 in Israel, Abbadi 1993). In fact, the capture rates obtained for the African wildcat in our study area (average RAI = 5.46, range = 0.55–14.67, for the areas with confirmed presence) were much higher than those obtained for the sand cat (average RAI = 0.31, range = 0.12–0.56, for the areas with confirmed presence). The resulting high confidence intervals for \(\psi\) and for the density estimates were related to the low sample size of sand cat records, a limitation that could be solved by (1) increasing the number of camera locations (Amin et al. 2021) and (2) increasing the repositioning of the lure, by hiring local field assistants to avoid the high logistical and economic efforts involved in new expeditions for checking the cameras. In any case, and despite the low number of sand cat records registered by the camera traps, we at least established optimal arrays to detect the species during 3.5 months of surveys in this very low-density scenario. This would allow for logistically feasible large-scale presence surveys through the simultaneous deployment of multiple 3 × 3 or 3 × 4 camera sampling blocks.
The use of bait notably increases the capture rate of small felids such as the Iberian lynx (Garrote et al. 2012) and the European wildcat (Gil-Sánchez et al. 2015) and probably was also true for sand cats (e.g., they were observed smelling the lure in 90.5% of the records in the ISA; see also Ahmed et al. 2016 and Al Zaabi et al. 2019), but this was not tested in our study, and therefore, our results in this regard should be taken with caution. In any case, two facts supported the efficiency of our luring as an attractant for the species: (1) the series of photographs clearly showing the sand cats going to the bait (SI Fig. 3) and (2) the decrease in the capture rate over the course of the survey (see Fig. 3) in relation to the dissipation of the urine. The use of blind camera locations (i.e., without any lure) probably would result in lower sand cat capture rates (see, e.g., the case of European wildcats in Gil-Sánchez et al. 2015 and cheetahs Acinonyx jubatus in Brassine and Parker 2015), particularly within a habitat where there are no evident wildlife paths (Matias et al. 2021). Therefore, the detection of sand cats would be an extremely rare event without attractants. Amin et al. (2021) did not use attractants in a large camera-trapping survey of sand cats in Saudi Arabia, but they placed the devices on drainage lines, pathways, and other locations likely to be used by mammals, which was not possible in the homogeneous habitat in our study area. Lures also increase the number of photographs per capture event, which simultaneously increases the chances for individual recognition (Garrote et al. 2012; Gil-Sánchez et al. 2015), as was the case for the sand cats in our study. However, a potential limitation to replicate our protocol by other researchers could be the use of Iberian lynx urine, which may be challenging to obtain. In the case of the European wildcat, the use of valerian extract and domestic cat urine (easier to obtain) has offered optimal results in density surveys (Matias et al. 2021). Further studies are needed to evaluate the efficiency of different attractants for sand cats that can be easily obtained by any researcher (see Ahmed et al. 2016 and Al Zaabi et al. 2019, who used canned fish and cat food as lures for sand cats).
Camera trapping was largely more efficient than live captures to obtain records of the rodent community in the reg habitat. The very low capture rate of Sherman’s traps could be related to the type of bait, but also to the behavior and/or size of the dominant rodent, the lesser jerboa, a species probably too large for our cage traps. In fact, we frequently observed jerboa tracks around the traps, but none were captured. Another advantage of cameras over Sherman’s traps is the valuable information on diel activity, which allows the study of predator–prey relationships.
Sand cat status and ecology
Beyond our methodological assessment, the data collected in the Atlantic Sahara contributed to enhance the knowledge of this poorly known species. We confirmed for the first time that the species is well distributed in Area #1, which represents the northwestern limit of its Atlantic Sahara range (Aulagnier et al. 2017); we cannot discard the presence of the sand cat in Area #2, as the method used there (only walking surveys) was not optimal, whereas in Area #3, its presence is well known (Aulagnier et al. 2017). We exclusively found the sand cat in flat areas of gravels and sandy areas (reg habitat), while we never detected the species in rocky hills or rocky mountains, demonstrating a strong first-order habitat selection (sense Johnson 1980). We also confirmed that rodent availability in areas of sand cat presence (the case of Reg Labyad) was apparently high.
Data on abundance showed a very low density of sand cats, likely being a frequent scenario (see previous sub-section). This abundance did not show a significant reduction in the ISA between 2019 and 2020, despite the significant decrease in rainfall (and primary production, pers. observ.). Drought periods have been confirmed to have negative effects on sand cat survival in the Arabian Peninsula (Sliwa et al. 2016), which are probably harsher than in the Atlantic Sahara, even considering the severe drought period of 2019–2020. In fact, rodents apparently did not decrease in the ISA (data from the camera surveys). On the other hand, the drought period could locally benefit sand cats, since nomadic herders are almost absent for long periods, whereas several hundred sheep and goats with dozens of herder dogs invade the ISA during the rainy years (e.g., 2016 and 2017), with dogs being a key problem for sand cat survival (Sliwa et al. 2016).
We only detected males in the ISA (two of them in both 2019 and 2020). In a review of felids, Anile and Devillard (2018) found that in large areas, the adult sex ratio was male-biased for the smaller species (but note that they did not study the Felis genus); they argued that some sampling designs are unsuitable for females due to “gaps” within the trapping grid. However, our sampled area could be considered small on the basis of the known spatial ecology of sand cats (see the sampling design of the ISA in the “Material and methods” section), a sampling scenario in which the adult sex ratio was balanced for all the species analyzed by Anile and Devillard (2018). In fact, the available information on the home range of one sand cat female in the Atlantic Sahara suggests that there were no relevant gaps in our grid (MCP 100% home range of 13.4 km2 within 4 days and 14 waypoints, Breton et al. 2016). The influence of the lure (i.e., females may avoid the lynx urine) is an alternative hypothesis that requires further research, although this effect has not been detected for another Felis genus species, the European wildcat, in a study that combined radio-tracking and camera trapping with Iberian lynx urine as a lure (Monterroso et al. 2009). The apparent case of a true absence of females deserves long-term monitoring to explain the reasons and/or dynamics that may be leading to such an unfavorable demographic scenario.
Although our results should be taken with caution due to the low sample size, the diel activity was similar to the pattern observed in central Arabia during the same season (Amin et al. 2021). In our study, we observed a large predator–prey overlap in the diel activity, 78%–84% depending on the rodent species, in concordance with the relationships obtained for red foxes and rodents in NE Africa, specifically in the Middle Atlas (83% of overlapping, Gil-Sánchez et al. 2021). Moreover, despite the high overlap, Tarabul’s and fat-tailed gerbils apparently tended to avoid the activity peaks of sand cats. The strictly nocturnal behavior of sand cats could also be a response to avoid the high temperatures of the desert (Allan and Warren 1993), since other potential prey such as lizards (Sunquist and Sunquist 2009; Sliwa et al. 2016) are active during daylight. It is important to note that, in the absence of nomadic herders and their dogs, nighttime still probably represents a higher mortality risk for sand cats in the study area, due to the nocturnal activity of predators such as African golden wolves (Canis anthus, data from our surveys: 310 captures), pharaoh eagle-owls (Bubo ascalaphus), and poachers using spotlights from cars (who were detected in our study during expeditions).
Conclusions
We compared for the first time the applications of two non-invasive methods to survey and study the sand cat in the remote areas typically inhabited by this elusive felid. Our results show that camera trapping can offer reliable data on several key aspects of its poorly known ecology, from habitat, presence, and density to activity patterns and interspecific relationships, within a logistically feasible framework. Furthermore, we provide key recommendations to improve the rate of records to achieve better density estimations of sand cats using camera trapping surveys, highlighting some methodological aspects that require further research, such as the use of attractants. Unfortunately, the non-invasive survey of samples to obtain molecular data failed completely in our case, but we recognize that further studies are necessary in other habitats and abundance scenarios. In conclusion, our study offers the following: (1) a guideline to improve knowledge about the sand cat through a non-intrusive and realistic field approach and (2) novel information on key aspects of its biology, collected from one of its most important populations in the world.
References
Abbadi M (1993) Israel’s elusive feline: sand cats. Cat News 18:15–16
Ahmed S, Alzaabi R, Soorae P, Shah J, Hammadi E, Pusey R, Dhaheri S (2016) Rediscovering the Arabian sand cat (Felis margarita harrisoni) after a gap of 10 years using camera traps in the Western Region of Abu Dhabi, United Arab Emirates. Eur J Wildl Res 62. https://doi.org/10.1007/s10344-016-1035-8
Al Zaabi R, Gubiani R, Soorae P (2019) Current distribution of Arabian Sand Cat Felis margarita harrisoni in Abu Dhabi, United Arab Emirates, via camera trapping. Tribulus 27:8–10
Allan T, Warren A (1993) Deserts: the encroaching wilderness. IUCN and Mitchell Beazley, London
Amin R, Wacher T, Bruce T, Barichievy C (2021) The status and ecology of the sand cat in the Uruq Bani Ma’arid Protected Area. Empty Quarter of Saudi Arabia Mammalia. https://doi.org/10.1515/mammalia-2020-0031
Anile S, Ragni B, Randi E, Mattucci F, Rovero F (2014) Wildcat population density on the Etna volcano, Italy: a comparison of density estimation methods. J Zool (lond) 293:252–261
Anille E, Devillard S (2018) Camera-trapping provides insights into adult sex ratio variability in felids. Mammal Rev 48:168–179
Aulagnier S, Cuzin F, Thévenot M (2017) Mammifères sauvages au Maroc. Peuplement, répartition, ecologie. Société Française pour l’Etude et la Protection des Mammifères, Paris
Barea-Azcón JM, Virgós E, Ballesteros-Duperón E, Moleón M, Chirosa M (2007) Surveying carnivores at large spatial scales: a comparison of four broad-applied methods. Biodivers Conserv 16:1213–1230
Brassine E, Parker D (2015) Trapping elusive cats: using intensive camera trapping to estimate the density of a rare African felid. PLoS One 10:e0142508. https://doi.org/10.1371/journal.pone.0142508
Breton G, Sliwa A, Azizi S, Essalhi A (2016) Sand cats in the Moroccan Sahara, preliminary results of a new study. Cat News 63:7–10
Brito JC, Durant SM, Pettorelli N, Newby J, Canney S et al (2018) Armed conflicts and wildlife decline: challenges and recommendations for effective conservation policy in the Sahara-Sahel. Conserv Lett 11:e12446
De Valpine P, Paciorek C, Turek D et al (2021) NIMBLE: MCMC, particle filtering, and programmable hierarchical modeling. R package version 0.11.1. URL: https://cran.r-project.org/package=nimble, https://doi.org/10.5281/zenodo.1211190
De Valpine P, Turek D, Paciorek C et al (2017) Programming with models: writing statistical algorithms for general model structures with NIMBLE. J Comput Graph Stat 26:403–413
Durant SM, Wacher T, Bashir S et al (2014) Fiddling in biodiversity hotspots while deserts burn? Collapse of the Sahara’s megafauna. Divers Distrib 20:114–122
Garrote G, Gil-Sánchez JM, McCain B, Lillo S, Tellería JL, Simón MA (2012) The effect of attractant lures in camera trapping: a case study of population estimates for the Iberian lynx (Lynx pardinus). Eur J Wildl Res 58:881–884
Gelman A, Carlin JB, Stern HS, Rubim DB (2013) Bayesian data analysis, 3rd edn. CRC Press, Boca Raton, FL, Third edit
Ghafaripour S, Naderi M, Rezaei HR (2017) Investigating abundance, density and potential threats of Sand cat in the South-Eastern parts of Iran. J Wildl Biodiver 1:47–55
Ghafaripour S, Naderi M, Riazi B, Rezaei HR (2018) How prey density and distribution can affect predator habitat usage pattern: a case study on sand cat (Felis margarita, Locke 1858) from Iran. Russ J Ecol 49:320–324
Gil-Sánchez JM, Barea-Azcón JM, Jaramillo J (2015) Strong spatial segregation between wildcats and domestic cats may explain low hybridization rates on the Iberian Peninsula. Zoology 118:377–385
Gil-Sánchez JM, Barea-Azcón JM, Jaramillo J, Herrera-Sánchez FJ, Jiménez J, Virgós E (2020b) Fragmentation and low density as major conservation challenges for the southernmost populations of the European wildcat. PLoS One 15:e0227708
Gil-Sánchez JM, Herrera-Sánchez F, Álvarez B et al (2017) Evaluating methods for surveying the Endangered Cuvier’s gazelle Gazella cuvieri in arid landscapes. Oryx 51:648–655
Gil-Sánchez JM, Herrera-Sánchez FJ, Rodríguez-Siles J et al (2020a) Feeding specialization of honey badgers in the Sahara Desert: a trial of life in a hard environment. Diversity 12:59. https://doi.org/10.3390/d12020a059
Gil-Sánchez JM, Mañá-Varela B, Herrera-Sánchez FJ, Urios V (2021) Spatio-temporal ecology of a carnivore community in Middle Atlas. NW of Morocco Zoology 146:125904
IUCN (2016) The IUCN red list of threatened species 2016:e. https://doi.org/10.2305/IUCN.UK.2016-2.RLTS.T8541A50651884.en
Jiménez J, Augustine BC, Linden DW, Chandler RB, Royle JA (2021) Spatial capture–recapture with random thinning for unidentified encounters. Ecol Evol 11:1187–1198
Johnson D (1980) The comparison of usage and availability measurements for evaluating resource preference. Ecology 61:65–71
Kottek M, Grieser J, Beck C, Rudolf B, Rubel F (2006) World map of the Köppen-Geiger climate classification updated. Meteorol Z 15:259–263
Linkie M, Ridout MS (2011) Assessing tiger-prey interactions in Sumatran rainforests. J Zool (lond) 284:224–229
Long RA, MacKay P, Zielinsky WJ, Ray JC (2008) Noninvasive survey methods for carnivores. Island Press, Washington DC
Mackenzie D, Nichols J, Royle J, Pollock K, Bailey L, Hines J (2006) Occupancy estimation and modeling. Academic Press, Oxford, Inferring patterns and dynamics of species occurrence
Matias G, Rosalino LM, Rosa JL, Monterroso P (2021) Wildcat population density in NE Portugal: a regional stronghold for a nationally threatened felid. Popul Ecol 63. https://doi.org/10.1002/1438-390X.12088
Mengüllüoğlu D, Fickel J, Hofer H, Förster DW (2019) Non-invasive faecal sampling reveals spatial organization and improves measures of genetic diversity for the conservation assessment of territorial species: Caucasian lynx as a case species. PLoS One 14:e0216549
Monterroso P, Alves PC, Ferreras P (2011) Evaluation of attractants for non-invasive studies of Iberian carnivore communities. Wildl Res 38:446–454
Monterroso P, Alves PC, Ferreras P (2013) Catch me if you can: diel activity patterns of mammalian prey and predators. Ethology 119:1044–1056
Monterroso P, Brito JC, Ferreras P, Alves PC (2009) Spatial ecology of the European wildcat in a Mediterranean ecosystem: dealing with small radio-tracking datasets in species conservation. J Zool (lond) 279:27–35
O’Connell AF, Nichols JD, Karanth KU (2011) Camera traps in animal ecology: methods and analyses. Springer, Tokyo, Dordrecht, Heildelberg, London, New York
Palomares F, Godoy JA, Piriz A, O’Brien SJ (2002) Faecal genetic analysis to determine the presence and distribution of elusive carnivores: design and feasibility for the Iberian lynx. Mol Ecol 10:1885–2194
Ridout MS, Linkie M (2009) Estimating overlap of daily activity patterns from camera trap data. J Agric Biol Environ Stat 14:322–337
Sher Shah M, Cunningham P (2008) Fences as a threat to Sand cats Felis margarita Loche, 1958, in Saudi Arabia. Zool Middle East 42:99–101
Sliwa A, Ghadirian T, Appel A, Banfield L, Sher Shah M, Wacher T (2016) Felis margarita. The IUCN Red List of Threatened Species 2016:e.T8541A50651884
Strauss WM, Shobrak M, Sher Shah M (2007) First trapping results from a new sand cat study in Saudi Arabia. Cat News 47:20–21
Sunquist M, Sunquist F (2002) Wild cats of the world. University of Chicago Press, Chicago
Sunquist M, Sunquist F (2009) Family felidae. In: Wilson DE, Mittermeier RA (eds) Handbook of the mammals of the world, vol 1. Carnivores. Lynx Edicions, Barcelona, pp 54–168
Tenan S, Pedrini P, Bragalanti N, Groff C, Sutherland C (2017) Data integration for inference about spatial processes: a model-based approach to test and account for data inconsistency. PLoS One 12:e0185588–e0185588
Tobler W, Powell G (2013) Estimating jaguar densities with camera traps: problems with current designs and recommendations for future studies. Biol Conserv 159:109–118
Waits LP, Paetkau D (2005) Noninvasive genetic sampling tools for wildlife biologists: a review of applications and recommendations for accurate data collection. J Wildl Manag 69:1419–1433
Acknowledgements
Salvador Castillo, Luis García-Cardenete, Aitor Rincón-García, Jesús Rodríguez-Osorio, Luis Llaneza, Ugo Melone, Marcos Mallo, Jennifer Leonard, and Carlos Sarabia helped in some surveys. We are especially grateful to the members of the association Tabia Biladohoud of Assa for their hospitality and support and to Abdalahi Hatari, who kept our camera devices safe during the survey of 2020 in the Reg Labyad. The Département des Eaux et Forêst of Morocco provided permission for these wildlife surveys.
Funding
Funding for open access publishing: Universidad de Granada/CBUA. This study was partially supported by Fundación Barcelona Zoo (PRIC Project 2017 grant).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gil-Sánchez, J.M., Herrera-Sánchez, F.J., Rodríguez-Siles, J. et al. Applications of non-intrusive methods to study the sand cat: a field study in the Sahara Desert. Eur J Wildl Res 69, 20 (2023). https://doi.org/10.1007/s10344-023-01645-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10344-023-01645-7