Abstract
Some deer species are of conservation concern; others are officially managed as a food source or for their trophies, whereas in many regions, deer are regarded as overabundant or even as a nuisance causing damages. Regardless of local management issues, in most cases, reliable data on deer population sizes and sex ratios are lacking. Non-invasive genetic approaches are promising tools for the estimation of population size and structure. We developed and tested a non-invasive genetic approach for red deer (Cervus elaphus) population size and density estimation based on faeces collected from three free-ranging red deer populations in south-western Germany. Altogether, we genotyped 2762 faecal samples, representing 1431 different individuals. We estimated population density for both sexes separately using two different approaches: spatially explicit capture-recapture (SECR) approach and a single-session urn model (CAPWIRE). The estimated densities of both approaches were similar for all three study areas, ranging between total densities of 3.3 (2.5–4.4) and 8.5 (6.4–11.3) red deer/km2. The estimated sex ratios differed significantly between the studied populations (ranging between 1:1.1 and 1:1.7), resulting in considerable consequences for management. In further research, the issues of population closure and approximation of the effectively sampled area for density estimation should be addressed. The presented approach can serve as a valuable tool for the management of deer populations, and to our knowledge, it represents the only sex-specific approach for estimation of red deer population size and density.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Assessing ungulate population size and density is of central importance for monitoring programmes and management decisions (Smart et al. 2004). Because many ungulate species show elusive behaviour and occur in habitats with dense vegetation, this is a challenging task for both rare (Barrio 2007; De Oliveira et al. 2019) and overabundant species, which can cause damages to silviculture (Putman and Moore 1998; Gordon et al. 2004; Allombert et al. 2005). Especially, management of red deer (Cervus elaphus) is of increasing interest because populations are spreading and increasing in many parts of Europe (Ward 2005; Milner et al. 2006; Mysterud et al. 2007; Acevedo et al. 2008). Although of high value for recreational, trophy or meat hunting, foresters complain of economic losses due to bark stripping or browsing effects of this large herbivore. However, red deer populations are particularly difficult to survey in forested areas because direct counts are difficult or yield only indices (Garel et al. 2010) and indirect methods like, e.g. pellet counts yield imprecise results (reviewed in Smart et al. 2004; Grignolio et al. 2020).
Non-invasive genetic approaches represent a powerful tool for population size and density estimation of animals that are elusive and therefore difficult to survey (Woods et al. 1999; Beja-Pereira et al. 2009; Ferreira et al. 2018; Skrbinšek et al. 2019). DNA contained in hair or faecal samples can be used for identification of individual animals, and these data can be used for population size estimation, e.g. implemented in a capture-recapture (CR) framework. Capture-recapture can yield accurate and precise population estimates (Otis et al. 1978; Seber 1982; Pollock et al. 1990), and non-invasive genetic CR offers the advantage that animals do not have to be captured physically, but are detected through their genotypes. This can reduce some sources of bias that are problematic in traditional CR, particularly those related to the behaviour of animals towards being captured like, e.g. ‘trap-shyness’ (McKelvey and Schwartz 2004; Petit and Valière 2006). However, several issues remain that can compromise non-invasive population size estimates and thus have to be carefully taken into account when conducting a study (Boulanger et al. 2004; Lukacs & Burnham 2005b; Ebert et al. 2010; Marucco et al. 2011). Detection probabilities may be heterogeneous amongst individuals or vary over time, and violation of the assumption of population closure can impede the definition of the effectively sampled area (ESA) relevant for the assessment of population density (Boulanger and McLellan 2001; Boulanger et al. 2004). Markers used to discriminate individuals have to be sufficiently informative; otherwise, a genotype may not represent a unique ‘mark’ (Pompanon et al. 2005). Genotyping errors such as allelic dropout or false alleles can lead to misidentification of individuals and thus to overestimation of population sizes (Prugh et al. 2005; Waits and Paetkau 2005). Therefore, care must be taken to reduce genotyping errors, e.g. by repeating analyses several times and by using effective error-checking protocols (Broquet and Petit 2004; McKelvey and Schwartz 2004).
Non-invasive population size estimation approaches have to date been applied for several ungulate species, amongst them different Odocoileus species (e.g. Brinkman et al. (2011) for Sitka black-tailed deer Odocoileus hemionus sitkensis in Alaska; Goode et al. (2014) and Brazeal et al. (2017) for mule deer O. hemionus and O. h. columbianus), argali sheep Ovis ammon (Harris et al. 2010), Sonoran pronghorn Antilocapra americana sonoriensis (Woodruff et al. 2016), roe deer Capreolus capreolus (Ebert et al. 2012) and mountain goats Oreamnos americanus (Poole et al. 2011). For red deer, a first evaluation of the technical background for non-invasive population monitoring was carried out in a pilot study (Valière et al. 2007). However, to our knowledge, the study presented here is the first successful attempt of applying this method on free-ranging red deer populations. We carried out non-invasive sampling of red deer populations in three different study areas in south-western Germany. All three areas are situated within large forest districts that are of high importance for silviculture and harbour large managed red deer populations. Browsing and bark stripping damages have been an ongoing issue, resulting in the need for an effective monitoring of the local red deer populations. As in many other areas in Germany, deer management goals are diverse, including regulating population size for damage control as well as meat and/or trophy hunting. Our aim was to investigate the applicability of the non-invasive genetic approach to monitor red deer, particularly with respect to sample size, genotyping reliability and potential sources of bias, but also to its costs.
Material and methods
Study areas and field sampling
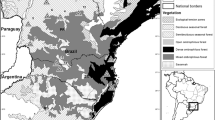
We conducted our study in three mainly forested areas, the Hunsrück (HU, sampling in March 2012), Soonwald (SO, sampling in March 2015) and Pfälzerwald (PF, sampling in March 2016; Fig. 1), situated in the federal state of Rhineland-Palatinate in south-western Germany. Each study area approximates 10,000–11,500 ha in size (Table 1) and is located within a larger forested area continuously populated by red deer. Elevation within the areas ranges from approx. 200 to 700 m above sea level. Forest cover is lowest in the SO area (83%) and higher in the PF (93%) and HU (95%) areas, with Pinus sp., Fagus sylvatica, Picea abies and Quercus petraea as well as Quercus robur as dominant tree species. Annual average temperature is 8–9 °C; annual precipitation is approximately 600–1000 mm. Three ungulate species occur in the study areas: red deer, roe deer (C. capreolus) and wild boar (Sus scrofa). Red deer are hunted during drive hunts, involving approx. 30–50 shooters and beaters and often dogs, between mid-October and the end of January. Single hunt, as a sit-and-wait-strategy of a single hunter using a high seat, is carried out between May 1 and January 31; hunting season differs between sex and age classes. Due to official but unpublished harvest records (every successful hunt has to be reported to the local hunting services), the average yearly hunting bag sums up to 1.8 harvested red deer per km2 and year in the HU area, 3.1 per km2 and year in the SO area and 1.1 per km2 and year in the PF area (mean over 5–9 years preceding the respective faeces collection; for details, see Fig. 2). Hunting quotas are based on damage levels. In case of unacceptable damage levels (acceptable levels, e.g. winter browsing rate for terminal shoots in regenerated tree species < 15–20% or last-year bark stripping rate below 1%, are fixed by Rhineland-Palatinate hunting regulations, § 31 game law of the federal state), quotas are increased.
Locations and transect designs of the three study areas in which red deer faeces were collected for non-invasive genetic population density estimation (HU Hunsrück area, SO Soonwald area, PF Pfälzerwald area). Transects are given as thin black lines, bold outlines indicate buffered study area boundaries, and black dots indicate locations of collected red deer faeces
Red deer faeces are easily distinguishable from wild boar faeces due to their differences in form, colour and texture. Roe deer faeces are similar to those of red deer in form and texture, but normally differ considerably in size and length/diameter ratio and thus can be distinguished in most cases (Mayle et al. 1999; Daniels 2006). However, a roe deer sample erroneously collected would be detected after genotyping because the microsatellites we used for red deer perform differently for roe deer: BM203, CSSM16 and BMC1009 show distinct alleles, whereas Haut14, IDVGA55, CSSM19 and TGLA53 do not amplify. AMELXY shows a different Y allele for male roe deer (236 bp) compared to red deer (228 bp).
We collected faeces during March after snowmelt, but before ground vegetation starts growing and with still rather low ambient temperatures in order to ensure adequate conditions concerning faecal detection and DNA quality (Lucchini et al. 2002; Maudet et al. 2004; Rea et al. 2016; Woodruff et al. 2015). In each area, transects were distributed as evenly as possible to represent different habitat types, microhabitats and altitudinal levels. Transects were spaced between 120 and 250 m from each other (Fig. 1). In the three areas, between 60 and 80 transects with lengths between 4.5 and 10 km were searched for red deer faeces, representing a total transect length of 2022 km (Table 1). The transect width, which a walking person could effectively search for faeces, was approximately 1.5 to 3 m depending on ground vegetation and slope of the transect.
The locations of all detected red deer faeces were recorded using handheld GPS devices. All field workers were trained to distinguish faeces between the different species and to evaluate the freshness of faeces. In order to increase the genotyping success rate, only fresh faeces (i.e. intact pellets with moist, shiny surfaces) were collected for analysis. To minimize cross-contamination, we sampled only faeces that were spatially segregated from other red deer faeces and thus were assumed to originate from one defecation event, and we avoided collecting samples from faeces with scattered pellets. One handful of pellets (approx. 5–15) of each fresh pellet group was collected using an inverted freezer bag, which was then reversed and closed. Samples were stored frozen (− 19 °C) in the sealed freezer bags until analysis.
In order to ensure a sufficiently large sample size, we collected all fresh faeces detected along the transects. As a first approximation of the desired sample size, we determined the number of samples for analysis as 2.5–3 times the ‘assumed’ number of red deer in each population (as recommended in Solberg et al. 2006). We based a rough estimate of the ‘assumed’ number of red deer based on the mean hunting bag data for each study area (for PF, see Lang et al. (2016) and Hohmann et al. (2018); for HU and SO, see Gräber et al. (2016) and Hohmann & Hettich (2018)). Therefore, we assumed that the average harvest rate per year is lower than 50% in our study areas (Clutton-Brock et al. 2002), and thus, the assumed population density in spring will be at least twice the harvest rate. We considered 2.5 to 3 times the harvest rate as a sufficient rough estimate of assumed number of deer. For each area, we aimed to analyse a number of fresh samples corresponding as a minimum to (mean of the harvest rate of the 3 years preceding the sampling × 2.5) × 2.5, e.g. for the HU area (1.9 × 2.5) × 2.5 = 11.87, i.e. a minimum of 1187 samples for the study area. As an additional measure of sampling intensity, we furthermore aimed at reaching a mean number of detections per individual of at least two (Miller et al. 2005). Compared to the assumed number of individuals, this measure does not rely on preliminary assumptions about population size. However, we were restricted in all three areas to a maximum number of samples due to budget reasons. From the total number of samples collected in each study area, we selected a subsample according to the preliminary sample size considerations (see above) for analysis. To achieve a representative spatial coverage of the subsample, we calculated the percentage of all samples found for each transect. We then drew the number of samples corresponding to this percentage randomly from the samples found on the respective transect. With this approach, we ensured that the spatial distribution of analysed samples reflects the spatial distribution of the detected samples.
In addition to faecal sampling, we collected tissue samples from all harvested red deer older than 1 year (and thus possibly present in the area during faecal sampling) in the hunting season after our faecal sampling in the PF study area. We obtained these samples in cooperation with the local forestry offices and hunters. Our aim was to check the coverage of the faecal sampling by comparing how many of the harvested individuals had been recorded before.
Genetic methods
We analysed faecal samples 2 to 4 months after collection. For isolation of DNA, we used a commercial kit (NucleoSpin Soil Kit, Macherey-Nagel, Baesweiler, Germany). To achieve a high proportion of target DNA, the standard protocol was modified in that two pellets of each sample were washed with 1.5 ml lysis buffer at room temperature in a 50 ml Falcon tube, thereby avoiding the destruction of the pellets and including mainly the mucosal layer on the pellet surface containing most of the intestinal cells. We isolated DNA from tissue samples of the harvested deer using the QiaAmp DNA Mini Kit (Qiagen, Hilden, Germany).
We used seven dinucleotide microsatellite markers for individual identification (Table 2). We initially tested 16 microsatellites that had already been used for amplification of red deer DNA and selected loci based on their polymorphism and their ability to produce consistent results with non-invasively obtained samples (data not shown; references for the used loci in Table 2). In tests with tissue samples, this set of loci ensured a sufficiently high discriminative power (i.e. probability of identity (PID) sib consistently well below 0.01; Waits et al. 2001). We furthermore used an x- and y-chromosome-specific region of the amelogenin gene as a sex marker according to Gurgul et al. (2010). The eight markers were combined and co-amplified in two separate multiplex PCRs (Table S1 in Supplemental Material). The thermocycling profile was as follows: 95 °C for 15 min, 45 cycles of 94 °C for 30 s, 57 °C for 90 s, 72 °C for 60 s and then 60 °C for 30 min. Amplification reactions were performed in triplicates in a total volume of 12 μl using the Qiagen Multiplex PCR Kit (Qiagen, Hilden, Germany). The primers were used at concentrations of 0.1 to 0.4 μM (for details of the PCR conditions, see Table S1). We separated fluorescently labelled DNA fragments on an ABI3730 DNA analyser and determined allele size using the ABI GS500LIZ size ladder (Applied Biosystems, Darmstadt, Germany). We included two negative PCR controls in every PCR set to detect potential contamination.
We deduced consensus genotypes from the triplicate results. Samples were typed as heterozygous at one locus if both alleles appeared at least twice and as homozygous when all replicates showed the same result. We repeated the genotyping another three times when results were ambiguous after the first three replicates. All samples that failed to amplify or to produce unambiguous results for more than two loci were discarded. For samples with one or two missing loci, we carefully re-checked raw data for plausibility in case of matching genotypes and excluded any samples matching with more than one genotype (Wilberg & Dreher 2004). We also excluded samples showing signs of cross-contamination (i.e. genotypes with more than two alleles) from further analyses.
For population size estimation, not only the genotyping error rates are relevant but also how often whole genotypes are incorrectly identified in spite of repeated analyses and error checking. Thus, we additionally estimated a misidentification rate (i.e. false identification of a whole sample due to genotyping errors or potential misinterpretation of data in the lab) by submitting 98 red deer faecal samples to the lab in duplicate without disclosing which samples were duplicates (i.e. a blind test). Duplicate samples that did not match their corresponding original sample (i.e. did not exhibit the same genotype) were defined as misidentifications. In order to increase our blind test sample size with samples of known individuals, we furthermore used faeces from 13 captive red deer. These samples were collected immediately after defecation under direct observation, so that we could record the defecating individual and its sex and age class for each sample. Each of the samples was divided arbitrarily into 2 to 6 aliquots yielding a total of 40 aliquots, which were numbered randomly (such that the lab personnel had no information how many different individuals had been sampled and which aliquots originated from which individual) and then sent to the lab for analysis. Genotyping in this case should result in correct identification of the number of different individuals and reveal which aliquot originated from which individual, if no misidentifications occurred. We genotyped all blind test samples using the same procedure as for other faecal samples, i.e. three PCR repeats per sample.
For all analysed samples, DNA extraction and PCR setup were carried out in separate rooms on different floors of the laboratory to avoid transfer of amplified DNA into the pre-amplification steps (Waits & Paetkau 2005).
Data analysis
Determination of matching genotypes was carried out using GENECAP (Wilberg and Dreher 2004) based on the PIDsib method with a match probability of 0.05. In order to confirm the power of the loci, we calculated the probability of identity (PID) and, being more conservative, PID for siblings (Waits et al. 2001) as well as heterozygosity using GIMLET (Valière 2002). We calculated genotyping error rates (allelic dropout [ADO] and false alleles [FA]) as recommended by Broquet & Petit (2004; Eqs. 1 and 3).
Estimation of population size and density
We calculated density estimates based on spatially explicit capture-recapture (SECR) modelling (Efford et al. 2004; Borchers & Efford 2008; Royle & Young 2008; Royle et al. 2014). This approach uses spatial information (i.e. where an animal was detected during a capture/sampling session) to estimate animal home range centres and spatially referenced capture probability (Borchers & Efford 2008). By fitting a spatial model of the population and the detection process to the spatial detection histories, SECR allows estimating population density directly from the sampling data and unbiased by edge effects and incomplete detection. We ran spatially explicit models for total population density as well as for the two sexes separately using the R package secr (Efford 2017a). Because we covered all study areas with a rather dense transect grid representing an area search, we used polygon as a detector type (Efford 2011; Efford 2017b). We used a hazard halfnormal detection function and defined the buffer for each data set as four times its initial sigma. For each data set, we ran a model assuming homogeneous sampling (null model M0, g0 ~ 1) and a model accounting for sampling heterogeneity (model Mh, g0 ~ h2). The Mh model assumes two different groups in the population that differ in their detection probability and estimates the probability to belong to each of these groups (pmix). We compared the fit of the two models via the Akaike information criterion with an additional bias correction term (AICc, Burnham & Anderson 2002).
As an additional approach, we estimated population size using the R package CAPWIRE (Miller et al. 2005; Pennel et al. 2013). CAPWIRE is a maximum likelihood–based approach designed for genetic data, enabling population size estimation with data from single sampling sessions. This is possible because in contrast to other approaches, CAPWIRE uses counts of detections and it is based on urn models that allow multiple captures per session (Miller et al. 2005). CAPWIRE enables the estimation of population size in the presence of sampling heterogeneity with the two-innate rates model (TIRM) and even performs particularly well when detection rates are heterogeneous (Miller et al. 2005). For the three study areas, we estimated population size for males and females separately. Thereby, we sought to minimize total sampling heterogeneity, since sex-specific differences, e.g. in space or habitat use or behaviour, can be a significant source of heterogeneity bias, particularly in a sexually dimorphic species like red deer (Clutton-Brock & Lonergan 1994; Harris et al. 2010). However, there remain multiple sources for sex-independent individual heterogeneity. A likelihood ratio test implemented in CAPWIRE allows selection between the equal catchability model (ECM, comparable to M0 in our SECR estimates) and the TIRM (comparable to Mh in our SECR estimates). We additionally tested for presence of sampling heterogeneity by simulating the sampling process under the assumption of homogeneity and comparing the expected number of captures with the observed number of captures per individual using the R package CMRPopHet (Puechmaille & Petit 2007).
Since in addition to population size, estimates of population density are of crucial interest for managers and for purpose of comparison, we also estimated population density based on the CAPWIRE estimates. However, density estimation is not straightforward when study areas are not geographically closed. In our study areas, the assumption of geographic closure is not met (however, we consider the populations as demographically closed since we conducted our sampling well out of the farrowing and the hunting season and the genotyped fresh faeces were not older than approx. 2 weeks). For density estimation, we approached the effectively sampled area (ESA) by adding the mean maximum recapture distance (MMRD) as a buffer around each study area. We calculated MMRD for each sampled individual using the R package secr (Efford 2017a). In a study by Parmenter et al. (2003) comparing different approaches, the MMRD showed the best performance for estimating the ESA. We calculated sex-specific ESAs for each study area separately by buffering the forested parts of the study area with the respective MMRDs and clipping the buffers where, e.g. motorways or settlements represent human-made borders (see, e.g. Fig. 1). We used these sex-specific ESAs to calculate population densities from the CAPWIRE population size estimates.
Results
Field sampling and genotyping
Of all the collected samples (Table 1), we included 1218, 1700 and 1000 for DNA analysis from study area HU, SO and PF, respectively, obtaining genotyping success rates of 67.6% (HU), 72.6% (SO) and 70.1% (PF, Table 3). Altogether, 2762 (70%) of the 3918 analysed samples yielded consensus genotypes useable for further analyses. Of the 1156 samples that did not yield useable red deer consensus genotypes, 83 (7.1%) were excluded because they had been identified by their genotypes as originating from roe deer. For all 2762 samples with consensus genotypes, eight loci amplified for 2536 samples (92%), seven loci for 146 samples (5.3%) and six loci for 80 samples (2.9%).
For the successfully analysed red deer samples, PID over all loci was estimated to be 3.184 × 10−11, and PIDsibs was 3.339 × 10−04. Mean expected heterozygosity (H exp) was 0.81 and mean observed heterozygosity (H obs) was 0.77 (Table 2). The mean ADO rate estimated over all markers was 4%, whereas the FA rate averaged 1% (Table 2).
Over all three study areas, we detected 1431 red deer individuals, of which 568 (40%) were classified as male and 833 (58%) as female, while for 30 individuals (2%), no sex could be assigned because the sex marker failed to amplify. When comparing the sex ratios (male to female) of the sampled deer between the three study areas, there were marked differences: In the HU area, the sex ratio was nearly even (1:1.1), while the red deer sampled in the SO area exhibited a considerably female-biased sex ratio (1:1.8). The red deer sampled in the PF area lay between these two extremes (1:1.3). The mean number of detections varied between the three study areas: In PF, males were sampled on average 2.04 times and females were slightly stronger represented with an average of 2.28 times (Table 4). In SO and HU, the two sexes showed almost equal mean detection numbers: SO averages: males 2.08 and females 2.07 and HU averages: males 1.64 and females 1.63. However, when examining the absolute numbers of sampled individuals (Table 3, Figs. S1, S2, and S3 in Supplemental Material), in SO and HU, significantly more female than male individuals were detected.
The total MMRD differed between the three study areas and within the study areas between the two sexes (Table 3). In all three areas, male red deer exhibited larger MMRDs compared to females, resulting in larger ESAs. The difference between the sexes was largest in the PF and smallest in the SO (Table 3).
Of the 51 tissue samples that were genotyped from deer > 1 year old harvested in the PF area, 31 (61%) matched a genotype present in the faecal sample.
Blind test
In the first blind test part, consensus genotypes were obtained for both original sample and duplicate in 63 of the 98 sample pairs. Of these, 62 pairs were found to match, and one pair showed differences in two loci as well in the sex marker and was thus considered as a mismatch. In the second part based on samples from captive deer, 34 of 40 subsamples yielded usable consensus genotypes. All 34 subsamples were assigned correctly to the right individual; thus, in this part, no misidentifications occurred. We then combined the samples of the two parts. Thus altogether, the blind test consisted of 97 samples, of which one was not assigned correctly, representing a total misidentification rate of 1.03%.
Estimation of population size and density
Miller et al. (2005) recommended an average of 2–2.5 observations per individual (corresponding to samples/individual in our study) to achieve estimates within 10–15% of the true population size. In the HU study, the sample size was below this threshold (mean number of samples/ind. 1.63). However, in the SO and PF studies, the sample sizes were within the recommended range (mean number of samples/ind. 2.07 and 2.16, respectively; Table 4).
For the SECR density estimates, the Mh model clearly fit the data better for all three study areas and for both sexes, indicating the presence of sampling heterogeneity (Table 4; see Table S2 in Supplemental Material for the results of both model M0 and Mh as well as the AICc results).
We compared the performance of the models ECM and TIRM by using the CAPWIRE-inherent likelihood ratio test. The results show that the TIRM better fits the data for both sexes in all three areas (Supplemental Material Table S4). Therefore, the CAPWIRE TIRM estimates were used for all data sets (Table 4).
The estimated population sizes differ strongly between males and females for the SO and PF areas, but are quite similar for the two sexes in the HU area (Table 4). The sex ratios of the estimated populations are 1:1.0 for HU, 1:1.7 for SO and 1:1.5 for PF. These ratios are very similar to those obtained when comparing the genotyped individuals instead of the estimated population sizes (HU: 1:1.1, SO: 1:1.8, PF: 1:1.3); only in the PF area, the difference is slightly more pronounced, probably an effect of differences in detection probability between the sexes (as reflected in the average numbers of detections, Table 4). In all three areas, sampling heterogeneity was detected for both sexes in the test following Puechmaille and Petit (2007), corroborating the result of the SECR goodness-of-fit tests and CAPWIRE likelihood ratio tests.
The population densities based on the CAPWIRE and the SECR estimates yield similar results for the HU and PF study areas and both sexes (Fig. 3, Table 4). When adding the CAPWIRE estimates for the two sexes to a total population density, the estimates also correspond well to the SECR total population densities; however, in the SO area, the total red deer density estimated with SECR is lower compared to the CAPWIRE estimate. Furthermore, the sex-specific CAPWIRE estimates exceed the SECR estimates for the SO area (Fig. 3, Table 4).
Red deer density estimates in three study areas (HU Hunrsück, SO Soonwald, PF Pfälzerwald) based on two different approaches: TIRM denotes estimates based on the CAPWIRE two-innate rates model (for details, see text); SECR represents densities estimated using a spatially explicit capture-recapture approach. The hatched bars behind the TIRM estimates represent the sum of the male and female density
Discussion
Our findings suggest that non-invasive genetic estimation of population density based on faeces is a practicable and effective monitoring approach for red deer in forested areas. Other approaches applied in our three study areas based on harvest statistics, terrestrial spot light counts and aerial surveys reveal significantly lower estimates compared with the genetic approach (for PF, see Lang et al. (2016) and Hohmann et al. (2018); for HU and SO, see Gräber et al. (2016) and Hohmann & Hettich (2018)). Population estimates obtained with these conventional methods tend to underestimate populations and are thus often exceeded considerably by DNA-based estimates (Kendall et al. 2009; Harris et al. 2010).
However, to yield reliable results, non-invasive genetic estimates need to be based on a sufficient sample size and a representative sampling design. Concerning sample size, Solberg et al. (2006) and Puechmaille and Petit (2007) recommend analysing 2.5–3 times as many samples as the assumed population size or the minimum population size assessed, e.g. using direct counts. Since minimum population sizes or other comparative numbers are often not readily available, it may be more practicable to use the average number of observations per sampled individual as recommended by Miller et al. (2005). In their simulation study, Miller et al. (2005) showed that in populations of up to 100 animals, an average of 2.0 observations per individual generated CAPWIRE population estimates in a range of ± 15% from the true N. In our study, the average number of observations per individual ranged from 1.63 in the HU area to 2.28 for the female part of the PF population. For SO and PF, both sexes showed average numbers above 2.0 (Table 4), indicating that the populations were adequately represented in the sample to obtain reliable estimates. Without the budget restrictions in the HU area, we may have been able to increase the number of analysed samples to reach the recommended average number of observations per individual.
Concerning the sampling design, from our experience, we consider sampling along transects to be a practicable and representative solution in areas where the terrain allows following a randomly placed transect. In our first sampling in the HU area, we spaced the transects quite densely (approximately 120 m apart). With this design, we collected far more samples than we would have needed considering the recommendations of Solberg et al. (2006). Therefore, we used a less dense transect grid in the following study areas with transects spaced 200–250 m apart, having in mind that this distance still is small compared to red deer home ranges in our study areas (Hamann et al. 1997).
In our study, the overall genotyping error rate lies in the range of those reported in other studies (reviewed by Valière et al. (2006)). With PIDsibs being well below 0.01, we consider the set of seven loci as sufficient for discrimination between individuals (Waits et al. 2001). By conducting three to six PCR replicates per sample, we were able to keep the total misidentification rate below 5%, which can be considered as sufficient for the purpose of population estimation (Taberlet and Luikart 1999; Lukacs and Burnham 2005a). However, even for genotyping error rates in the range of 5%, population estimates can be considerably biased (Roon et al. 2005). In a simulation study, Valière et al. (2006) showed that with residual errors of 1–3%, population estimates with a small relative bias (below 10%) can be obtained. Due to the rigorous genotyping protocol, the misidentification rate (1.03%) in our study lies in this range, and thus, we expect the resulting bias to be minor.
Heterogeneity in capture/sampling probability is a problematic issue for population estimation in traditional and in non-invasive CR approaches and can result in negative bias of population size estimates (Otis et al. 1978; Boulanger et al. 2004; Ebert et al. 2010). Individual sampling heterogeneity is considered to be present in nearly all CR data sets (Pledger & Efford 1998). Different sources of heterogeneity can bias non-invasive genetic population density estimates, e.g. sex, age class, social status, individual differences in behaviour or space use (Boulanger et al. 2004; Bischof et al. 2020). Furthermore, differences in defecation rates can result in heterogeneous detection probabilities (Marucco et al. 2009; Lunt & Mhlanga 2011), an aspect that in our opinion should be addressed in future studies. In our study, we tried to minimize heterogeneity by treating the two sexes separately. However, all the implemented tests still detected considerable heterogeneity, indicating the presence of non-sex-related sources of bias in the data.
One potential source of sampling heterogeneity concerning the male part of the population in particular could be the fact that there is an age-related difference in male spatial behaviour: juvenile and subadult male red deer normally stay with the female groups and therefore share the same spatial behaviour (Clutton-Brock et al. 1982). In contrast, adult stags live solitary or in bachelor herds most of the year and therefore potentially show a different space use compared to the juvenile and subadult males. This aspect could introduce bias (Hickey & Sollmann 2018; Bischof et al. 2020) that would not be accounted for by sex-specific estimation of population density. Further research, e.g. based on telemetry data of red deer of both sexes and different age classes, is needed to clarify if such age-class-related behavioural differences could introduce bias in sex-specific population density estimates. However, we consider estimating population density for the two sexes separately to be an important step to reduce overall bias. Furthermore, novel estimation approaches for social species that can take into account group structure like those proposed by Hickey and Sollmann (2018) and Bischof et al. (2020) could help to mitigate bias introduced by social structure.
An approach to decrease individual- and sex-based heterogeneity for red deer population estimation and to increase the sample size could be to use not only one but multiple different sampling strategies and/or sample sources. This can result in an increase in overall detection probability and reduction of overall bias (Boulanger et al. 2008; DeBarba et al. 2010; Dreher et al. 2007). Dreher et al. (2007) sampled harvested bears in addition to hair sampling. Sampling of harvested individuals would be easily feasible in many deer populations. The fact that 61% of the harvested red deer sampled in our PF study area matched with genotypes found in the faeces sampling suggests that this approach is promising. Furthermore, it provides a rough evaluation of the coverage of our sampling, when comparing the proportion of known genotypes (i.e. represented before in the faecal sample) in the hunting bag to the proportion of known individuals in the estimated population: The number of individuals detected via faecal sampling in the PF area (327) corresponds to 67% of the estimated total PF population size (when adding the male and female CAPWIRE estimates: 487; see Table 4). This corresponds quite well to the proportion of matches in the harvest sample, corroborating the representation of the population in our faecal sampling.
Population closure is a major concern in DNA capture-recapture studies like in traditional CMR (Boulanger and McLellan 2001). Closure violation can result in positive bias of population estimates, when animals move in and out of the sampling grid. The alternative use of open-population CR models suited for estimation of population size is not applicable for our data, since open models cannot account for individual heterogeneity and their use requires larger sample sizes and longer study times compared to closed models (Pollock 1990; Boulanger and McLellan 2001; Luikart et al. 2010). However, population estimates can still be meaningful when closure is violated. In that case, they can be considered as superpopulation estimates, i.e. the sampled population extends beyond the sampling grid boundaries (Kendall 1999; Roon et al. 2005; Tsaparis et al. 2014). Closure violation however represents a considerable source of bias for the determination of population density. Nevertheless, population density, i.e. the relation to the area for which a given population estimate is valid, is needed for most management purposes (Wilson and Anderson 1985). ‘Naïve’ density estimates (i.e. when the area of sampling is used as ESA for density estimation without any correction or buffer) can be severely biased when the closure assumption is not met (Wilson and Anderson 1985; Harris et al. 2010; Dupont et al. 2019). Since our study areas were not geographically closed, we added the MMRD as a buffer to the study areas for density calculation as one approach to reduce bias. However, different approaches to define the ESA exist and the size of the buffer has a significant impact on the estimated density (Parmenter et al. 2003; Tioli et al. 2009). Furthermore, the suitability of a given approach may differ between species and study areas. Parmenter et al. (2003) stated that MMRD can be constrained by the size of the sampling grid and therefore suggested the use of telemetry data when available for calculating the ESA, which was not the case for our study areas. However, to obtain valid data for ESA calculation via telemetry, a large number of tracked animals would be necessary, making this difficult to implement in many studies.
Spatially explicit methods hold the potential to mitigate spatial sources of bias in density estimates and thus represent a superior alternative to methods in which density has to be inferred by approximating ESA (Obbard et al. 2010). Potential bias introduced due to disturbance by human field personnel however is not avoided by using SECR; thus, sampling with minimal disturbance remains important. In our study, we sampled each transect only once and tried to cover parts of the area en bloc to avoid repeated disturbance of the animals. Another solution to reduce disturbance might be to sample in larger time intervals, e.g. every 5 to 10 days instead of collecting samples on consecutive days (Brinkman et al. 2011; Dupont et al. 2019). Overall, we believe spatially explicit approaches should be the estimators of choice when the aim is estimation of population density. Nevertheless, the application of a second approach, as in our case CAPWIRE, in our opinion can be useful to gain more insight into the data and to allow comparison with a wider range of other studies.
In our study, the density estimates based on the CAPWIRE TIRM with the MMRD as a buffer correspond quite well with the SECR estimates (Fig. 3, Table 4). The fact that two statistically independent approaches yield similar results might indicate that the estimated red deer densities are not too far from reality. The densities estimated via SECR exhibit considerably larger confidence intervals in most cases; however, the SECR estimates already account for a certain spatial heterogeneity and thus can be considered as more robust compared to the CAPWIRE estimates. Furthermore, CAPWIRE has been originally designed and performs best with small populations (Miller et al. 2005); thus, we cannot completely rule out a reduced performance of the CAPWIRE estimator in our studies. However, several studies report good performance of the CAPWIRE estimator in larger populations, e.g. around 200 (Granjon et al. 2016) and 450 (Miller et al. 2005, taxicab populations). Our study indicates that for large populations of around 1000 or more individuals, CAPWIRE might exhibit reduced performance: We calculated total population sizes for the three areas using CAPWIRE, and even though the TIRM was recommended for all areas, the estimated TIRM population size was not included in the confidence interval for HU and SO (Table S7). This has not been the case when calculating population sizes for the two sexes separately in both areas and thus using smaller data sets.
Conclusion and management implications
The population densities in the HU and SO areas both exceed the densities estimated in the PF area considerably, whereas HU and SO exhibit rather similar densities, however with the SO area showing a considerably higher proportion of females in its population. For setting hunting quotas, usually the reproductive output for the next fawning season is taken into account. If hunting is supposed to reduce the population sustainably, the harvest rate must be above the reproductive output. Assessments of reproductive output are often based on total spring female population including females born the previous year (Bertouille & De Crombrugghe 2002; Bonenfant et al. 2003). Hence, based on our genetic data set, which excluded samples from newborns (in all three areas, we finished sampling at least a month before the beginning of the fawning season) for the SO population, a higher reproductive potential can be stated. This finding might be an explanation for the fact that – compared with the HU area – in the SO area, a considerably higher harvest rate has been kept up sustainably for years (see Fig. 2).
For a mammal with distinct morphological and ethological differences between sexes, non-invasive genetic approaches are able to deliver estimates of sex ratio in contrast to often highly biased estimates based on hunting bag analysis or visual observations (Hagen et al. 2018). As far as management of red deer is management of two sexes (Loe et al. 2009; Cywicka at al. 2019; Harris et al. 2010), reliable insights into the population structure are crucial. The results of our study – particularly the example of the SO versus the HU population, both showing similar densities but quite different sex ratios – furthermore underline the importance of considering the two sexes separately for density estimation.
The non-invasive genetic approach presented here holds the potential to obtain reliable data on red deer populations, which have until now been lacking in many regions (Smart et al. 2004; Apollonio et al. 2017). This lack of reliable information is hampering the assessment of hunting management effectiveness. In our study areas, hunting quotas are adjusted based on damage levels. Our non-invasive genetic density estimates allowed basing the degree of adjustment on sound sex-specific population data for the first time.
However, the question remains if a genetic approach is feasible on a larger scale, especially for species like red deer occurring in high densities and thus requiring the analysis of many samples. Including lab consumables and labour input as well as collection of samples in the field, our studies cost approximately 40–45 € per sample (with lab work and consumables representing approximately 75 to 80% of the total costs). In comparison to other non-invasive genetic population estimation studies, the costs for our study lie in the lower range (costs for other ungulate studies ranged between 60 and 100$ per sample; see, e.g. Harris et al. (2010), Poole et al. (2011) and Goode et al. (2014)). Compared to other approaches applied in our study areas, the non-invasive genetic method is rather expensive. Spotlight counts, e.g. cost approximately 1 €/ha; this corresponds to only 20% of the costs of a genetic estimate. The costs for aerial counts ranged from 1 to 4.5 €/ha depending on the coverage (Ehrhart et al. 2016). Further research could try to lower the expenses for genotyping, e.g. by optimizing the laboratory protocol or by improving the pre-selection of samples according to their freshness (Luikart et al. 2020). Besides this, the genetic method could be regarded as a tool to calibrate other approaches: It could be applied in larger time intervals (e.g. every 5 to 10 years) and in between, e.g. spotlight or aerial counts, which are much less expensive, but also provide less informative value compared to genetic estimates (Garel et al. 2010; Collier et al. 2013; Ehrhart et al. 2016), could be used. Thus, a reliable long-term monitoring could be based on such a combination of different methods.
References
Allombert S, Stockton S, Martin JL (2005) A natural experiment in the impact of overabundant deer on forest invertebrates. Conserv Biol 19:1917–1929
Apollonio M, Belkin VV, Borkowski J, Borodin OI, Borowik T, Cagnacci F, Danilkin AA, Danilov PI, Faybich A, Ferretti F, Gaillard JM, Hayward M, Heshtaut P, Heurich M, Hurynovich A, Kashtalyan A, Kerley GIH, Kjellander P, Kowalczyk R, Kozorez A, Matveytchuk S, Milner JM, Mysterud A, Ozoliņš J, Panchenko DV, Peters W, Podgórski T, Pokorny B, Rolandsen CM, Ruusila V, Schmidt K, Sipko TP, Veeroja R, Velihurau P, Yanuta G (2017) Challenges and science-based implications for modern management and conservation of European ungulate populations. Mammal Research 62(3):209–217
Barrio J (2007) Population viability analysis of the Taruka, Hippocamelus antisensis (D’Orbigny, 1834) (Cervidae) in southern Peru. Revista Peruana de Biologia 14:193–200
Beja-Pereira A, Oliveira R, Alves PC, Schwartz MK, Luikart G (2009) Advancing ecological understandings through technological transformations in noninvasive genetics. Mol Ecol Resour 9:1279–1301
Bertouille SB, De Crombrugghe S (2002) Fertility of red deer in relation to area, age, body mass and mandible length. Z Jagdwiss 48:87–98
Bischof R, Dupont P, Milleret C, Chipperfield J, Royle JA (2020) Conequences of ignoring group association in spatial capture-recapture analysis. Wildl Biol 2020. https://doi.org/10.2981/wlb.00649
Borchers DL, Efford MG (2008) Spatially explicit maximum likelihood methods for capture-recapture studies. Biometrics 64:377–385
Brazeal JL, Weist T, Sacks BN (2017) Noninvasive genetic spatial capture-recapture for estimating deer population abundance. J Wildl Manag 81:629–640
Brinkman TJ, Person DK, Chapin FS III, Smith W, Hundertmark KJ (2011) Estimating abundance of Sitka black-tailed deer using DNA from fecal pellets. J Wildl Manag 75:232–242
Broquet T, Petit E (2004) Quantifying genotyping errors in noninvasive population genetics. Mol Ecol 13:3601–3608
Bonenfant C, Gaillard JM, Loison A, Klein F (2003) Sex-ratio variation and reproductive costs in relation to density in a forest-dwelling population of red deer (Cervus elaphus). Behav Ecol 14:862–869
Boulanger J, McLellan B (2001) Closure violation in DNA-based mark-recapture estimation of grizzly bear populations. Can J Zool 79:642–651
Boulanger J, Stenhouse G, Munro R (2004) Sources of heterogeneity bias when DNA mark-recapture sampling methods are applied to grizzly bear (Ursus arctos) populations. J Mammal 85:618–624
Boulanger J, Kendall KC, Stetz JB, Roon DA, Waits LP (2008) Multiple data sources improve DNA-based mark-recapture population estimates of grizzly bears. Ecol Appl 18:577–589
Burnham KP, Anderson DR (2002) Model selection and multimodel inference: a practical information-theoretic approach, 2nd edn. Springer Verlag, New York
Collier BA, Ditchkoff SS, Ruth CR, Raglin JB (2013) Spotlight surveys for white - tailed deer: monitoring panacea or exercise in futility? J Wildl Manag 77:165–171
Clutton-Brock TH, Guiness FE, Albon SD (1982) Red deer – behaviour and ecology of two sexes. The University of Chicago Press, Wildlife behaviour and ecology series, 378 pp
Clutton-Brock TH, Lonergan ME (1994) Culling regimes and sex ratio biases in highland red deer. J Appl Ecol 31:521–527
Clutton-Brock TH, Coulson TN, Milner-Gulland EJ, Thomson D, Armstrong HM (2002) Sex differences in emigration and mortality affect optimal management of deer populations. Nature 415:633–637
Daniels MJ (2006) Estimating red deer Cervus elaphus populations: an analysis of variation and cost-effectiveness of counting methods. Mammal Rev 36:235–247
De Barba M, Waits LP, Genovesi P, Randi E, Chirichella R, Cetto E (2010) Comparing opportunistic and systematic sampling methods for non-invasive genetic monitoring of a small translocated brown bear population. J Appl Ecol 47:172–181
De Oliveira ML, Zarate do Couto HT, Barbanti Duarte JM (2019) Distribution of the elusive and threatened Brazilian dwarf brocket deer refined by non-invasive genetic sampling and distribution modelling. Eur J Wildl Res 65: 21
Dreher BP, Winterstein SR, Scribner KT, Lukacs PM, Etter DR, Rosa GJM, Lopez VA, Libants S, Filcek KB (2007) Non-invasive estimation of black bear abundance incorporating genotyping errors and harvested bear. J Wildl Manag 71:2684–2693
Dupont P, Milleret C, Gimenez O, Bischof R (2019) Population closure and the bias-precision trade-off in spatial capture-recapture. Methods Ecol Evol 2019:1–12
Ebert C, Knauer F, Storch I, Hohmann U (2010) Individual heterogeneity as a pitfall in population estimates based on non-invasive genetic sampling: a review and recommendations. Wildl Biol 16:225–240
Ebert C, Sandrini J, Spielberger B, Thiele B, Hohmann U (2012) Non-invasive genetic approaches for estimation of ungulate population size: a study on roe deer (Capreolus capreolus) based on faeces. Anim Biodivers Conserv 35:267–275
Efford MG, Dawson DK, Robbins S (2004) DENSITY: software for analysing capture-recapture data from passive detector arrays. Anim Biodivers Conserv 27:217–228
Efford MG (2011) Estimation of population density by spatially explicit capture-recapture analysis of data from area searches. Ecology 92:2202–2207
Efford MG (2017a) secr: spatially explicit capture-recapture models. R package version 3.0.1 https://CRAN.R-project.org/package=secr
Efford MG (2017b) Polygon and transect detectors in secr:3.0 www.otago.ac.nz/density/pdfs/secr-polygondetectors.pdf
Ehrhart S, Lang J, Simon O, Hohmann U, Stier N, Nitze M, Heurich M, Wotschikowsky U, Burghart F, Gerner J, Schraml U (2016) Wildmanagement in deutschen Nationalparken. BfN Skripten 434. Script of the Federal Agency for Nature Conservation, Bonn, Germany [in german]: 180 pp.
Ferreira CM, Sabino-Marques H, Paupério J, Barbosa S, Costa P, Encarnação C, Alpizar-Jara R, Pita R, Beja P, Mira A, Searle JB, Paupério J, Alves PC (2018) Genetic non-invasive sampling (gNIS) as a cost-effective tool for monitoring elusive small mammals. Eur J Wildl Res 64 No. 46
Garel M, Bonenfant C, Hamann JL, Klein F, Gaillard JM (2010) Are abundance indices derived from spotlight counts reliable to monitor red deer Cervus elaphus populations? Wildl Biol 16:77–84
Goode MJ, Beaver JT, Muller LI, Clark JD, van Manen FT, Harper C, Basinger PS (2014) Capture-recapture of white-tailed deer using DNA from fecal pellet groups. Wildl Biol 20:270–278
Gordon IJ, Hester AJ, Festa-Bianchet M (2004) The management of wild large herbivores to meet economic, conservation and environmental objectives. J Appl Ecol 41:1021–1031
Granjon A-C, Rowney C, Vigilant L, Langergraber KE (2016) Evaluating genetic capture-recapture using a chimpanzee population of known size. J Wildl Manag 81:279–288
Gräber R, Ronnenberg K, Strauss E, Siebert U, Hohmann U, Sandrini J, Ebert C, Hettich U, Franke U (2016) Vergleichende Analyse verschiedener Methoden zur Erfassung von freilebenden Huftieren. Endbericht Deutsche Bundesstiftung Umwelt [in german]: 117 pp. https:// www.dbu.de/projekt_30413/01_db_2409.html
Grignolio S, Apollonio M, Brivio F, Vicente J, Acevedo P, Palencia P, Petrovic K (2020) Keuling O (2020) guidance on estimation of abundance and density data of wild ruminant population: methods, challenges, possibilities. EFSA supporting publication EN-1876:54 pp
Gurgul A, Radko A, Slota E (2010) Characteristics of X- and Y-chromosome specific regions of the amelogenin gene and a PCR-based method for sex identification in red deer (Cervus elaphus). Mol Biol Rep 37:2915–2918
Hamann JL, Klein F, Saint-Andrieux C (1997) Domaine vital diurne et déplacements des biches (Cervus elaphus) sur le secteur de La Petite Pierre (Bas-Rhin). Gibier Faune Sauvage 14:1–17
Harris RB, Winnie J Jr, Amish SJ, Beja-Pereira A, Godinho R, Costa V, Luikart G (2010) Argali abundance in the Afghan Pamir using capture-recapture modelling from fecal DNA. J Wildl Manag 74:668–677
Hickey JR, Sollmann R (2018) A new mark-recapture approach for abundance estimation of social species. PLoS One 13:e0208726
Hohmann U, Hettich U, Ebert C, Huckschlag D (2018) Evaluierungsbericht zu den Auswirkungen einer dreijährigen Jagdruhe in der Kernzone „Quellgebiet der Wieslauter “im Wildforschungsgebiet „Pfälzerwald“. Mitteilungen aus der Forschungsanstalt für Waldökologie und Forstwirtschaft FAWF, Trippstadt Nr. 84/18 [in german]: 152 pp.
Hohmann U, Hettich U (2018) Standards für nächtliche Scheinwerferzählungen von Rotwild in waldgeprägten Gebieten. Webpage FAWF. https://fawf.wald-rlp.de/index.php?eID=dumpFile&t=f&f=41091&token=7c1b21d6d190357606755aa7bc0a3c15cc906065. Accessed 17 December 2020
Kendall WL (1999) Robustness of closed capture-recapture methods to violations of the closure assumption. Ecology 80:2517–2525
Kendall KC, Stetz JB, Boulanger J, Macleod AC, Paetkau D, White GC (2009) Demography and genetic structure of a recovering grizzly bear population. J Wildl Manag 73:3–17
Lang J, Huckschlag D, SIMON O (2016) Möglichkeiten und Grenzen der Wildbestandsschätzung für Rotwild mittels retrospektiver Kohortenanalyse am Beispiel des Rotwildgebietes “Pfälzerwald”. Beiträge zur Jagd-und Wildforschung [in german] 41:351–360
Lucchini V, Fabbri E, Marucco F, Ricci S, Biotani L, Randi E (2002) Noninvasive molecular tracking of colonizing wolf (Canis lupus) packs in the western Italian Alps. Mol Ecol 11:857–868
Luikart G, Ryman N, Tallmon DA, Schwartz MK, Allendorf FW (2010) Estimation of census and effective population sizes: the increasing usefulness of DNA-based approaches. Conserv Genet 11:355–373
Lukacs PM, Burnham KP (2005a) Estimating population size from DNA-based closed capture-recapture data incorporating genotyping error. J Wildl Manag 69:396–403
Lukacs PM, Burnham KP (2005b) Review of capture-recapture methods applicable to non-invasive genetic sampling. Mol Ecol 14:3909–3919
Lunt N, Mhlanga MR (2011) Defecation rate variability in the common duiker: importance of food quality, season, sex and age. S Afr J Wildl Res 41:29–35
Maudet C, Luikart G, Dubray D, von Hardenberg A, Taberlet P (2004) Low genotyping error rates in wild ungulate faeces sampled in winter. Mol Ecol Notes 4:772–775
Marucco F, Pletscher DH, Boitani L, Schwartz MK, Pilgrim KL, Lebreton J-D (2009) Wolf survival and population trend using non-invasive capture-recapture techniques in the Western Alps. J Appl Ecol 46:1003–1010
Marucco F, Boitani L, Pletscher DH, Schwartz MK (2011) Bridging the gap between non-invasive genetic sampling and population parameter estimation. Eur J Wildl Res 57:1–13
Mayle BA, Peace AJ, Gill RMA (1999) How many deer? A field guide to estimating deer population size. Forestry Commission Archive Field Book 18, London: Her Majesty’s Stationery Office, 96 pp.
McKelvey KS, Schwartz MK (2004) Genetic errors associated with population estimation using non-invasive molecular tagging: problems and new solutions. J Wildl Manag 68:439–448
Miller CR, Joyce P, Waits LP (2005) A new method for estimating the size of small populations from genetic mark-recapture data. Mol Ecol 14:1991–2005
Milner J, Bonenfant C, Mysterud A, Gaillard J-M, Csányi S, Stenseth NC (2006) Temporal and spatial development of red deer harvesting in Europe: biological and cultural factors. J Appl Ecol 43:721–734
Mysterud A, Meisingset EL, Veiberg V, Langvatn R, Solberg E, Loe LE, Stenseth NC (2007) Monitoring population size of red deer Cervus elaphus: an evaluation of two types of census data from Norway. Wildl Biol 13:285–298
Obbard ME, Howe EJ, Kyle CJ (2010) Empirical comparison of density estimators for large carnivores. J Appl Ecol 47:76–84
Otis DL, Burnham KP, White GC, Anderson DR (1978) Statistical inference for capture-recapture experiments. Wildl Monogr 62
Parmenter RR, Yates TL, Anderson DR, Burnham KP, Dunnum JL, Franklin AB, Friggens MT, Lubow BC, Miller M, Olson GS, Parmenter CA, Pollard J, Rexstad E, Shenk TM, Stanley TR, White GC (2003) Small-mammal density estimation: a field comparison of grid-based vs. web-based density estimators. Ecol Monogr 73:1–26
Pennel MW, Stansbury CR, Waits LP, Miller CR (2013) Capwire: a R package for estimating population census size from non-invasive genetic sampling. Mol Ecol Resour 13:154–157
Petit E, Valière N (2006) Estimating population size with non-invasive capture-recapture data. Conserv Biol 20:1062–1073
Pledger S, Efford M (1998) Correction of bias due to heterogeneous capture probability in capture-recapture studies of open populations. Biometrics 54:888–898
Pollock KH, Nichols JD, Brownie C, Hines JE (1990) Statistical inference for capture-recapture experiments. Wildl Monogr 107:1–97
Pompanon F, Bonin A, Bellemain E, Taberlet P (2005) Genotyping errors: causes, consequences and solutions. Nat Rev Genet 6:847–859
Poole KG, Reynolds DM, Mowat G, Paetkau D (2011) Estimating mountain goat abundance using DNA from fecal pellets. J Wildl Manag 75:1527–1543
Puechmaille SJ, Petit E (2007) Empirical evaluation of non-invasive capture-capture-recapture estimation of population size based on a single sampling session. J Appl Ecol 44:843–852
Putman RJ, Moore NP (1998) Impact of deer in lowland Britain on agriculture, forestry and conservation habitats. Mammal Rev 28:141–164
Rea RV, Johnson CJ, Murray BW, Hodder DP, Crowley SM (2016) Timing moose pellet collections to increase genotyping success of fecal DNA. Journal of Fish and Wildlife Management 7:461–466
Roon DA, Waits LP, Kendall KC (2005) A simulation test of the effectiveness of several methods for error-checking non-invasive genetic data. Anim Conserv 8:203–215
Royle JA, Young KV (2008) A hierarchical model for spatial capture-recapture data. Ecology 9:2281–2289
Royle JA, Chandler RB, Sollmann R, Gardner B (2014) Spatial capture-recapture. Academic Press, San Diego, CA, 612 pp
Seber GAF (1982) The estimation of animal abundance and related parameters, 2nd edn. Charles Griffin, London
Skrbinšek T, Lustrik R, Majić-Skrbinšek A, Potočnik H, Kljun F, Jelenčič M, Kos I, Trontelj P (2019) From science to practice: genetic estimate of brown bear population size in Slovenia and how it influenced bear management. European Journal of Wildlife Research 65. No.29
Smart JCR, Ward A, White PCL (2004) Monitoring woodland deer populations in the UK: an imprecise science. Mammal Rev 34:99–114
Taberlet P, Luikart G (1999) Non-invasive genetic sampling and individual identification. Biol J Linn Soc 68:41–55
Tioli S, Cagnacci F, Stradiotto A, Rizzoli A (2009) Edge effect on density estimates of a radiotracked population of yellow-necked mice. J Wildl Manag 73:184–190
Tsaparis D, Karaiskou N, Mertzanis Y, Triantafyllidis A (2014) Non-invasive genetic study and population monitoring of the brown bear (Ursus arctos) (Mammalia: Ursidae) in Kastoria region – Greece. J Nat Hist 49:393–410
Valière N (2002) GIMLET: a computer program for analysing genetic individual identification data. Mol Ecol Notes 2:377–379
Valière N, Bonenfant C, Toigo C, Luikart G, Gaillard J-M, Klein F (2007) Importance of a pilot study for non-invasive genetic sampling: genotyping errors and population size estimation in red deer. Conserv Genet 8:69–78
Waits LP, Luikart G, Taberlet P (2001) Estimating the probability of identity among genotypes in natural populations: cautions and guidelines. Mol Ecol 10:249–256
Waits LP, Paetkau D (2005) Noninvasive genetic sampling tools for wildlife biologists: a review of applications and recommendations for accurate data collection. J Wildl Manag 69:1419–1433
Ward AI (2005) Expanding ranges of wild and feral deer in Great Britain. Mammal Rev 35:165–173
Wilberg MJ, Dreher BP (2004) GENECAP: a program for analysis of multilocus genotype data for non-invasive sampling and capture-recapture population estimation. Mol Ecol Notes 4:783–785
Wilson KR, Anderson DR (1985) Evaluation of two density estimators of small mammal population size. J Mammal 66:31–21
Woodruff SP, Johnson TR, Waits LP (2015) Evaluating the interaction of faecal pellet deposition rates and DNA degradation rates to optimize sampling design for DNA-based mark-recapture analysis of Sonoran pronghorn. Mol Ecol Resour 15:843–854
Woodruff S, Lukacs P, Waits LP (2016) Estimating Sonoran pronghorn abundance and survival with fecal DNA and capture-recapture methods. Conserv Biol 30:1102–1111
Woods JG, Paetkau D, Lewis D, McLellan BN, Proctor M, Strobeck C (1999) Genetic tagging of free ranging black and brown bears. Wildl Soc Bull 27:616–627
Acknowledgements
F. Feind and S. Peters provided very valuable help in the field. We also thank all those who helped collecting samples. Many thanks to K. Schifferle for her help with ArcGIS and graphics and to F. Klein for his fruitful discussion. K. Schifferle, C. Tröger and two anonymous reviewers provided helpful comments on this manuscript. The project is supported by the foundation ‘Rheinland-Pfalz für Innovation’ and the Ministry of Environment and Forestry, Rhineland-Palatinate. C. Ebert was supported by the FAZIT Foundation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ebert, C., Sandrini, J., Welter, B. et al. Estimating red deer (Cervus elaphus) population size based on non-invasive genetic sampling. Eur J Wildl Res 67, 27 (2021). https://doi.org/10.1007/s10344-021-01456-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10344-021-01456-8