Abstract
Health assessment of individuals is an important aspect of monitoring endangered wildlife populations. Haematological and biochemical values are a common health assessment tool, and whilst reference values are well established for domestic species, they are often not available for wild animal species. This study established 31 haematological and biochemical reference intervals for golden eagle (Aquila chrysaetos) nestlings in Scotland, in order to improve the understanding of the species’ health and support conservation efforts. Reference intervals were created from 47 nestlings (ages 2–7.5 weeks old) across 37 nests, to date, the largest sample of wild individuals of this species and age cohort sampled for these purposes. Upper reference intervals for concentrations of lymphocytes, total protein, cholesterol, triglycerides, uric acid, and monocytes, calculated in this study, are higher than those found for adult raptors and the interval span is higher than that observed in adult raptors for concentrations of AST, albumin, eosinophil, LDH, and monocyte count. Statistically significant positive correlations were found with age and concentrations of haemoglobin, lymphocytes, serum pH, and creatine kinase, and significant negative correlations with age for concentrations of thrombocytes, heterophils, total protein, globulin, and lactate dehydrogenase. Packed cell volume was significantly higher for females than males, and concentration of calcium and eosinophils were higher for individuals in good body condition than those in moderate body condition. The reference intervals produced by this study will be of important use to the veterinary and conservation management communities and will aid the long-term monitoring of the Scottish golden eagle population health.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Haematological and biochemical reference intervals are commonly used to evaluate individual animal health (Mathieu-Denoncourt et al. 2015; Barrows et al. 2017; Morandini et al. 2018; Townsend et al. 2018). Health assessment of individuals can be used for diagnosing disease and informing individual treatment. Whilst haematological and biochemical values are well established for domestic species, information is not always available or easy to obtain for free-ranging wild species (Polo et al. 1992; Hernández and Margalida 2010; Mello et al. 2016) or free-ranging or domestic nestlings, juveniles, and sub-adults. Blood reference values developed from individuals under human care may lack the natural variation presented by a wild environment, as conditions can be more easily controlled (Perry et al. 1986). It is known that haematological and biochemical values can be influenced by age, nutritional status, sex, or external factors such as chemicals or contaminants as well as seasonal changes (Gee et al. 1981; Perry et al. 1986; Gessaman et al. 1986; Maxwell et al. 1990; Dobado-Berrios and Ferrer 1997; Dickinson et al. 2002; Hollamby et al. 2004; Peinado et al. 2007; Sonne et al. 2010; Greig et al. 2010; Muñoz et al. 2012; Græsli et al. 2014; Stoffel et al. 2020).
The golden eagle (Aquila chrysaetos) is a key apex predator, and the second largest and one of the most charismatic of the UK’s birds of prey (Royal Society for the Protection of Birds 2011; Riley 2012; Taylor 2016). It is also widely spread in the northern hemisphere, spanning across the Palearctic and Nearctic regions (Watson 2010; Taylor 2016). Historically, golden eagles were distributed across the whole of Europe but continuous persecution and pollution during the nineteenth century drove the species to near extinction in the UK (Lockie and Ratcliffe 1964; Lockie et al. 1969; Newton and Galbraith 1991). Despite the golden eagle legal protection since 1954 and an increase in breeding pairs (Hayhow et al. 2017), the 2015 Scottish golden eagle survey and subsequent annual monitoring have reported a decrease in productivity in some areas despite a reduction in persecution (Hayhow et al. 2017) and evidence of food supply. This unexplained low productivity for certain areas of Scotland has anecdotally been suggested as being due to high nestling mortality (pers.com Scottish Raptor Study Group, SRSG). Direct observations of high nestling mortality and poor health in the 2018 nesting season by one author of this study (GP) supports this anecdotal suggestion.
Clinical examination and blood sampling to assess the health of free-ranging golden eagle nestlings in Scotland have never been carried out across the population and existing published haematological values for golden eagles in general are limited.
Representative information from free-ranging individuals is required as housing, diet, management, and handling under captive conditions can alter natural behaviours, blood values, and parasite range, providing an inaccurate view of the health and ecology of wild species (Forbes and Simpson 1997; Park 2003; Spagnolo et al. 2008). Age has also been described as a factor affecting blood values (Viñuela et al. 1991; Dobado-Berrios et al. 1998; Ferrer and Dobado-Berrios 1998). Currently, the few references on golden eagles relate mainly to studies of captive adult individuals or free-ranging individuals which include only a small sample of nestlings (Nazifi et al. 2008; Sonne et al. 2010, 2012; Teare 2013).
The development of blood reference intervals for Scottish golden eagle nestlings will add valuable information for individual health assessment and management of the species and facilitate health studies of the wider raptor assemblage. Golden eagle nests in Scotland are well monitored and the population size can be estimated through yearly counts of successful breeding territories and ringing (also known as banding) of nestlings, which are also the more physically accessible of the species cohort (Hayhow et al. 2017). Nestlings are also often the group of choice for reintroduction, reinforcement, or translocation programmes (Love and Ball 1979; Molenaar et al. 2017) and blood reference values for this age group will facilitate health evaluation and choice of individuals. The rationale for this research was developed during the early stages of a Scottish golden eagle reinforcement project using nestlings for translocation, highlighting the management importance and value of this work. Comprehensive health examinations, including haematology and biochemistry, are used in this reinforcement project to inform the selection of individuals and decide the course of interventions. Blood reference values for this cohort would assist with these decisions before and during the quarantine of the eagles, and before their release.
The aim of this study was to establish reference intervals for haematological and biochemical values of free-ranging golden eagle nestlings using birds from across Scotland, in order to inform future health assessments of this age group and improve the understanding of a well monitored but vulnerable population.
Materials and methods
Sampling strategy and ethical approval
A total of 53 golden eagle nestlings between two and seven and a half weeks old from 38 nests across Scotland were visited. Two nestlings could not be sampled due to small size and thus blood samples were collected from 51 nestlings from 37 nests, with pairs of nestlings found in 14 nests, between 9 am and 8 pm during daylight summer hours of June and July in the 2017 and 2018 breeding seasons during visits to the nests for population marking (via leg ringing) as part of routine management and monitoring of the species. All procedures took place under Home Office licence authority (project licence PB8A1D5C7, local AWERB review identifier PL10-17), and ethical approval from the University of Edinburgh.
Sampling area
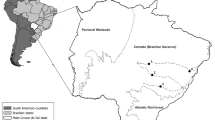
The nests were distributed across Scotland, from the Western Isles across to the North East Glens and down as West Argyll and Isles (Fig. 1). These areas have been designated and developed by Scottish Natural Heritage (SNH) as natural heritage zones (NHZs) (Scottish Natural Heritage 1998).
Scottish biogeographic zones, termed natural heritage zones by Scottish Natural Heritage (1998). The eight areas and their respective names sampled in 2017 and 2018 during this study appeared in grey and names in bold
Health assessment and blood collection
A clinical health examination protocol was designed and performed on each individual following general raptor medicine and management recommendations (Heidenreich 1997; Redig and Ackermann 2000). As part of this examination, body weight was obtained and age, body condition, feather development, mentation, body posture, observation of obvious lesions, abnormal body discharges, breathing abnormalities, and general appearance were evaluated. Points were given for each negative observation and with two points or more, the individuals were considered not healthy and thus not considered for reference range calculations. See Supplementary Table 1 for details of observations and scoring. Age was estimated based on a protocol created from descriptions of weekly feather development of American and Scottish golden eagle nestlings (Driscoll 2010; Watson 2010). Body condition was assessed and categorised as good, moderate, or poor, based on prominence of the keel bone and pectoral muscle mass (Meredith 2016), accounting for less developed pectoral muscle mass due to pre-flight status. Finally, individuals were categorised as ‘healthy’ or ‘not healthy’ based on this full clinical examination protocol.
Blood was obtained from the brachial vein and used for haematological, biochemical, and molecular sexing analysis, using a 5 ml disposable syringe and a 23 gauge needle, whilst the bird was held in dorsal recumbency at an angle of approximately 45° to minimise pressure on dorsal air sacs. A maximum of 5.5 ml of blood was obtained from each wing, by filling all usable space in the syringe, beyond the graduated 5 ml, to a maximum of 11 ml per individual (never exceeding 1% bodyweight). Pressure was applied to the venipuncture site after sampling to allow clotting and prevent hematoma formation. Handling time was limited to approximately 5 min per bird. Individuals were returned to their nest as soon as possible after handling. The time the birds were out of the nest varied depending on the number of people available to assist at the nest site and the accessibility of the nest; time spent at the nest ranged from 10 to 30 min, and on average 20 min, during which routine protocols for species monitoring were performed (ringing, measuring), along with the blood sampling and health examination for this study. The same procedures were conducted on all nestlings present in the nest.
Sample handling and storage
Blood was collected into a lithium heparin tube (minimum 1.5 ml) and the remaining drops of fresh blood in the syringe were placed on a Whatman FTA ® DNA card (GE Healthcare Live Sciences UK Ltd. Amersham Place, Buckinghamshire HP7 9NA, UK) for molecular sexing and additionally to make two fresh whole-blood, air-dried smears, using the slide on slide technique (Campbell and Ellis 2015). The dried smears and the lithium heparin tube samples were stored at ambient temperature (15–17 °C) and sent to Greendale Veterinary Diagnostics Ltd., Surrey, England, UK, for arrival within 48 h, or 72 h for cases sampled on a Saturday. Lithium heparin tubes were spun on arrival to the lab. Each Whatman FTA ® DNA card was stored in a SceneSafe™ tamper evident bag containing moisture indicator silica gel beads (Scientific Laboratory supplies Ltd. 22–23 Ruddington Ln, West Bridgford, Nottingham NG11 7EP, UK), providing a moisture-free environment for cards to dry out and remain dry whilst working in the field.
Analytical method
An extended avian profile (Greendale Veterinary Diagnostics Ltd.) was performed on every lithium heparin sample, volume permitting. The plasma biochemistry profile analytes included proteins: albumin, haemoglobin, total globulin, and total protein; enzymes: aspartate aminotransferase (AST), creatinine kinase (CK), and lactate dehydrogenase (LDH); metabolites: bile acids, uric acid, glucose, cholesterol, and triglycerides; minerals: calcium, potassium, and sodium; and ionised calcium. All biochemical analytes were photometrically measured with an Olympus AU640 at 37 °C (Olympus Corporation, Southend-on-Sea, SS2 5QH, UK) except for the haemoglobin and ionised calcium, for which different analysers were used (HemoCue, Prospect Diagnostics, Derbyshire, S18 2LX, UK; and ABAXIS i-STAT® analyser (Abaxis UK Ltd. Chessingham Park, Dunnington, York, UK)) respectively. White blood cell (WBC) counts were determined manually with an improved Neubauer haemocytometer and the use of phase contrast microscopy. A solution of 0.38 ml of 1% ammonium oxalate and 0.02 ml of lithium heparin blood was prepared and used to fill the counting chamber and a total of four large squares (64 small squares) were counted with the × 40/0.65 phase objective. The total number of cells was divided by 20 to obtain WBC × 109/L. Packed cell volume (PCV) was obtained from plain capillary tubes filled with blood at centrifuged at 10,000 g for 5 min and read manually with a microhaematocrit reader. Counts of avian red blood cells which have large and nucleated cells are not accurate enough to be useful (Harvey 2012); thus, counts were not done for erythrocytes. However, the blood smears, stained with Wright Giemsa (Hematek 2000 stainer, Siemens Healthcare Ltd.), were used to evaluate thrombocytes, red and white blood cell morphology, haemoparasites, and evidence of anisocytosis, polychromasia, and anaemia. The WBC differential count was based on 100 counted leucocytes. Polychromasia and anisocytosis were estimated to assess erythropoietic activity. The serum pH was measured with an ABAXIS i-STAT® analyser (Abaxis UK Ltd., Chessingham Park, Dunnington, York, UK). Extraction and amplification of DNA for molecular sexing were performed by the Science and Advice for Scottish Agriculture (SASA) forensics laboratory (Roddinglaw Road Edinburgh EH12 9FJ, Scotland, UK). Most values tested here would have remained within allowed deviations under the imposed conditions prior to analysis; nevertheless, any time-sensitive values likely to change prior to analysis will be presented with a notification in the results (Thoresen et al. 1992; Day et al. 2001; Van_Balveren et al. 2017).
Data analysis
Statistical analysis, including reference limit calculation and confidence intervals, was conducted for each haematological and biochemical value. This was performed using R version 3.5.1 and the ‘referenceIntervals’ R package (Finnegan 2014; RStudio Team 2016; R Core Team 2018). A Shapiro–Wilk test was performed for exploration and normality testing of the data. A robust reference interval calculation that would comply with basic reference interval calculation principles (Friedrichs et al. 2012) could not be performed due to small sample size; therefore, a conservative approach to determine the use of a parametric or nonparametric reference interval calculation was adopted in order to determine 95% reference interval, with 90% confidence intervals (CI). Fourteen out of a total of 37 nests contained a pair of individuals per nest; thus, the calculation of reference intervals using all nestlings would therefore potentially introduce pseudo-replication in the values. To account for this, nest-level datasets were created via bootstrapping methodologies prior to reference interval calculation. For each nest, a single nestling from that nest was selected at random (same nestling for single nests) to create a dataset of one nestling per nest, and this was repeated 1000 times. These 1000 datasets were used to calculate the reference intervals.
For each dataset produced, depending on data distribution, different methods for calculating the reference intervals were followed: If data were normally distributed (Shapiro–Wilk normality test p value ≥ 0.05), then reference intervals were calculated using parametric methods. If raw data were not normally distributed (p values < 0.05), a Box-Cox transformation was attempted. If this Box-Cox transformation resulted in normally distributed data, then reference intervals were calculated using parametric methods on the transformed data. Values obtained were subsequently back-transformed to obtain the real reference limits based on the raw data. If data were not normally distributed (p values < 0.05), whether Box-Cox transformed or not, then reference intervals were calculated using a nonparametric method via bootstrapping (NP). All outliers detected by the Horn method were retained for calculations, based on clinical evaluation (Geffré et al. 2011; Friedrichs et al. 2012). The mean and upper and lower 5% quantile reference values for the lower and upper reference interval for each value were then calculated from the output from the 1000 bootstrap data sets.
Differences in blood values between males and females were investigated by means of a t-test for values that presented a normal distribution on first examination (Shapiro–Wilk ≥ 0.05) or where transformation resulted in a normal distribution and by Wilcoxon test for data with a non-normal distribution (Shapiro–Wilk ≤ 0.05). A two-sample t-test of parametric, otherwise Wilcoxon was used to compare values between the different body condition categories. Pearson product moment correlations were calculated to look at the association of age with the different blood values.
Results
Following the clinical examinations, four individuals were considered not healthy resulting in 47 healthy individuals being used for reference interval calculation (Table 1, Supplementary Fig. 1). Individual mean values and reference intervals are presented in the context of previous studies on other populations in the discussion. No blood parasites were detected in any of the samples and mild to moderate polychromasia was reported for 8 individuals of which two cases presented the occasional metarubricyte. The ages of the individuals used in this study ranged between 2 and 7.5 weeks of age (mean = 5.4) (Fig. 2), whilst the weight of healthy and unhealthy individuals ranged from 1400 to 4650 g across the females (mean = 3320.5 g) and from 1500 to 4300 g across the males (mean = 2890.5 g) (Table 2). Association of age with the different blood values found 11 statistically significant correlations (Table 3).
Body condition scores were assessed on all examined birds (including four deemed as unhealthy) and the effects of good and moderate body conditions were analysed. Analysis of birds in poor body condition was not possible because only two birds were observed in such condition. Positive statistically significant associations were observed between body condition and two blood values (PCV (L/L) and eosinophils (× 109/L)) (Table 3).
Out of 47 healthy individuals examined, 28 were members of a twin pair and 19 were single individuals in a nest. When analysing the effect of sex on all values measured, the only statistically significant difference found was for packed cell volume (Welch two sample t-test p-value = 0.009, female mean = 0.420, male mean = 0.381, Table 3). No effect of presence of twins vs. single individuals in each nest was detected on any of the blood variables measured (Welch two sample t-test, smallest p-value = 0.08).
Discussion
This work has estimated reference interval values for 31 haematological and biochemical values in golden eagle nestlings from Scotland adding considerably to the currently sparse literature for this species. Previous studies have been limited to either only adults (Nazifi et al. 2008), captive individuals (Polo et al. 1992; Pollock 2005; Teare 2013) or have been on a limited number of nestlings (N = 12) (Sonne et al. 2012). The haematologic and biochemical values reported from a study using a small sample (N = 5) of adult captive golden eagles (Polo et al. 1992) generally fit within the ranges that are presented here, with a few notable variations or exceptions. Upper reference intervals for lymphocytes, monocyte percentage, total protein, cholesterol, triglycerides, and uric acid calculated in this study (Table 1, Supplementary Fig. 1) are higher than those found for adults (Polo et al. 1992), although the lower interval remains within the range presented for adults. In contrast, the span of the interval in the present study is higher than the range of values observed in adult raptors (Polo et al. 1992) for AST (present study 175.11–575.40 U/L vs Polo et al. (1992) study 55–71 U/L), albumin (12.10–17.49 g/L vs 8–10 g/L), eosinophil percentage (5–24.0% vs 1–3%), eosinophil count (0.60–5.40 × 109 vs 0–0 × 109), LDH (861.55–3658.56 U/L vs 374–683 U/L), and monocyte count (0.30–2.0 × 109 vs 0–0 × 109).
The authors recommend that the reference limits provided for evaluation of golden eagle nestlings are used in combination with clinical experience for decision-making; these conservative intervals are shown in Table 1. Animals with values found outside the conservative ranges presented here should merit further examination and investigation of possible causes such as presence of toxins or invisible chronic illness.
The width of ranges presented in this study are more similar to those previously presented for a few free-ranging golden eagle nestlings (Sonne et al. 2012) than those presented for captive adult golden eagles (Polo et al. 1992). The values comparable between Sonne et al. (2012) study of free-ranging golden eagle nestlings and the present study provide similar means for albumin (14.08 g/L vs 14.33 g/L); Na (155.7 mmol/L vs 154.2 mmol/L); potassium (3.06 mmol/L vs 2.12 mmol/L); total protein (30.07 g/L vs 38.72 g/L); and calcium (1.3 mmol/L vs 2.75 mmol/L) where the second reading represents the mean for the present study. These parallels are probably not surprising given the age similarity and the fact that captive conditions impose control over various values such as diet, anaesthesia, and fixed sampling times (Dressen et al. 1999; Ferrer et al. 1987). The values presented by this paper are likely to be more representative of the natural range of golden eagle nestlings exposed to a non-captive environment. A larger sample size and the use of health examinations improve the suitability of the reference intervals for assessing health of free-ranging golden eagle nestlings.
Whilst an important tool, blood reference values should not be the only factor used to determine an animal’s health. Values such as lymphocytes, monocytes, eosinophils, and basophils can be used as indicators of chronic, systemic, or parasitic infections, tissue damage, disease, or stress (Maxwell et al. 1990; Maxwell 1993; Clark and Raidal 2009), but must be interpreted whenever possible in conjunction with patient history, other clinical findings, and clinical experience for a more complete health assessment.
Sampling of large free-ranging species in remote locations means that it is not possible to fully meet ideal sample processing standards. All measures were taken to obtain, transport, and process the samples in order to obtain meaningful and reliable results. Most values tested here would have remained within allowed deviations under the imposed conditions prior to analysis; nevertheless, glucose could have decreased and potassium could have increased (Thoresen et al. 1992; Day et al. 2001; Van_Balveren et al. 2017). However, this is not likely to be to a clinical degree or critical change value (Guder et al. 2010; Van_Balveren et al. 2017).
Plasma enzymes regulate metabolic reactions and can reflect avian health (Hanauska-Brown et al. 2003). The authors suspect that the high values observed for CK, AST, LDH, triglycerides, and uric acid may be due to factors such as dehydration, skeletal muscle damage, or postprandial effect (Joseph 2006; Lumeij and Remple 2007). Furthermore, to the intervention of handling and sampling, this may reflect the additional natural stresses imposed on free-ranging wild animals. It is generally accepted that brachial vein sampling as well as muscle damage associated with handling may cause an elevation of CK and LDH (Joseph 2006; Meredith et al. 2012). As with previous work with young raptors (Meredith et al. 2012), the nestlings sampled by this study are yet to fledge and the majority are yet to start exercising their wings. The lack of exercise could mean that their muscles are more prone to cellular disruption (Knuth and Chaplin 1994), and thus high CK levels could be observed as a result of handling. In an effort to reduce the possibility of muscle damage, every effort was made to reduce handling stress. Samples were collected by skilled veterinary practitioners and birds handled by experienced bird handlers.
Glucose levels (0.60–15.40 mmol/L), found by this study for the majority of the golden eagle nestlings sampled, have a wider range and the upper reference limit is comparable to that found for golden eagle nestlings in Norway (6–17 mmol/L) (Sonne et al. 2012), and results for both of these studies contrast with more elevated glucose readings (13.6–25.6 mmol/L) reported for adult golden eagles (Polo et al. 1992). Whilst an increase in the glucose readings with age has been reported for other raptor species (Ferrer and Pablo 1998), reinforcing the importance of acknowledging differences in age when referring to blood reference intervals for the species, no such association was found with the current study with nestlings whilst in the nest (from 2 to 7.5 weeks old). This suggests that glucose age differences may not be apparent under seven and a half weeks of age.
However, statistically significant positive correlations were observed between age and haemoglobin, lymphocyte percentage, lymphocyte count, serum pH, CK, and thrombocytes, and a positive trend between age and sodium. A positive trend for age and sodium has also been observed in five species of nocturnal raptors (Agusti Montolio et al. 2018). Statistically significant negative correlations between age and heterophil percentage, heterophil count, LDH, and globulin were observed. Again, the negative correlation between age and globulin has been reported by Agusti Montolio et al. (2018) for five species of owl, likely reflecting the maturation of the immune system in growing individuals. The higher levels of haemoglobin and CK towards fledging age can be explained by increasing muscle usage. This muscle usage requires more oxygen, and thus haemoglobin, resulting in cellular disruption to the untrained muscles, potentially increasing CK (Knuth and Chaplin 1994; Cornell et al. 2017). The positive association in haemoglobin levels with age has been previously recorded for long-lived avian species and haemoglobin concentration has been suggested as a strong indicator of physiological condition related to habitat quality, parasitism, moult, and breeding state (Bańbura et al. 2007; Minias 2015), as well as a useful indicative of fledging time due to its constant concentration in avian blood (Bańbura et al. 2007).
A decrease in LDH with age found in this study, seemingly connected with the eminent fledging, has been observed in pre-migrating birds, where low LDH levels seem to increase the glycolytic capacity in the muscles, in preparation for the coming endeavour (Banerjee and Chaturvedi 2016). The likely increase in food competition between siblings may contribute to increased levels of stress and might be the reason behind the observed increase in lymphocytes and reduction in heterophils (Moreno et al. 2016). No correlation was observed between PCV and age in this study and the mean for the same value was lower for individuals in this study than that reported for adult golden eagles (0.395 L/L vs 0.421 L/L) (Polo et al. 1992; Hollamby et al. 2004).
Recognising a 15.3% shift of the mean of the ranges between nestlings and adults could help identify a potential nutritional problem across a population, which could be missed if one single range is used for all cohorts in the population. This provides further evidence that reference intervals for adult individuals may not be applicable for use on individuals below 7.5 weeks of age, despite their grown appearance and early resemblance to the adult of the species. The rapid growth and weight increase in golden eagle nestlings are typical of raptors and altricial birds in general, which are born highly dependent on parents for body warmth and food (Ricklefs 1984). The intensive care and food supplied by the parents translates into great physical differences between a newly hatched nestling and a 10-week-old nestling that is ready to fledge.
There were no between-sex differences in metabolite or enzyme levels, total white cell estimates, or individual cell type; the only statistically significant difference was found for packed cell volume where the female mean PCV (0.41 L/L) was higher than that for males (0.38 L/L). Both means lie within the reported range for free-ranging nestling raptors (Hollamby et al. 2004; Meredith et al. 2012) and these findings follow the common trend observed in nature of a higher red cell content carried by larger veins and arteries, possibly explained by the reverse sexual dimorphism in raptors, where females are larger (Murphy 2014).
Haematocrit or packed cell volume (PCV) is often documented as an indicator of the condition of wild birds (Saino et al. 1997; Ots et al. 1998). A higher PCV was observed in this study for birds with good body condition over that for birds in moderate body condition. On its own, the use of haematocrit or packed cell volume (PCV) as an indicator of body condition in wild birds can be a contentious topic due to the fact that there are many factors able to contribute to a PCV reading (Dawson and Bortolotti 1997a, b; Fair et al. 2007) and variations in PCV values within the day can even be associated with fasting and feeding (García-Rodríguez et al. 1987). Whilst higher energy expenditure (i.e., moulting, temperature regulation) or dehydration can contribute to higher PCV values (Hõrak et al. 1998), chronic disease or long-term presence of certain chemicals such as DDT can result in lower PCV values (Harrison and Harrison 1986). Whilst the difference in the mean PCV between males and females in this study is only 0.04, this represents 24% of the overall the healthy reference interval for this value; therefore, awareness of the differences between sexes could help identify population level health issues. As per any health assessment, an overall clinical examination, history (if available) together with a suite of blood variables should be used to guide a health assessment (Joseph 2006).
No differences were found for any of the blood variables between individuals in single and twin nests, suggesting the cost of rearing twins over single individuals does not appear to be as significant as that found for raptor species with brood sizes of four or more individuals (Castaño 1997; Limiñana et al. 2009). Previous studies have observed differences in development throughout the nesting period between individuals in a twin nest versus those found in a single nest, as well as between the two individuals found in a twin nest (Donazar and Ceballos 1989; Limiñana et al. 2009). In the same studies, differences observed are believed to be determined by prehatching constraints and/or parental investment constraints affecting broods with multiple nestlings. Whilst we detected no evidence of differences in health between single and twin brood individuals, there may be health differences found between the first and second hatch individual in any one nest. However, due to the complexity and number of variables (hatch date, food provision, sex, body weight) that may drive such a pattern, our data of 47 individuals, where 28 were members of a twin pair and 19 were single individuals in a nest, cannot be used to determine the validity of such an assertion with any statistical rigour.
The haematological and biochemical reference values produced by this study were obtained from the largest sample size of free-ranging golden eagles nestlings to date. Blood chemistry can be an indirect way to assess health; it transports metabolic products, nutrients, hormones, enzymes, and immune cells (Stein et al. 1998; Hanauska-Brown et al. 2003), and for free-ranging individuals, when combined with environmental information such as habitat quality or environmental stressors, can provide a valuable tool for health assessment of individuals and their populations. Thus, baseline metabolite, enzyme, and blood cytology levels for free-ranging golden eagle nestlings will be of use to the veterinary and conservation management communities when assessing individual and population health of golden eagle nestlings in Scotland. The baseline information created by this work will permit health monitoring of golden eagles based on variations in blood levels in connection to their habitat, especially important for a species of conservation concern. Whilst targeting the Scottish population, these blood reference intervals can be used for golden eagle nestlings outside their range with due considerations of geographical and environmental differences. Long-term recording of blood value values could allow an improved ability to study population health trends of the species. Currently, the feasibility of golden eagle population health monitoring is limited by a lack of baseline health data which this study remedies.
Availability of data and material
Not applicable.
Code availability
Not applicable.
References
Agusti Montolio S, Cuenca Valera R, Lav S et al (2018) Plasma biochemistry RIs and age effect in European Strigiformes Background: blood biochemistry and hematology are essential in the laboratory. Vet Clin Pathol 47:78–93
Bańbura J, Bańbura M, Kaliński A et al (2007) Habitat and year-to-year variation in haemoglobin concentration in nestling blue tits (Cyanistes caeruleus). Comp Biochem Physiol Part A Mol Integr Physiol 148:572–577
Banerjee S, Chaturvedi CM (2016) Migratory preparation associated alterations in pectoralis muscle biochemistry and proteome in Palearctic-Indian emberizid migratory finch, red-headed bunting, (Emberiza bruniceps). Comp Biochem Physiol - Part D Genomics Proteomics 17:9–25
Barrows M, Killick R, Saunders R, Tahas S, Day C, Wyatt K, Horspool T, Bingaman Lackey L, Cook J (2017) Retrospective analysis of elective health examinations as preventative medicine interventions at a zoological collection. J Zoo Aquarium Res 5:25–32
Campbell T, Ellis C (2015) Blood sample collection and preparation in birds. In: Avian and exotic animal hematology and cytology, Fourth. John Wiley & Sons, Ltd, Ames, Iowa USA, pp 170–171
Castaño JP (1997) Fenología de puesta y parámetros reproductivos en una población de aguilucho cenizo (Circus pygargus) en el Campo de Montiel. Ardeola 44:51–59
Clark P, Raidal SR (2009) Haematological indicators of inflammation exhibited by Australian Falconiformes. Comp Clin Path 18:1–6
Cornell A, Gibson KF, Williams TD (2017) Physiological maturity at a critical life-history transition and flight ability at fledging. Funct Ecol 31:662–670
Dawson R, Bortolotti G (1997a) Are avian hematocrits indicative of condition? American kestrels as a model. J Wildl Manage 61:1297–1306
Dawson R, Bortolotti G (1997b) “Are avian hematocrits indicative of condition? American kestrels as a model,” J Wildlife Manage 1297–1306
Day RL, Heard DJ, La Blanc D (2001) The Effect of time at which plasma separation occurs on biochemical values in small island flying foxes (Pteropus hypomlanus). J Zoo Wildl Med 32:206–208
Dickinson VM, Jarchow JL, Trueblood MH (2002) Hematology and plasma biochemistry reference range values for free-ranging desert tortoises in Arizona. J Wildl Dis Assoc 38:143–153
Dobado-Berrios PM, Ferrer M (1997) Age-related changes of plasma alkaline phosphatase and inorganic phosphorus, and late ossification of the cranial roof in the Spanish imperial eagle (Aquila adalberti C. L. Brehm, 1861). Physiol Zool 70:421–427
Dobado-Berrios PM, Tella JL, Ceballos O, Donázar JA (1998) Effects of age and captivity on plasma chemistry values of the Egyptian Vulture. Condor 100:719–725
Donazar JA, Ceballos O (1989) Growth rates of nestling Egyptian Vultures. Ardea 77:217–226
Dressen P, Wimsatt J, MB-J of AM (1999) The effects of isoflurane anesthesia on hematologic and plasma biochemical values of American kestrels (Falco sparverius). J Avian Med Surg 1:173–179
Driscoll DE (2010) Protocol for golden eagle occupancy, reproduction, and prey population assessment. American Eagle Research Institute, Apache Jct. Arizona, USA
Fair J, Whitaker S, Pearson B (2007) Sources of variation in haematocrit in birds. Ibis 149:535–552
Ferrer M, Dobado-Berrios P (1998) Factors affecting plasma chemistry values of the Spanish Imperial Eagle, Aquila adalberti. Comp Biochem Physiol 120:209–217
Ferrer M, García-Rodríguez T, Carrillo JC, Castroviejo J (1987) Hematocrit and blood chemistry values in captive raptors (Gyps fulvus, Buteo buteo, Milvus migrans, Aquila heliaca). Comp Biochem Physiol A Comp Physiol 87(4):1123–1127
Finnegan D (2014) ReferenceIntervals: Reference Intervals. R package. Version 1.1.1. Version 1.1.1
Forbes NA, Simpson GN (1997) A review of viruses affecting raptors. Veterinary Record 141:123–126
Friedrichs KR, Harr KE, Freeman KP et al (2012) American Society Veterinary Clinical Pathology reference interval guidelines: determination of de novo reference intervals in veterinary species and other related topics. Vet Clin Pathol 41:441–453
García-Rodríguez T et al (1987) Metabolic responses of Buteo buteo to long-term fasting and refeeding. Comp Biochem Physiol 87:381–386
Gee GF, Carpenter JW, Hensler GL (1981) Species differences in hematological values of captive cranes, geese, raptors, and quail. J Wildl Manage 45:463–483
Geffré A, Geffré G, Concordet D et al (2011) Reference Value Advisor: a new freeware set of macroinstructions to calculate reference intervals with Microsoft Excel. Vet Clin Pathol 40:107–112
Gessaman JA, Johnson JA, Hoffman SW (1986) Hematocrits and erythrocyte numbers for cooper’s and sharp-shinned hawks. Condor 88:95–96
Græsli AR, Fahlman Å, Evans AL et al (2014) Haematological and biochemical reference intervals for free-ranging brown bears (Ursus arctos) in Sweden. BioMed Cent Vet Res 10:1–9
Greig DJ, Gulland FMD, Rios CA, Hall AJ (2010) Hematology and serum chemistry in stranded and wild-caught harbor seals in central California: reference intervals, predictors of survival, and parameters affecting blood variables. J Wildl Dis 46:1172–1184
Guder WG, da Fonseca-Wollheim F, Heil W et al (2010) Quality of diagnostic samples: recommendations of the working group preanalytical quality of the German society for clinical chemistry and laboratory medicine. German WHO/DIL/LAB, Bonn, Germany
Hanauska-Brown UA, Dufty AM, Roloff G (2003) Blood chemistry, cytology and body condition in adult northern goshawks (Accipiter gentilis). J Raptor Res 37:299–306
Harrison G, Harrison L (1986) Clinical avian medicine and surgery. W.B. Saunders, London, UK
Harvey J (2012) Hematology Procedures. In Veterinary Hematology (pp. 11–32). Elsevier Inc, St. Louis, Missouri, USA
Hayhow DB, Benn S, Stevenson A et al (2017) Status of golden eagle (Aquila chrysaetos) in Britain in 2015. Bird Study 64:281–294
Heidenreich M (1997) Clinical examination. Birds of prey: medicine and management. Blackwell Science, Oxford, UK, pp 70–83
Hernández M, Margalida A (2010) Hematology and blood chemistry reference values and age-related changes in wild bearded vultures (Gypaetus barbatus). J Wildl Dis 46:390–400
Hollamby S, Afema-Azikuru J, Sikarskie JG et al (2004) Clinical pathology and morphometrics of African fish eagles in Uganda. J Wildl Dis 40:523–532
Hõrak P, Ots I, Murumägi A (1998) Haematological health state indices of reproducing great tits: a response to brood size manipulation. Funct Ecol 12:750–756
Joseph V (2006) Raptor medicine: an approach to wild, falconry, and educational birds of prey. Vet Clin North Am Exot Anim Pract 9:321–345
Knuth ST, Chaplin SB (1994) The effect of exercise on plasma activities of lactate dehydrogenase and creatine kinase in red- tailed hawks (Buteo jamaicensis). J Raptor Res 28:27–33
Limiñana R, López-Olvera JR, Gallardo M et al (2009) Blood chemistry and hematologic values in free-living nestlings of Montagu’s harriers (Circus pygargus) in a natural habitat. J Zoo Wildl Med 40:687–695
Lockie JD, Ratcliffe DA (1964) Insecticides and Scottish golden eagles. Br Birds 57:89–101
Lockie JD, Ratcliffe DA, Balharry R (1969) Breeding success and organo-chlorine residues in golden eagles in west Scotland. J Appl Ecol 6:381–389
Love JA, Ball ME (1979) White-tailed sea eagle (Haliaeetus albicilla) reintroduction to the Isle of Rhum, Scotland, 1975–1977. Biol Conserv 16:23–30
Lumeij JT, Remple JD (2007) Plasma urea, creatinine and uric acid concentrations in relation to feeding in peregrine falcons (Falco peregrinus). Avian Pathol 20:79–83
Mathieu-Denoncourt J, Wallace SJ, de Solla SR, Langlois VS (2015) Plasticizer endocrine disruption: highlighting developmental and reproductive effects in mammals and non-mammalian aquatic species. Gen Comp Endocrinol 219:74–88
Maxwell MH (1993) Avian blood leucocyte responses to stress. Worlds Poult Sci J 49:34–43
Maxwell MH, Robertson GW, Spence S, McCorquodale CC (1990) Comparison of haematological values in restricted-and ad libitum-fed domestic fowls: white blood cells and thrombocytes. Br Poult Sci 31:399–405
Mello RH, Martins RF, Bonissi DA et al (2016) Haematological reference for red-browed parrot (Amazona rhodocorytha, Salvadori, 1890) captive in the Atlantic Forest in Eastern Brazil. Arq Bras Med Vet Zootec, v 68:1275–1282
Meredith A, Surguine K, Handel I et al (2012) Hematologic and biochemical reference intervals for wild osprey nestlings (Pandion haliaetus). J Zoo Wildl Med 43:459–465
Meredith AL (2016) Chapter 4. Wildlife triage and decision making. In: Mullineaux E, Keeble EJ, Cooper JE (eds) British Small Animal Veterinary A manual of wildlife casualties, 2nd Ed. British Small Animal Veterinary Association, pp 27–36
Minias P (2015) The use of haemoglobin concentrations to assess physiological condition in birds: a review. Conserv Physiol 3:1–7
Molenaar FM, Jaffe JE, Carter I et al (2017) Poisoning of reintroduced red kites (Milvus Milvus) in England. Eur J Wildl Res 63:1–8
Morandini V, Ferrer M, Perry L, Bechard (2018) Blood chemistry values in nestlings of rockhopper penguins (Eudyptes chrysocome): the effect of sex and body condition. Polar Biol 41(12):4533–4541
Moreno J, Merino S, Martínez J et al (2016) Heterophil/lymphocyte ratios and heat-shock protein levels are related to growth in nestling birds. Ecosience 9:434–439
Muñoz A, Riber C, Trigo P, Castejón F (2012) Age- and gender-related variations in hematology, clinical biochemistry, and hormones in Spanish fillies and colts. Res Vet Sci 93:943–949
Murphy WG (2014) The sex difference in haemoglobin levels in adults — mechanisms, causes, and consequences. Blood Rev 28:41–47
Nazifi S, Nabinejad A, Sepehrimanesh M et al (2008) Haematology and serum biochemistry of golden eagle (Aquila chrysaetos) in Iran. Comp Clin Path 17:197–201
Newton I, Galbraith E (1991) Organochlorines and mercury in the eggs of golden eagles (Aquila chrysaetos) from Scotland. Ibis (Lond 1859) 133:115–120
Ots I, MurumÄgi A, HÕrak P (1998) Haematological health state indices of reproducing Great Tits: methodology and sources of natural variation. Funct Ecol 12:700–707
Park F (2003) Behavior and behavioral problems of Australian raptors in captivity. Semin Avian Exot Pet Med 12:232–241
Peinado VI, Polo FJ, Viscor G, Palomeque J (2007) Haematology and blood chemistry values for several flamingo species. Avian Pathol 21:55–64
Perry MC, Obrecht HH III, Williams BK (1986) Blood chemistry and hematocrit of captive and wild canvasbacks. J Wildl Manage 50:435–441
Pollock CJW (2005) Hematologic and serum biochemical values of selected raptors. In: Exotic Animal Formulary, 3rd edn. Elsevier Saunders, St. Louis, Missouri, USA
Polo FJ, Celdrán JF, Peinado VI et al (1992) Hematological values for four species of birds of prey. Source: The Condor 94:1007–1013
R Core Team (2018) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. https://www.R-project.org
Redig PT, Ackermann J (2000) Raptors. In: Tully TNJ, Lawton MPC, Dorrestein GM (eds) Avian medicine, 1st edn. Elsevier Health Sciences, London, UK, p 448
Ricklefs RE (1984) The optimization of growth rate in altricial birds. Ecology 65:1602–1616
Riley H (2012) Raptors-naturally Scottish. Publications, Scottish Natural Heritage, Regorton, Perth, UK
Royal Society for the Protection of Birds (2011) Birds of prey in the UK on a wing and a prayer, 1st edn. Royal Society for the Protectio of Birds, London, UK
RStudio Team (2016) RStudio: integrated development environment for R. Boston, MA
Saino N, Cuervo JJ, Ninni P et al (1997) Haematocrit correlates with tail ornament size in three populations of the barn swallow (Hirundo rustica). Funct Ecol 11:604–610
Scottish Natural Heritage S (1998) Annual report 1997–1998. Battleby, Scotland UK
Sonne C, Bustnes JO, Herzke D et al (2012) Blood plasma clinical-chemical parameters as biomarker endpoints for organohalogen contaminant exposure in Norwegian raptor nestlings. Ecotoxicol Environ Saf 80:76–83
Sonne C, Bustnes JO, Herzke D et al (2010) Relationships between organohalogen contaminants and blood plasma clinical-chemical parameters in chicks of three raptor species from Northern Norway. Ecotoxicol Environ Saf 73:7–17
Spagnolo V, Crippa V, Marzia A et al (2008) Hematologic, biochemical, and protein electrophoretic values in captive tawny owls (Strix aluco). Vet Clin Pathol 37:225–228
Stein RW, Yamamoto JT, Michael D, Wilson BW (1998) Comparative hematology and plasma biochemistry of red-tailed hawks and American kestrels wintering in California. J Raptor Res 32:163–169
Stoffel MA, Acevedo-Whitehouse K, Morales-Durán N et al (2020) Early sexual dimorphism in the developing gut microbiome of northern elephant seals. Mol Ecol 11:2109–2122
Taylor M (2016) Royal Society for the Protection of Birds: British birds of prey. Bloomsbury Publishing Plc, London, UK
Teare JA (2013) International Species Information System reference ranges for physiological values in captive wildlife. International Species Information System, Apple Valley, MN
Thoresen SI, Havre GN, Morberg H, Mowinckel P (1992) Effects of storage time on chemistry results from canine whole blood, heparinized whole blood, serum and heparinized plasma. Vet Clin Pathol 21:88–94
Townsend AK, Wheeler SS, Freund D, Sehgal RNM, Boyce WM (2018) Links between blood parasites, blood chemistry, and the survival of nestling American crows. Ecol Evol 8:8779–8790
Van_Balveren JA, Huijskens MJAJ, Gemen EFA et al (2017) Effects of time and temperature on 48 routine chemistry, haematology and coagulation analytes in whole blood samples. Ann Clin Biochem 54:448–462
Viñuela J, Ferrer M, Recio F (1991) Age-related variations in plasma levels of alkaline phosphatase, calcium and inorganic phosphorus in chicks of two species of raptors. Comp Biochem Physiol - Part A Physiol 99:49–54
Watson J (2010) The golden eagle, 2nd edn. Bloomsbury Publishing, London, UK
Acknowledgements
The authors are very grateful to Paola Cazzini, Jennifer Harris, Claire Taylor, Jenny Weston, Margaret Pearson, and Heather Brain, for logistical support before and during fieldwork, to Pius Boso for microbiological technical support, and to Ian Handel and Simon Dures for technical support and data analysis advice.
Funding
Gabriela Peniche was funded by a Natural Environment Research Council (NERC) CASE studentship in partnership with NatureScot, previously Scottish Natural Heritage (SNH), and a European Wildlife Disease Association (EWDA) grant supported this research.
Author information
Authors and Affiliations
Contributions
Study conception and design were done by Gabriela Peniche and Anna L Meredith. Material preparation was done by Gabriela Peniche. Data collection was facilitated by JC Brain, R Reid, E Weston, S Benn, J Grant, and L Pate and performed by Gabriela Peniche. Data analysis and reference interval calculations were done by Gabriela Peniche and Darren J Shaw. The first draft of the manuscript was written by Gabriela Peniche and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
All procedures took place under the necessary Natural England/British Trust for Ornithology licence authority, Home Office licence authority (Project licence PB8A1D5C7), and ethical approval from the University of Edinburgh.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
G., P., D.J., S., D.B.A., T. et al. Establishing haematological and biochemical reference intervals for free-ranging Scottish golden eagle nestlings (Aquila chrysaetos). Eur J Wildl Res 68, 43 (2022). https://doi.org/10.1007/s10344-022-01586-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10344-022-01586-7