Abstract
In this study, we examine the spatial interrelation between different carnivore species (Carnivora, Mammalia) and ground-nesting birds in a Special Protection Area (SPA) in Brandenburg, Germany. Camera- and live-trapping of carnivores during an 18-month period revealed that the SPA hosts most mesocarnivore species that occur in Germany. Since raccoon (Procyon lotor), red fox (Vulpes vulpes), and domestic cat (Felis silvestris f. catus) showed highest abundance-activity indices, we used GPS telemetry for a detailed analysis of spatial behavior of nine raccoons and five red foxes over a 22-month period. Spatial distribution patterns showed a strong difference between both species: raccoons showed a clear preference for reed swamps and shrub swamps, clustering in high concentrations along the edges of water bodies. Although this behavior is likely due to the high and year-round availability of aquatic food sources, overlap of raccoon core areas with high densities of wetland birds likely results in a high level of sublethal predation effects particularly on waterfowl breeding in reed beds. Red foxes showed much more evenly distributed movement patterns and a high intraspecific variability in habitat preference, revealing a general preference for woodlands and an avoidance of wetlands. Thus, predation pressure by foxes on ground-breeders seems to be lower and focusing on grassland-associated bird species in close proximity to woodlands. Consequently, our study highlights the need for a differentiated view on the predatory potential of particular mesocarnivore species on the endangered bird species in the SPA, leading to future management implications with a focus on swampland habitats.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In central Europe, agricultural practices such as crop production and livestock farming by humans have led to the conversion of structurally uniform forests into cultural landscapes with biodiversity-rich habitat types (Naveh 1998). Many faunal elements of European agroecosystems (e.g., grassland-associated arthropods and songbirds) only exist due to this anthropogenic transformation process (Zeller et al. 2017). Therefore, nature conservation regulations (e.g., European Bird Directive, Fauna and Flora Directive) aim at promoting biodiversity by protecting cultural landscapes. During decades, however, an increasing agricultural intensification process causes a dramatic decline across many taxonomic groups. Negative impacts of intensive agriculture on populations have already been reported for several groups of epigeic arthropods (Rushton et al. 1989; Aebischer 1991; Sotherton and Self 2000), as well as for mammals, such as European hare (Lepus europaeus) (Smith et al. 2005), European hamster (Cricetus cricetus) (Nechay 2000), and several bat species (Starik 2016). Another example is the well-documented negative relationship between agricultural intensification and the species richness of birds adapted to open areas (Hagemeijer and Blair 1997; Chamberlain et al. 2000; Donald et al. 2001; Robinson and Sutherland 2002; Haupt et al. 2009). Apart from direct effects, such as intense machine processing, advanced mowing dates, or frequent cuts per year, agricultural intensification also indirectly affects reproductive performance of bird populations through land consolidation, melioration, and increasing eutrophication, resulting in a micro-climatic deterioration of the quality of nesting sites (Hudson et al. 1990; Haupt et al. 2009). In addition, climatic changes and increasingly humid springtimes can increase this effect (Haupt et al. 2009), illustrating the complexity of the interrelation between land use and decreasing populations of particularly ground-nesting bird species. In fact, most of the bird species listed as extinct or threatened in Germany are ground-breeders (Langgemach and Bellebaum 2005). Already in 1979, the European Union designed a pan-European network of Special Protection Areas (SPA) in order to protect bird species (particularly migratory birds) and their habitats (e.g., wetlands of international importance for migratory waterfowl). However, management actions in this areas, such as habitat improvements and extensive farming practices, could not yet stop decreasing population trends of many bird species (Südbeck and Krüger 2004; Haupt et al. 2009).
For this reason, predation on ground-nesting birds is receiving considerable attention for its potential effect on the reproductive success and consequently the population development of ground-nesting bird species (Baines 1990; Newton 1993; Grant et al. 1999; Bellebaum 2002; Langgemach and Bellebaum 2005). In wetlands, predation has been reported an important factor for declining population trends of several wader species (Grant et al. 1999; Macdonald and Bolton 2008; Milsom 2010). Predation has been shown to also negatively affect the breeding success of grassland-associated songbirds, such as corn bunting (Emberiza calandra) (Brickle et al. 2000), yellowhammer (Emberiza citrinella) (Bradbury et al. 2000), and skylark (Alauda arvensis) (Donald et al. 2002). Apart from avian predators, numerous studies highlight the role of carnivores (Carnivora, Mammalia) in this context (Crooks and Soulé 1999; Sanders and Maloney 2002; Engl et al. 2004). However, often the specific carnivore species remains unidentified, resulting in surprisingly little information regarding the specific role of particular carnivore species. According to the current state of knowledge, the red fox (Vulpes vulpes) seems to be the most important mammalian predator of ground-nesting birds in Europe (Isaksson et al. 2007; Pedersen et al. 2009; Fletcher et al. 2010; Kämmerle et al. 2017; Roos et al. 2018). However, this seems to be simply a result of the species’ high degree of occurrence in a vast distribution range and its ability to occasionally attack and kill even large bird species, such as adult mute swan (Cygnus olor) (Litzbarski 1998; Kube et al. 2005). In addition, many studies simply link decreasing populations of ground-nesting birds to increasing numbers of red foxes. Despite an increasing awareness regarding the predatory potential of alien carnivores, such as raccoon (Procyon lotor), raccoon dog (Nyctereutes procyonoides), or American mink (Neovison vison) in Europe, surprisingly little scientific attention has been paid on the predatory potential of alien carnivores on a species level. Although the raccoon is commonly regarded as a nest predator of waterfowl (Cowardin et al. 1983; Johnson et al. 1989, 1992; Sargeant et al. 1993; Heske et al. 2001; Schmidt 2003; Ellis et al. 2007), even in its native range in the USA and Canada, the predation risk caused by raccoons is still poorly investigated. In a European context, the relative predatory potential of alien carnivore species compared to native carnivores has not received much attention (Langgemach and Bellebaum 2005; Salo et al. 2007). In addition, the few studies focusing on this issue could not find evidence for negative impacts on ground-nesting bird populations concerning raccoons (Engelmann et al. 2011; Michler 2017) and raccoon dogs (Lavrov 1971; Nasimovič and Isakov 1985; Kauhala 2004). Nevertheless, against the background of still increasing population densities and in view of their omnivorous feeding habits and their association to water, there is need for further research concerning in particular raccoons and their predatory potential on ground-nesting bird species in Europe.
The overall objective of this study is to investigate the interrelation between different carnivore species and the ground-nesting bird community in the SPA “Mittlere Havelniederung” (MH-SPA) in Brandenburg, Germany. The first objective is to examine the composition and distribution of the local carnivore community associated with this protected area. On this basis, the next objective is to analyze the spatial behavior (home ranges, core areas, dispersal patterns) and habitat preferences of selected individuals representing the most common mesocarnivore species. In a third step, we aim at analyzing how this spatial behavior is locally related with the occurrence and spatial distribution of selected ground-nesting bird species. Collectively, a better understanding of the relation between spatial behavior of mammalian carnivores and the occurrence and distribution of selected ground-nesting bird species will help to derive future management implications for the conservation of ground-nesting bird species and a sustainable predator management, also addressing the requirements and ecological function of different carnivore species in this SPA.
Methods
Study area
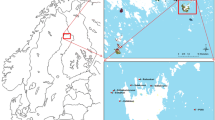
The study was conducted at the northern border of the MH-SPA which is located around 10 km west of Berlin in the western part of the federal state of Brandenburg, Germany (12.651205 E, 52.490064 N). The MH-SPA (249 km2) and its surrounding area were shaped by the Weichselian Glaciation and thus are characterized by glacier moraines, outwash plains, and a largely open landscape (Küster 2007). Due to numerous former meltwater discharge streams, today, the area is one of the largest continuous areas of rivers and lakes in Germany. Apart from waters (15.3%), the protection area is covered by large areas of reed beds (3.4%), which are surrounded by a mosaic of human settlements (3.7%), woodland (11.7%), arable land (36.9%), and grassland (30.0%) (LUGV 2013). The area is strongly influenced by seasonal water levels of the river Havel. Thus, especially during springtime, large areas of grassland are flooded. This landscape pattern makes the Protection Area an important breeding and resting site for many endangered ground-nesting bird species, in particular waterfowl and waders. A total of more than 250 bird species have already been reported in this area, including 30 ground-nesting bird species listed on Annex I to the Bird Directive (26 reported breeding) (Rudolph 2005). Notably, common crane (Grus grus), Eurasian bittern (Botaurus stellaris), little bittern (Ixobrychus minutus), and bluethroat (Luscinia svecica) are represented in this area by a huge part of the federal state’s total population (Rudolph 2005). Located in the northern part of the MH-SPA, the case study area (12.5 km2) is bounded by Lake Beetzsee in the south and a large forest area in the north, including the village Gortz. The northern part of the study area is mainly covered with forest, while the lakeshores in the south are dominated by swampland and floodplains. The rest of the study area mainly comprises arable farmland and pasture land (Fig. 1).
a Location of the MH-SPA in Germany. b Location of the case study area in the MH-SPA. c Case study area. Data of habitat mapping adapted from the European Regional Development Fund (ERDF) and the federal district of Brandenburg 2009 (LUGV 2013)
Data collection and data analysis
Camera trapping
In order to investigate the composition and distribution of the carnivore community, we deployed 20 infrared camera traps from mid-June to September 2015 (12 Moultrie® M100 and 8 Moultrie® M-990i). Our trapping design was set up to detect mesocarnivores with a predatory potential for ground-nesting birds in three different habitat types (swampland, woodland, open land). Thus, we deployed cameras in an opportunistic way, close to signs of carnivore activity such as tracks, feces, or dens. Camera traps were mounted on trees or timber stakes around 40–50 cm off the ground, facing potentially occupied sites. We did not use any attractant. Locations of camera traps were changed if carnivore activity at camera trap sites was low. In total, we conducted camera surveys at 39 locations (n = 15 in swampland, n = 17 in woodland, n = 7 in open land), resulting in 1356 trap nights. Average length of camera deployment at each site was 34.8 days (SD = 34.7; min = 10; max = 147).
Camera traps were active 24 h/day and took bursts of three pictures with a delay of 30 s between trigger activations. To avoid overestimation of species, camera trap detections were converted to camera-trap events (Meek et al. 2014). An event was recorded where an individual of a species was photographed with a minimum absence of 5 min between two detections. As in some cases, more than one individual of the same species was photographed at the same time, we later multiplied camera-trap events for each species and habitat type by the species’ average group size of the respective habitat type. In order to compare abundance-activity indices between habitat types, we computed a relative abundance index (RAI) for each species and habitat type (O’Brien et al. 2003; Jenks et al. 2011). For each habitat type, all camera-trap events of each species were summed for all camera traps over all days, multiplied by 100 and divided by the total number of camera trap nights in each habitat type (O’Brien et al. 2003).
where n refers to the total number of camera traps, E refers to the number of camera-trap events, and TN is the total number of trap nights per habitat.
Since most pictures were black and white and therefore did not always allow distinguishing beech martens (Martes martes) and stone martens (Martes foina), we grouped both species. Calculations were done with R 3.4.1 (R Core Team 2017) and Microsoft® Excel 2010.
Live-trapping and radio tracking
Live-trapping of carnivores took 18 months (October 2015 to March 2017) and 2543 trap nights. Since we used a maximum relative transmitter weight of 3% (Kenward 2000; Sikes 2016) for collaring, our trapping design was set up to trap carnivores with a body weight larger than 5.7 kg (raccoon, raccoon dog, red fox, badger). For capturing, we used 11 wooden walk-through box traps, that were complemented with seven additional walk-through box traps in May 2016 (length = 200 cm, height = 40 cm, width = 33 cm). Box traps were deployed in three different habitat types (swampland, woodland, open land) along obligatory passages such as ditches, game trails, or lakeshores. Locations of live traps were frequently changed depending on trapping success and activity of selected carnivores at trap sites. In order to measure carnivore activity and trapping success, trap sites were surveilled with a total of 20 camera traps (12 Moultrie® M100 and 8 Moultrie® M-990i), resulting in 3018 trap nights. Camera traps were active 24 h/day and took bursts of three pictures with a delay of 30 s between trigger activations. In total, we mounted live traps at 88 different trap sites (n = 28 in swampland, n = 27 in woodland, n = 33 in open land). Traps were controlled twice a day and baited with liver sausage, cat food, or dried dates. As done for camera trapping, we also calculated relative abundance indices for live captures by using the relative abundance index and the acronym CRAI.
Captured animals were transferred into a holding cage and then checked for parasites, body condition, and injuries. Animals in bad conditions as well as juveniles and lactating females were not handled but released immediately. Adult animals were defined by body weight (Fiero and Verts 1986; Stubbe 1989; Wandeler and Lüps 1993), putting the transfer box on a mobile scale. If animals were heavy enough, they were immobilized with an intramuscular injection of a ketamine-xylazine hydrochloride combination via a blowpipe. This mixture has been applied in numerous previous studies due to its high therapeutic index (Wiesner and von Hegel 1985) and is suitable for red foxes (Travaini et al. 1992; Kaphegyi 2002) and raccoons (Belant 1995; Michler et al. 2003, 2015). For immobilization of raccoons, we used doses of 15 mg ketamine and 3 mg xylazine per kg body weight (Michler et al. 2015). Red foxes were immobilized with slightly higher doses. Depending on level of aggression, we used 20–25 mg ketamine and 4–5 mg xylazine per kg body weight. After immobilization, animals were retrieved from the holding cage, weighted to the nearest 10 g, measured, sexed, and ear-tagged (Dalton® Rototag). Besides, all animals were fitted with GPS collars (e-obs®, Grünwald, model 1C-heavy, 170 g) that were programmed to an interval of 40 min (36 fixes per day). Since animals were arranged in safe boxes to recover, we did not use any antidotes. Besides, we wanted to avoid possible adverse effects by antidotes (e.g., tachycardia) (Michler et al. 2015). Animals were released at the same locations after 4–6 h. GPS data was stored on the tags and was downloaded weekly with a handheld receiver and a yagi antenna which had a range of 100 to 1000 m, depending on vegetation structure. To locate collared individuals from high distance, GPS tags were also reachable via UHF for 2 h/day. Prior to live-trapping, accuracy of all GPS tags was tested for 8 h by mounting the collars on an open field. For each collar, we then calculated the average position of all GPS fixes (ArcGIS 10.1, Function: Median Center) and the mean deviation of all single fixes to this point. Mean deviance was 3.4 m (median = 2.56; SD = 2.72 m). There was no significant difference in accuracy between the different tags (ANOVA: F = 1.6677, p = 0.074).
Total home range of carnivores was calculated using R 3.4.1 (R Core Team 2017) and the package “adehabitat” (Calenge 2006). For calculating home ranges, we used three methods: (1) minimum convex polygon (MCP) (Mohr 1947; White and Garrott 1990), (2) fixed kernel density estimation (KDE) (Worton 1989), and (3) adapted local convex hull (A-LoCoH) (Getz et al. 2007). According to Getz et al. (2007), LoCoH methods and especially the A-LoCoH method are superior to parametric kernel methods and minimum polygons due to their ability to identify hard boundaries such as lakes or rivers. For calculating adapted local convex hulls, the parameter a was defined as the maximum distance between any two individual’s GPS points (Getz et al. 2007). For KDE, we used the reference bandwidth h ref as the smoothing parameter (Worton 1995), since smoothing with other methods (e.g., least squares cross-validation) resulted in excessive fragmentation and underestimates of home range sizes (see also Blundell et al. 2001). According to (Burt 1943), single excursions to the area outside the animal’s normal area should not be considered as part of the home range. Thus, for calculation of home ranges, the concept of utilization distribution (UD) (Van Winkle 1975) is often used. The UD describes the use of space by a bivariate probability density function, commonly using a value of 95% to define the total home range (Worton 1989; White and Garrott 1990). Individual core areas instead were estimated by calculating home ranges at a level of 50% utilization distribution (Dixon and Chapman 1980; Laver and Kelly 2008; Byrne and Chamberlain 2011; Drygala and Zoller 2013). In two cases, when red foxes established new home ranges within the first 3 weeks, we only used GPS fixes of the second home range. Intraspecific spatial overlap between two individuals A and B was calculated using the 95% Kernel isopleths of both animals and Sørensen’s coefficient (Sørensen 1948; Poole 1995).
where a refers to the area used by individual A, b refers to the area used by individual B, and c is the area shared by both individuals.
Habitat mapping
To quantify habitat selection of the equipped carnivores, vegetation was mapped by measuring the shapes of different vegetation types with a GPS device (Garmin® e-Trex 20). Dynamic shorelines between waters and reed swamps were mapped using a kayak. Data of different woodland types were taken from public data of the European Regional Development Fund (ERDF) and the Land Brandenburg (MI 09-046L/80140147). Mapped data was digitalized with ArcGIS 10.4 (ESRI®) and adjusted with the help of satellite imagery. We differentiated between four broad habitat types (woodland, open land, swampland, human settlements) and 14 detailed habitat types. Woodland was divided into deciduous, coniferous, and mixed forests as well as into reforestation areas. Open land was grouped into hedges and thickets, meadows, fallow land, arable fields, permanent pastures, and shifting pastures, which were only grazed by cattle during summertime from May to August. Swampland was grouped into carrs, shrub swamps, and reed swamps. To calculate habitat preferences, we used Chesson’s electivity index ɛ (Chesson 1983), which is an often used method for calculating habitat preferences (Krebs 1989; Pebsworth et al. 2012; Cristescu et al. 2013; Schai-Braun et al. 2013). We chose this index because it guarantees a comparability of cases with different number of habitat types. Chesson’s electivity index is based on Manly’s selection index ɑ (Manly et al. 1972), which computes a utilization probability ɑi for any vegetation type i:

where m is the total number of vegetation types available to an individual, ri refers to the portion of vegetation type i used, and pi refers to the portion of vegetation type i available to an individual. Utilization ratio ɑi is the probability that the next vegetation used will be of type i. Chesson’s selectivity index Ɛ is scaled to values from − 1 to + 1. Neutral selection is indicated by values of 1 / m. Values larger than 1 / m mean preference while values smaller than 1 / m mean avoidance (Chesson 1983). For computing pi, we calculated the available portions of every vegetation type inside the 95% kernel isopleths of each individual, whereas ri was calculated by analyzing the single GPS fixes of every individual inside the 95% kernel isopleths. The preference or avoidance of a vegetation type was regarded significant, when the values of the lower and upper 95% confidence interval featured the same algebraic sign (Schai-Braun et al. 2013). We first computed Chesson’s electivity index for the four broad habitat types and afterwards again for the 14 detailed habitat types. GPS tags recorded 3d-acceleration data for 10 s in intervals of 5 min. Acceleration data was analyzed with the software Firetail® (Schäuffelhut Berger GmbH) to investigate diurnal activity. Calculations were done with R 3.4.1 (R Core Team 2017) and Microsoft® Excel 2010.
Bird survey
To identify trends in predation risk of ground-nesting birds, we calculated a predator–prey spatial overlap with the spatial distribution of red foxes and raccoons and bird sightings.
Therefore, a post hoc bird mapping survey was conducted between 1 March 2017 and 31 June 2017. For bird mapping, we selected an area of 269 ha in the southern part of the study area where GPS-tagged raccoons and red foxes occurred. Ground-nesting birds were defined as birds that are nesting directly on or near the ground. Birds were mapped once a week following the guidelines of the European Ornithological Atlas Committee (EOAC). Positions of bird observations were recorded on a map and later digitalized with ArcGIS 10.4 (ESRI®). For estimating relative abundance of bird species, MacKinnon lists (MacKinnon et al. 1993) were produced with a maximum number of 10 different species on each list (MacLeod et al. 2011). Although the MacKinnon list technique is typically used to estimate species richness, it has also been suggested as a rapid and time-efficient method to estimate relative abundance indices of birds (Bibby et al. 2000; MacLeod et al. 2011). The method briefly consists of a chronological listing of all species encountered during birding. This master list is then chronologically split into smaller list, such that each list comprises a predetermined number of different species (e.g., 10). In contrast to simple species lists, the MacKinnon list technique allows to estimate an abundance index by calculating the portion of lists in which each species occurs.
To calculate predator–prey spatial overlap, we compared the individual core areas of red foxes and raccoons (A-LoCoH 50%) during breeding time (1 March to 31 June) with locations of bird sightings (ArcGis Function: Near). Since positioning of bird observations had some inaccuracy, we calculated spatial overlap with a tolerance of 10 m, adding a respective buffer on the core areas first. For each bird species, we then calculated the percentage of sightings that overlapped with core areas of red foxes and raccoons, respectively.
Results
Carnivore community
Camera traps produced 7740 carnivore pictures (1994 events) in 4374 camera trap days (n [swampland] = 1485, n [grassland] = 1031, n [woodland] = 1858). A total of 12 carnivore species (including the domestic cat Felis silvestris f. catus) have been proven to occur in the study area. Five species (Mustela nivalis [RAI/swampland = 0.13/grassland = 0/woodland = 0.05], Mustela erminea [RAI = 0.13/0.1/0], Mustela putorius [RAI = 0/0.1/0], N. vison [RAI = 0.47/0/0], and Lutra lutra [RAI = 0.27/0/0]) were only recorded on less than five or on even one single event. Meles meles (RAI = 0.47/1.55/2.15), N. procyonoides (RAI = 3.50/2.33/0.91), and Martes spec. (RAI = 6.60/5.14/3.98) show higher abundance-activity indices, at least in some habitat types. Red fox (V. vulpes), raccoon (P. lotor), and domestic cat, by contrast, reveal abundance-activity indices, which are about factor 10 times higher than the average abundance-activity score of the remaining carnivore species photographed. While raccoons were mainly photographed in swampland (RAI = 27.21) and domestic cats were mainly photographed in grassland (RAI = 19.30), red foxes show high indices of relative abundance-activity in grassland (RAI = 22.41) and forest (RAI = 28.47) as well (Fig. 2).
Relative abundance index (RAI) of photographed carnivore species at trapping sites in different habitat types versus trapping success rate (number of captured animals per 100 camera-trap events) and total numbers of captured individuals. Capture indices refer only to trap sites that were surveilled with camera traps
During live-trapping, we captured a total of six carnivore species: M. martes (n = 2), M. foina (n = 6), N. procyonoides (n = 3 juveniles), V. vulpes (n = 11 adults, 13 juveniles), P. lotor (n = 31 adults, 16 juveniles), and F. silvestris f. catus (n = 22). Among these species, trapping success (capture index) was highest for raccoons (CRAI = 13.9), while red foxes (CRAI = 3.4) reveal the lowest degree of trapping success (Fig. 2). Focusing on the most detected carnivore species and following the requirements in terms of body weight (see “Methods”), we equipped a total of nine raccoons and six red foxes with GPS collars in order to study their spatial behavior in detail.
Dispersing activity
Except for two juvenile individuals, raccoons were only captured in the swampland habitats of the study area’s southern part and stayed in relatively close distance to their trapping sites all year (maximum distance from trapping site = 4.1 km, median = 0.87 km, n = 61,237 fixes). Red foxes, in contrast, were mainly captured in woodland and open land habitats (Fig. 2b) and showed a much higher post-capture dispersing activity than raccoons. Out of the six GPS-collared red foxes, two females (rf1and rf3) moved away (4.1 km, 12.1 km) from the study area and established new home ranges at the northern border of the MH-SPA, shortly after the GPS collar was fitted to the animals. During this dispersing, one red fox (Rf1) even traveled a total distance of more than 120 km during just five consecutive nights to Berlin and back again. Home ranges of raccoons showed a high degree of intraspecific overlap (mean = 33.6%, maximum = 82.8%), while red foxes’ home ranges did not overlap at all (Fig. 3).
Home ranges and core areas
The mean size of individual home ranges and core areas of raccoons and red foxes reveals a pronounced intraspecific variability (Table 1). In addition, the mean sizes of individual home ranges and core areas of red foxes are larger than the respective areas of raccoons. This applies for all three estimation methods (MCP, KDE, and A-LoCoH) and calculated probability ranges (50 and 95%), except for the KDE50%. However, significantly larger areas of red foxes compared to raccoons were only proven in terms of the A-LoCoH90% (Student’s t test: p = 0.04, t = 2.27) and the A-LoCoH50% (Student’s t test: p = 0.01, t = 3.01). All home range sizes of raccoons did not significantly depend on telemetry period (Pearson’s correlation: p > 0.05), while in red foxes, there was a significant correlation between the size of the A-LoCoH95% and the duration of telemetry (Pearson’s correlation: p = 0.04). In addition, median number of individual raccoon core areas (A-LoCoH50%) during night hours was significantly (Wilcoxon test: p = 0.046, W = 6) higher than in red foxes (Table 1).
Spatial distribution patterns
Spatial distribution patterns according to individual GPS fixes reveal a pronounced interspecific difference in habitat use (Fig. 4). Whereas raccoons show a strong tendency to cluster along the fringes of water bodies, resulting in a rather linear distribution pattern and elongated home ranges, red foxes were much more evenly distributed within their home ranges, resulting in a more homogenous distribution pattern within a rather planar-shaped area. As a result, the elongated shape of raccoon’s home ranges along water bodies leads to an overestimation of the size of individual kernel home ranges, as the KDE does not allow for an identification of hard geographic boundaries. Individual total home ranges of raccoons therefore contain large proportions of water (mean = 27.3%; max = 42.3%), which needs consideration when estimating the home range size of raccoons (Fig. 4.). True home ranges of raccoons (excluding non-used water areas) were about one third smaller than estimated by KDEs, leading to clearly smaller home range sizes of raccoons when compared to red foxes. This difference becomes apparent, when using the A-LoCoH method at the 95% probability level. In contrast to parametric KDE, the A-LoCoH method includes such borders in the estimation of spatial dimensions, resulting in significantly smaller total home ranges of raccoons when compared to red foxes (red foxes = 193.8 ha, raccoons = 81.7 ha, Table 1). In addition, the species-specific difference in spatial dimensions of activity areas becomes even clearer on the level of core areas. While individual raccoons maintained several (up to 13) small and aligned core areas, the core areas of red fox individuals were significantly larger (Table 1). Moreover, the spatial distribution of fixes of red foxes shows a much more evenly distributed pattern within a core area, while raccoons show distinct clusters of activity and little movement between these clusters.
Total GPS fixes of nine raccoons and five red foxes. The total visible area is defined by each KDE95%. For the lower two maps, home ranges of all raccoons and one red fox have been merged. GPS fixes: nred fox RF4 = 6303, nraccoons = 58,745. Note, the red fox home ranges are shown in actual relative positions. For exact locations, please refer to Fig. 3
Habitat use
Habitat use of raccoons revealed a significant preference for swampland, in particular reed swamps, while, compared to swamp land, open land (p = 0.00004, W = 0, Wilcoxon signed-rank test) and woodland (p = 0.0027, W = 0, Wilcoxon signed-rank test) were significantly avoided. However, some individuals showed a preference for carrs. The proportion of human settlements on the home ranges of raccoons varied considerably among individuals. Compared to raccoons, red foxes showed a higher level of intraspecific variation concerning their habitat preference. While the general habitat category “woodland” was moderately preferred, the analysis of specific habitat categories revealed a clear difference in the preference of foxes for different types of woodland: foxes significantly preferred deciduous forests and avoided coniferous forests. As in raccoons, open land was significantly avoided, while in some individuals, there was a preference of swamplands in particular carrs. Similar to raccoons, red foxes showed a high degree of intraspecific variability concerning the use of human settlements (Fig. 5a).
a Chesson’s electivity indices of n = 9 raccoons and n = 5 red foxes (medians with 25th/75th and 10th/90th percentiles). The grouped habitat types are indicated by gray shading. Not significant results are indicated with the abbreviation “n.s.” b Year-round composition of vegetation in core areas (A-LoCoH50%) dependent on activity period. Values are shown as medians of n = 9 raccoons and n = 5 red foxes
Red foxes and raccoons showed a strong nocturnal activity, which was closely linked to sunrise, while activity was extended into the early morning hours during summer (Figs. 1S and 2S). During resting time (sunrise to sunset), core areas (LoCoH 50%) of raccoons were mainly located in reed swamps and—to a much less extent—in shrub swamps. In contrast, core areas during activity period (sunset to sunrise) encompassed a smaller proportion of reed swamps and a higher portion of shrub swamps. Compared to raccoons, red foxes showed a high intraspecific variability in the use of habitat structures during resting period. While one individual spend most of its daytime in human settlements, other red foxes preferred deciduous wood, coniferous wood, or reed beds. During activity period, core areas of red foxes were also mainly located in woodland, human settlements, meadows, shifting pastures, and arable fields (Fig. 5b).
Interrelation between ground-nesting birds and carnivores
In total, we observed 1796 birds of 31 ground-nesting bird species with a significant (binominal test: p < 0.001) higher proportion of waterfowl (65.45%). Thirteen bird species have been observed only a few times (MacKinnon = 1–6), whereas the most common bird species were Greylag goose (Anser anser, MacKinnon = 45), Eurasian skylark (A. arvensis, MacKinnon = 40), and Great reed warbler (Acrocephalus arundinaceus, MacKinnon = 29) (Table S1).
On average, we observed 1.67 grassland birds and 5.0 waterfowl per hectare, which makes a total of 6.67 ground-nesting birds per hectare. However, in the core areas of the nine collared raccoons, we recorded more than the double (14.87) of bird observations per hectare with a significantly (binominal test: p < 0.001) higher proportion of waterfowl (13.88 observations per hectare) than grassland birds (0.99 observations per hectare) (Fig. 6). Since four red foxes either moved away or maintained their home ranges aside of swampland habitats, only one collared red fox remained in the area, where the collared raccoons were occurring and bird observations have been conducted. In the core areas of this single red fox, we recorded 8.3 bird observations per hectare, with a higher proportion of waterfowl than grassland birds (5.05 and 3.25 observations per hectare for waterfowl and grassland birds, respectively). On species level, highest relative overlap with red foxes’ core areas was found for Greylag goose (3.47 individuals per hectare) and European skylark (A. arvensis, 2.26 individuals per hectare). In raccoons’ core areas, highest relative overlap was found for Greylag goose (4.69 individuals per hectare), Savi’s warbler (Locustella luscinioides, 2.63), Nothern shoveler (Anas clypeata, 1.60), and Eurasian coot (Fulica atra, 1.32) (Fig. 6).
During nesting time, marked raccoons mostly preferred shrub swamps (median Ɛ = 0.58) and reed swamps (median Ɛ = 0.76), while red foxes preferred fallow (median Ɛ = 0.24) and also shrub swamp (median Ɛ = 0.29). Observed birds during nesting time preferred flooded grasslands (median Ɛ = 0.46) and reed swamps (median Ɛ = 0.20) as well as shrub swamps (median Ɛ = 0.06). Thus, largest number of detections of ground-nesting birds was also found in flooded grasslands (1.3 individuals per ha) and reed swamps (0.8 individuals per ha) (Fig. S1).
Discussion
Declining population trends of many ground-nesting bird species in Europe currently pose a high concern in nature conservation (Südbeck and Krüger 2004; Haupt et al. 2009). It is widely accepted that agricultural intensification plays a major role in this context (Hagemeijer and Blair 1997; Chamberlain et al. 2000; Donald et al. 2001; Robinson and Sutherland 2002; Haupt et al. 2009). However, the relationship between land use and the ongoing decline in ground-nesting bird numbers is complex. Several studies reveal a high predatory potential of mammalian carnivores (Carnivora, Mammalia) resulting in a reduced breeding and hatching success of ground-breeding bird species in different European countries (Crooks and Soulé 1999; Sanders and Maloney 2002; Engl et al. 2004; Südbeck and Krüger 2004). This problem is even more pronounced because several alien (and partly invasive) carnivore species, most importantly the raccoon (P. lotor), bear a considerable risk for increasing predation pressure on ground-breeders. However, studies dealing with the predatory potential of different medium-sized carnivore species (mesocarnivores) in Europe reveal that a native species—the red fox (V. vulpes) —is the most important predator of ground-nesting birds (Isaksson et al. 2007; Pedersen et al. 2009; Fletcher et al. 2010; Kämmerle et al. 2017; Roos et al. 2018).
Terrestrial carnivore species occurring in the MH-SPA
A total of 13 small- and medium-sized terrestrial carnivore species occur in Germany (Meining et al. 2009; Teubner et al. 2015). Camera- and live-trapping revealed that—except for wildcat (F. silvestris) and golden jackal (Canis aureus)—all of these species are also present in our study area. Among the carnivore species indirectly observed via camera traps, red fox and raccoon as well as the domestic cat (F. silvestris f. catus) clearly show highest levels of detection rates, at least in certain habitat types. Several studies draw special attention on the impact of domestic cats on songbird populations and breeding performance (Sanders and Maloney 2002; Woods et al. 2003; Tschanz et al. 2011). Accordingly, our study highlights the need for further studies on the predatory potential of domestic cats on ground-nesting birds in this area.
Red fox and raccoon in the MH-SPA
The camera- and live-trapping design was set up to detect mesocarnivores with a predatory potential for ground-nesting birds. It is thus not surprising that carnivore species with an ichthivorous feeding strategy (e.g., European otter (L. lutra)) (Webb 1975) as well as small species (e.g., weasels) were only sporadically observed. However, among the target (mesocarnivore) species European badger (M. meles), raccoon dog, red fox, and raccoon, the latter two species appear the main sources of predation risk for ground-nesting birds in the MH-SPA. High abundances of raccoons, red foxes, and domestic cats have also been reported from protected areas in close vicinity to our study area (Parker 2014; Wicke 2014). However, the level of detection of raccoon dogs (N. procyonoides) in our study is surprisingly low given that Parker (2014) reported raccoon dogs as the dominant mesocarnivore species. While raccoons showed the highest live-trapping success among all captured carnivore species, capturing red foxes with live traps turned out to be difficult although red foxes regularly inspected the live traps and were regularly observed in front of live traps via camera traps. This interspecific difference in the likeliness of capturing foxes and raccoons via live traps has also been reported in several other studies (Stiebling 2000; Janko et al. 2011; Börner 2014; Wicke 2014). Moreover, the remarkable level of dispersal activity in red foxes could lead to the assumption that some wide-ranging foxes may have been subordinates from previous years that were exhibiting dispersal activity. However, since we only collared animals with a minimum body mass of 5.7 kg, we consider all collared raccoons and red foxes older than 1 year. Since the adult, resident settled individuals are usually those most difficult to be captured, it is also possible that this study missed an important part of the red fox population and therefore underestimates the predatory potential of red foxes. On the other hand, in contrast to raccoons, camera trapping revealed very low trapping rates of red foxes in swampland areas, indicating low presence of red foxes in this habitat type.
Home range size and predatory potential of red foxes and raccoons on ground-nesting birds
The spatial dimensions of an animal’s home range and activity areas are strongly related with the quality of the respective habitat (Kenward 2000; McLoughlin et al. 2000). According to the resource dispersion hypothesis, the spatial scale of animal movement is strongly linked with the ecological diversity and availability of feeding resources within a habitat, as shown for different mesocarnivores (Macdonald 1983). This has also been proven for many taxa, including mammal herbivores (Tufto et al. 1996; Göttert et al. 2010), large carnivores (Powell et al. 1996; Mcloughlin et al. 2000), "reptiles" (Simon 1975), and birds (Hixon 1980). In areas of low resource availability, an animal has to move greater distances to find sufficient food, water, and shelter compared to areas of high resource availability (Kenward 2000). In Europe, the average home range size of red foxes varies from 14.2 ha in urban areas of Brighton (UK) (Tolhurst et al. 2016) up to 660 ha in less-structured rural areas of Finland (Kauhala et al. 2006). Average home ranges of raccoons vary between 129 ha in the city of Kassel (Michler et al. 2003) and 864 ha in agrarian landscapes of Brandenburg (Vorndran 2012) (Table 2). Besides the intraspecific variability as an expression of resource availability in different habitat types, these studies suggest similar spatial dimensions of home ranges of red foxes and raccoons. Accordingly, we found a similar level of the average home range size (KDE95%) for both species. Moreover, there is a very pronounced level of intraspecific variability in home range size for both species. The smallest home ranges are maintained by individuals spending most of their life in close distance to villages, which applies for red foxes and raccoons. Small home ranges in close vicinity to human settlements have also been reported from various other studies (Harris 1980; Gloor et al. 2001; Prange et al. 2004; Börner 2014).
Differences of home range use between red foxes and raccoons
To our knowledge, this is the first GPS telemetry study on red foxes and raccoons in the same study area and one of the first studies reporting on GPS telemetry-based home ranges of raccoons. This allowed investigating the spatial behavior of both species even on the micro-habitat level, recovering strong differences in the species’ movement behaviors: While raccoons showed a strong tendency to cluster along the fringes of water bodies, red foxes showed a rather even and more homogenous distribution pattern and different home range and core area features. We showed that comparing home range sizes of the two species using only KDE method on a level of 95% does not accurately reflect their actual spatial behavior. Thus, our data supports the importance of a combination of different home range and core area estimation methods (KDE and A-LoCoH) to capture the true spatial profile of the studied individuals. Moreover, while raccoons maintained a high degree of spatial overlap between individual home ranges over the entire study period, red foxes show a strong tendency to shift home ranges and core areas towards the northern border of the SPA. Against the background of this difference in the spatial dimensions of actual home ranges and core areas between red foxes and raccoons, this leads to a more differentiated assessment of their relation to ground-nesting birds.
Differential habitat preferences of red foxes and raccoons and potential impact on ground-nesting birds
Raccoons show a high affinity for water (Fritzell 1978; Sherfy and Chapman 1980; Winter et al. 2005; Fisher 2007; Hermes et al. 2011) and are most abundant in hardwood swamps, mangroves, flood forests, and marshes (Timm et al. 2015). Accordingly, our study reveals distinct activity clusters of raccoons located along the fringes of rivers and water bodies in the MH-SPA. In line with the camera trap data, GPS telemetry confirms a strong positive selection of raccoons for swampland habitats near water, while woodland (except for carrs) and open land were strongly avoided. A similar pattern has been shown by Hermes et al. (2011), reporting higher levels of raccoon abundance in swampland compared to open land and woodland. Hermes et al. (2011) and Gehrt and Fritzell (1998) argue that the preference of raccoons for water could be linked with a broad and year-round availability of water-associated food resources, such as fish, shells, and amphibians. Michler (2017) highlighted the high tactile abilities of raccoons, allowing them to fish for food and to occupy food niches in swampland habitat, which are not accessible to other mesocarnivore species. Besides these nutritional aspects, water-associated habitats, such as reed beds, also provide sufficient structures for resting and shelter seeking. This is supported by the preference of raccoons for dense reed swamps over shrub swamps during daytime in our study. It highlights the suitability of reed swamps as an important shelter-providing structure for raccoons (Wicke 2014). Also, the strong avoidance of open land habitats by raccoons in our study corresponds with literature data (Fritzell 1978; Sherfy and Chapman 1980; Glueck et al. 1988; Hohmann and Bartussek 2011). Thus, we suggest that raccoons pose a high predatory potential on ground-nesting birds in reed beds, while having a rather moderate potential impact on grassland birds (e.g., Eurasian skylark (A. arvensis) or Northern lapwing (Vanellus vanellus)). In contrast to raccoons and according to literature (Weber and Meia 1996; Janko et al. 2012), red foxes in our study show a moderate preference for woodland and swampland, suggesting a more even distributed predatory potential of red foxes when compared to raccoons. While camera trapping supports a high level of activity of red foxes in open land, GPS telemetry revealed the opposite. This is linked with the complexity and intraspecific variability in movement patterns of studied red foxes. Red foxes show a clearly different movement and spatial behavior when compared to the sedentary and highly predictable movement behavior of strictly water-associated raccoons.
High abundance of raccoons suggests a high level of sublethal effects on ground-nesting bird species
As discussed above, raccoons seem to pose the highest predatory pressure on waterfowl species in the MH-SPA. However, since raccoons maintained similar home ranges and movement patterns during the entire year, the water-associated spatial behavior of raccoons is more likely due to the high and year-round availability of aquatic food resources than due to the distribution of ground-nesting birds. However, the high abundance of raccoons in swampland habitats likely leads to a high predatory pressure for ground-nesting bird species during the season of breeding and rearing the young. This in turn can lead to changing behavior of different bird species, which might respond to high levels of predation risk by poor reproductive performance (Cresswell 2008; Peckarsky et al. 2008; Bonnington et al. 2013). Given the highly concentrated raccoon activity in swamplands, therefore simply the presence of raccoons could strongly deteriorate the habitat quality for ground-nesting birds in this habitat type. In contrast to this, a number of studies from Germany could not find evidence for negative effects of raccoons on ground-nesting birds (Engelmann et al. 2011; Michler and Michler 2012; Michler 2017). However, most of these studies have been conducted in the Müritz National Park and its surroundings: an area that contains large portions of riparian woodland where birds and raccoons find sufficient shelter and food such as mollusks and fruits (Engelmann et al. 2011). In the MH-SPA however, habitat types that appear suitable for raccoons and waterfowl are limited to narrow reed belts along the edges of water bodies as a result of grassland management. The cultivation of agricultural areas in close proximity to the edge of water is leading to relatively small habitat patches with high overlap rates of predators and their potential prey, generating an ecological trap that may even increase the level of sublethal effects of raccoons on ground-nesting birds in the reed belts of our study area. Accordingly, the high predatory potential of raccoons on ground-nesting birds, particularly waterfowl, in our study seems strongly related with the habitat characteristics and the land use practices in the MH-SPA. Thus, the predatory pressure caused by raccoons on ground-nesting birds may vary between different regions, limiting a transfer of these observations to other study sites. In order to better understand and further discuss our findings, it is also recommendable to perform detailed studies of feeding behaviors of raccoons and red foxes. Corresponding data on the diet of red foxes and raccoons have been collected and are currently under analysis.
In summary, our study reveals that raccoons in the SPA-MH show a high preference for the narrow reed beds along the edges of water bodies, causing a high level of sublethal effects on waterfowl species and in particular those breeding in reed beds. In the SPA-MH, the function of reed beds as an ecological trap for ground-breeders is especially pronounced, since grassland management restricts the expansion of reed beds (areas are managed until short before the edge of water bodies). For this reason, management measures for ground-breeding birds in the SPA should primarily focus on swampland habitats, including habitat improvements leading to larger areas with sufficient habitat for waterfowl. For example, decreasing the grazing intensity or restricting cattle from access to areas close to the edge of waters should already lead to an expansion of reed beds by natural succession. This in turn would directly translate into reducing the predatory pressure caused by raccoons on many ground-breeding bird species in this SPA. Furthermore, raccoons’ well-predictable movement behavior along the edges of water bodies results in a high susceptibility to management measures in swamplands. Data on movement of red foxes have to be interpreted carefully, as certain methodological limitations (low sample size, dispersal from study area) reduce the level of significance when compared to raccoons. However, our data suggest that red foxes in the SPA highly prefer woodland habitats and show lower preference for water-associated habitat types than raccoons. Since water-associated birds are among the most endangered bird species in this SPA (Rudolph 2005), we suppose that red foxes have a lower predatory potential on the ground-nesting birds in the SPA when compared to raccoons. In the SPA-MH, red foxes presumably pose the highest threat on grassland-associated bird species in close proximity to woodlands. In addition, considering the less predictable and skewed nature of the collared red foxes’ spatial behavior and movement patterns, we rather doubt that the attempt to sustainably regulate a red fox subpopulation in such an open system (unfenced SPA) is actually possible with justifiable efforts (e.g., sink effects). Consequently, we highlight the need to focus on the level of the carnivore species and habitat type when investigating the potential predatory impact of carnivores on ground-nesting birds. In addition, we also suggest that the predatory potential of carnivore species on ground-nesting birds is closely linked with availability of suitable habitat and intensity of agricultural land use practices.
References
Aebischer N (1991) Twenty years of monitoring invertebrates and weeds in cereal fields in Sussex. In: Firbank LG, Carter N, Darbyshire JF, Potts GR (eds) The ecology of temperate cereal fields. Blackwell Scientific Publications, Oxford, pp 305–331
Baines D (1990) The roles of predation, food and agricultural practice in determining the breeding success of the lapwing (Vanellus vanellus) on upland grasslands. J Anim Ecol 59:915–929. https://doi.org/10.2307/5022
Bartoszewicz M, Okarma H, Zalewski A, Szczęsna J (2008) Ecology of the raccoon (Procyon lotor) from Western Poland. Ann Zool Fenn 45:291–298. https://doi.org/10.5735/086.045.0409
Belant JL (1995) Field immobilization of raccoons with ketamine hydrochloride and xylazine hydrochloride. Acta Theriol 40:327–330. https://doi.org/10.4098/AT.arch.95-32
Bellebaum J (2002) Prädation als Gefährdung bodenbrütender Vögel in Deutschland - eine Übersicht. Vogelschutz 39:95–117
Bibby C, Burgess N, Hill D, British Trust for Ornithology, Royal Society for the Protection of Birds (2000) Bird census techniques. Serbiula
Blundell GM, Maier JAK, Debevec EM (2001) Linear home ranges: effects of smoothing, sample size, and autocorrelation on kernel estimates. Ecol Monogr 71:469–489. https://doi.org/10.1890/0012-9615(2001)071[0469:LHREOS]2.0.CO;2
Bonnington C, Gaston KJ, Evans KL (2013) Fearing the feline: domestic cats reduce avian fecundity through trait-mediated indirect effects that increase nest predation by other species. J Appl Ecol 50:15–24. https://doi.org/10.1111/1365-2664.12025
Börner K (2014) Untersuchungen zur Raumnutzung des Rotfuchses, Vulpes vulpes (L., 1758), in verschiedenen anthropogen beeinflussten Lebensräumen Berlins und Brandenburgs. Dissertation, Humboldt-Universität zu Berlin, Germany
Bradbury RB, Kyrkos A, Morris AJ, Clark SC, Perkins AJ, Wilson JD (2000) Habitat associations and breeding success of yellowhammers on lowland farmland. J Appl Ecol 37:789–805. https://doi.org/10.1046/j.1365-2664.2000.00552.x
Brickle NW, Harper DGC, Aebischer NJ, Cockayne SH (2000) Effects of agricultural intensification on the breeding success of corn buntings Miliaria calandra. J Appl Ecol 37:742–755. https://doi.org/10.1046/j.1365-2664.2000.00542.x
Burt WH (1943) Territoriality and home range concepts as applied to mammals. J Mammal 24:346–352. https://doi.org/10.2307/1374834
Byrne ME, Chamberlain MJ (2011) Seasonal space use and habitat selection of adult raccoons (Procyon lotor) in a Louisiana bottomland hardwood forest. Am Midl Nat 166:426–434. https://doi.org/10.1674/0003-0031-166.2.426
Calenge C (2006) The package “adehabitat” for the R software: a tool for the analysis of space and habitat use by animals. Ecol Model 197:516–519. https://doi.org/10.1016/j.ecolmodel.2006.03.017
Cavallini P (1996) Ranging behaviour of red foxes during the mating and breeding seasons. Ethol Ecol Evol 8:57–65. https://doi.org/10.1080/08927014.1996.9522935
Chamberlain DE, Fuller RJ, Bunce RGH, Duckworth JC, Shrubb M (2000) Changes in the abundance of farmland birds in relation to the timing of agricultural intensification in England and Wales. J Appl Ecol 37:771–788. https://doi.org/10.1046/j.1365-2664.2000.00548.x
Chesson J (1983) The estimation and analysis of preference and its relationship to foraging models. Ecology 64:1297–1304. https://doi.org/10.2307/1937838
Cowardin LM, Sargeant AB, Duebbert HF (1983) Problems and potentials for prairie ducks. Naturalist 34:411
Cresswell W (2008) Non-lethal effects of predation in birds. Ibis 150:3–17. https://doi.org/10.1111/j.1474-919X.2007.00793.x
Cristescu B, Bernard RTF, Krause J (2013) Partitioning of space, habitat, and timing of activity by large felids in an enclosed South African system. J Ethol 31:285–298. https://doi.org/10.1007/s10164-013-0376-y
Crooks KR, Soulé ME (1999) Mesopredator release and avifaunal extinctions in a fragmented system. Nature 400:563–566. https://doi.org/10.1038/23028
Dekker JJA, Stein A, Heitkönig IMA (2001) A spatial analysis of a population of red fox (Vulpes vulpes) in the Dutch coastal dune area. J Zool 255:505–510. https://doi.org/10.1017/S0952836901001583
Dixon KR, Chapman JA (1980) Harmonic mean measure of animal activity areas. Ecology 61:1040–1044
Donald PF, Evans AD, Muirhead LB, Buckingham DL, Kirby WB, Schmitt SIA (2002) Survival rates, causes of failure and productivity of skylark Alauda arvensis nests on lowland farmland. Ibis 144:652–664. https://doi.org/10.1046/j.1474-919X.2002.00101.x
Donald PF, Gree RE, Heath MF (2001) Agricultural intensification and the collapse of Europe’s farmland bird populations. Proc Biol Sci 268:25–29. https://doi.org/10.1098/rspb.2000.1325
Drygala F, Zoller H (2013) Spatial use and interaction of the invasive raccoon dog and the native red fox in Central Europe: competition or coexistence? Eur J Wildl Res 59:683–691. https://doi.org/10.1007/s10344-013-0722-y
Ellis JC, Shulman MJ, Jessop H, Suomala R, Morris SR, Seng V, Wagner M, Mach K (2007) Impact of raccoons on breeding success in large colonies of great black-backed gulls and herring gulls. Waterbirds 30:375–383. https://doi.org/10.1675/1524-4695(2007)030[0375:IOROBS]2.0.CO;2
Engelmann A, Köhnemann B, Michler F-UF (2011) Nahrungsökologische Analyse von Exkrementen gefangener Waschbären (Procyon lotor L., 1758) aus dem Müritz-Nationalpark (Mecklenburg-Vorpommern) unter Berücksichtigung individueller Parameter. Beitr Jagd- u Wildforsch 36:587–604
Engl M, Leibl F, Mooser K (2004) Bestandsentwicklung, Brutbiologie und Reproduktionserfolg des Großen Brachvogels Numenius arquata im Mettenbacher und Grießenbacher Moos. Kreis Landshut Ornithologischer Anzeiger 43:217–235
Fiero BC, Verts BJ (1986) Comparison of techniques for estimating age in raccoons. J Mammal 67:392–395
Fisher C (2007) Ecology of raccoons in central Alabama: a study of survival, space use, and habitat selection. Dissertation, Auburn University
Fletcher K, Aebischer NJ, Baines D, Foster R, Hoodless A (2010) Changes in breeding success and abundance of ground-nesting moorland birds in relation to the experimental deployment of legal predator control. J Appl Ecol 47:263–272. https://doi.org/10.1111/j.1365-2664.2010.01793.x
Frantz AC, Cyriacks P, Schley L (2005) Spatial behaviour of a female raccoon (Procyon lotor) at the edge of the species’ European distribution range. Eur J Wildl Res 51:126–130. https://doi.org/10.1007/s10344-005-0091-2
Fritzell EK (1978) Habitat use by prairie raccoons during the waterfowl breeding season. J Wildl Manag 42:118–127. https://doi.org/10.2307/3800698
Gehrt SD, Fritzell EK (1998) Resource distribution, female home range dispersion and male spatial interactions: group structure in a solitary carnivore. Anim Behav 55:1211–1227. https://doi.org/10.1006/anbe.1997.0657
Getz WM, Fortmann-Roe S, Cross PC, Lyons AJ, Ryan SJ, Wilmers C (2007) LoCoH: nonparameteric kernel methods for constructing home ranges and utilization distributions. PLoS One 2:e207. https://doi.org/10.1371/journal.pone.0000207
Gloor S, Bontadina F, Hegglin D, Deplazes P, Breitenmoser U (2001) The rise of urban fox populations in Switzerland. Mamm Biol 66:155–164
Glueck TF, Clark WR, Andrews RD (1988) Raccoon movement and habitat use during the fur harvest season. Wildl Soc Bull (1973-2006) 16:6–11
Göttert T, Schöne J, Zinner D, Hodges JK, Böer M (2010) Habitat use and spatial organisation of relocated black rhinos in Namibia. Mammalia 74(1):35–42. https://doi.org/10.1515/mamm.2010.012
Grant MC, Orsman C, Easton J, Lodge C, Smith M, Thomps G, Rodwell S, Moore N (1999) Breeding success and causes of breeding failure of curlew Numenius arquata in Northern Ireland. J Appl Ecol 36:59–74. https://doi.org/10.1046/j.1365-2664.1999.00379.x
Hagemeijer JM, Blair MJ (1997) The EBCC atlas of European breeding birds: their distribution and abundance. T & A D Poyser
Harris S (1980) Home ranges and patterns of distribution of foxes (Vulpes vulpes) in an urban area, as revealed by radio tracking. In: Amlaner C J A handbook on biotelemetry and radio tracking. Pergamon, pp 685–690
Haupt H, Ludwig G, Gruttke H, Binot-Hafke M, Otto C, Pauly A (2009) Rote Liste gefährdeter Tiere, Pflanzen und Pilze Deutschlands. Band 1: Wirbeltiere. Bundesamt für Naturschutz, Bonn-Bad Godesberg
Hermes N, Köhnemann BA, Michler FU, Roth M (2011) Radiotelemetrische Untersuchungen zur Habitatnutzung des Waschbären (Procyon lotor L., 1758) im Müritz-National Park. Beitr Jagd- u Wildforsch 36:557–572
Heske E, Robinson SD, Brawn J (2001) Nest predation and Neotropical migrant songbirds: piecing together the fragments. Wildl Soc Bull 29:52–61. https://doi.org/10.2307/3783980
Hixon MA (1980) Food production and competitor density as the determinants of feeding territory size. Am Nat 115:510–530. https://doi.org/10.1086/283577
Hohmann U, Gerhard R, Kasper M (2000) Home range size of adult raccoons (Procyon lotor) in Germany. Z Säugetierk 65:124–127
Hohmann U, Bartussek I (2011) Der Waschbär. Oertel & Spörer, Reutlingen
Hudson AV, Stowe TJ, Aspinall SJ (1990) Status and distribution of corncrakes in Britain in 1988. British Birds 83:173–187
Isaksson D, Wallander J, Larsson M (2007) Managing predation on ground-nesting birds: the effectiveness of nest exclosures. Biol Conserv 136:136–142. https://doi.org/10.1016/j.biocon.2006.11.015
Janko C, Börner K, König A, Schneider R (2011) Fang, Immobilisation und Besenderung von Rotfüchsen (Vulpes vulpes L.) - ein Erfahrungsbericht aus der Praxis. Beitr Jagd- u Wildforsch 36:265–275
Janko C, Schroder W, Linke S, Konig A (2012) Space use and resting site selection of red foxes (Vulpes vulpes) living near villages and small towns in Southern Germany. Acta Theriol 57:245–250. https://doi.org/10.1007/s13364-012-0074-0
Jenks KE, Chanteap P, Kanda D, Peter C, Cutter P, Redford T, Antony JL, Howard J, Leimgruber P (2011) Using relative abundance indices from camera-trapping to test wildlife conservation hypotheses—an example from Khao Yai National Park, Thailand. Trop Conserv Sci 4:113–131. https://doi.org/10.1177/194008291100400203
Johnson DH, Nichols JD, Schwartz MD (1992) Population dynamics of breeding waterfowl. In: Batt BDJ, Afton AD, Anderson MG, Ankney CD, Johnson DH, Kadlec JA, Krapu GL (eds) Ecology and management of breeding waterfowl. University of Minnesota Press, Minneapolis, pp 446–485
Johnson DH, Sargeant AB, Greenwood RJ (1989) Importance of individual species of predators on nesting success of ducks in the Canadian Prairie Pothole Region. Can J Zool 67:291–297. https://doi.org/10.1139/z89-043
Kämmerle J-L, Coppes J, Ciuti S, Suchant R, Storch I (2017) Range loss of a threatened grouse species is related to the relative abundance of a mesopredator. Ecosphere 8:e01934. https://doi.org/10.1002/ecs2.1934
Kaphegyi T (2002) Untersuchungen zum Sozialverhalten des Rotfuchses (Vulpes vulpes L.). Dissertation, Albert-Ludwigs Universität Freiburg i Brsg, Germany
Kauhala K (2004) Removal of medium-sized predators and the breeding success of ducks in Finland. Folia Zool 53:367–378
Kauhala K, Holmala K, Lammers W, Schregel J (2006) Home ranges and densities of medium-sized carnivores in south-east Finland, with special reference to rabies spread. Acta Theriol 51:1–13. https://doi.org/10.1007/BF03192650
Kenward RE (2000) A manual for wildlife radio tagging, 2nd edn. Academic Press, San Diego, CA; London
Krebs CJ (1989) Ecological methodology. Addison Wesley, Menlo Park, Calif
Kube J, Brenning U, Kruch W, Nehls HW (2005) Bestandsentwicklung von bodenbrütenden Küstenvögeln auf Inseln in der Wismar-Bucht (südwestliche Ostsee): Lektionen aus 50 Jahren Prädatorenmanagement. Vogelwelt 126:299–320
Küster H (2007) Die Elbe: Landschaft und Geschichte, 1st edn. C.H. Beck, München
Langgemach T, Bellebaum J (2005) Prädation und der Schutz bodenbrütender Vogelarten in Deutschland. Vogelwelt 126:259–298
Laver PN, Kelly MJ (2008) A critical review of home range studies. J Wildl Manag 72:290–298. https://doi.org/10.2193/2005-589
Lavrov NP (1971) The results of reintroductions of the raccoon dog (Nyctereutes procyonoides) in different provinces of the USSR. Trudy kafedry biologii MGZPI 29:101–160
Litzbarski H (1998) Prädatorenmanagement als Artenschutzstrategie. Natursch Landschaftspfl Brandenburg 7:92–97
LUGV (Landesamt für Umwelt, Gesundheit und Verbraucherschutz) (2013) Flächendeckende Biotop- und Landnutzungskartierung im Land Brandenburg (BTLN) CIR-Biotoptypen
Macdonald DW (1983) The ecology of carnivore social behaviour. Nature 301:379–384. https://doi.org/10.1038/301379a0
MacDonald MA, Bolton M (2008) Predation on wader nests in Europe. Ibis 150:54–73. https://doi.org/10.1111/j.1474-919X.2008.00869.x
MacKinnon J, Phillipps K, Andrew P (1993) A field guide to birds of Borneo, Sumatra, Java and Bali: the Greater Sunda Islands. Oxford University Press, Oxford; New York
MacLeod R, Herzog SK, Maccormick A, Ewing SR, Bryce R, Evans KL (2011) Rapid monitoring of species abundance for biodiversity conservation: consistency and reliability of the MacKinnon lists technique. Biol Conserv 144:1374–1381. https://doi.org/10.1016/j.biocon.2010.12.008
Manly BFJ, Miller P, Cook LM (1972) Analysis of a selective predation experiment. Am Nat 106:719–736
McLoughlin PD, Ferguson SH, Messier F (2000) Intraspecific variation in home range overlap with habitat quality: a comparison among brown bear populations. Evol Ecol 14:39–60. https://doi.org/10.1023/A:1011019031766
Meek PD, Ballard G, Claridge A, Kays R, Moseby K, O’Brien T, O’Connell A, Sanderson J, Swann DE, Tobler M, Townsend S (2014) Recommended guiding principles for reporting on camera trapping research. Biodivers Conserv 23:2321–2343. https://doi.org/10.1007/s10531-014-0712-8
Meining H, Boye P, Hutterer R (2009) Rote Liste und Gesamtartenliste der Säugetiere (Mammalia) Deutschlands. In: Rote Liste gefährdeter Tiere, Pflanzen und Pilze Deutschlands, Bd. 1 Wirbeltiere. Bundesamt für Naturschutz, pp 115–153
Michler BA (2017) Koproskopische Untersuchungen zum Nahrungsspektrum des Waschbären Procyon lotor (Linné, 1758) im Müritz-Nationalpark (Mecklenburg-Vorpommern) unter spezieller Berücksichtigung des Artenschutzes und des Endoparasitenbefalls. Dissertation, Technische Universität Dresden, Germany
Michler FU (2016) Säugetierkundliche Freilandforschung zur Populationsbiologie des Waschbären (Procyon lotor Linnaeus, 1758) in einem naturnahen Tieflandbuchenwald im Müritz-Nationalpark (Mecklenburg-Vorpommern). Dissertation, Technische Universität Dresden, Germany
Michler FU, Hohmann U, Stubbe M (2003) Aktionsräume, Tagesschlafplätze und Sozialsystem des Waschbären (Procyon lotor Linné 1758) im urbanen Lebensraum der Großstadt Kassel (Nordhessen). Beitr Jagd- u Wildforsch 29:257–273
Michler FU, Michler BA (2012) Ökologische, ökonomische und epidemiolgische Bedeutung des Waschbären (Procyon lotor) in Deutschland – eine aktuelle Übersicht. Beitr Jagd- u Wildforsch 37:389–397
Michler FU, Michler BA, Rieger S, Stubbe M, Roth M (2015) Medikamentöse Feldimmobilisation von Waschbären (Procyon lotor) mit Ketamin- und Xylazinhydrochlorid. Beitr Jagd- u Wildforsch 40:45–56
Milsom TP (2010) Decline of northern lapwing Vanellus vanellus breeding on arable farmland in relation to loss of spring tillage: capsule A decrease in the area of spring tillage was not the primary cause of a decline to extinction of a local breeding population of lapwing. Bird Study November 1:297–306. https://doi.org/10.1080/00063650509461403
Mohr CO (1947) Table of equivalent populations of North American small mammals. Am Midl Nat 37:223–249. https://doi.org/10.2307/2421652
Nasimovič AA, Isakov JA (1985) Arctic fox, red fox and raccoon dog: distribution of populations, ecology and preservation. Nauka, Moscow: 116–145.
Naveh Z (1998) From biodiversity to ecodiversity—holistic conservation of the biological and cultural diversity of Mediterranean landscapes. In: Rundel P, Montenegro G, Jaksic F (eds) Landscape disturbance and biodiversity in Mediterranean-type ecosystems. Springer, Berlin, Heidelberg, pp 23–53
Nechay G (2000) Status of hamsters Cricetus cricetus, Cricetus migratorius, Mesocricetus newtoni, and other hamster species in Europe. Council of Europe Pub
Newton I (1993) Predation and limitation of bird numbers. In: Power DM (ed) Current ornithology. Springer US, Boston, MA, pp 143–198
O’Brien TG, Kinnaird MF, Wibisono HT (2003) Crouching tigers, hidden prey: Sumatran tiger and prey populations in a tropical forest landscape. Anim Conserv 6:131–139. https://doi.org/10.1017/S1367943003003172
Pandolfi M, Forconi P, Montecchiari L (1997) Spatial behaviour of the red fox (Vulpes vulpes) in a rural area of central Italy. Ital J Zool 64:351–358. https://doi.org/10.1080/11250009709356222
Parker K (2014) Utilizing camera traps to evaluate the effects of artificial enclosures to ground-breeding bird species and their predators on Special Protection Areas in Brandenburg, Germany. Master thesis, Texas Tech University
Pebsworth PA, MacIntosh AJJ, Morgan HR, Huffman MA (2012) Factors influencing the ranging behavior of chacma baboons (Papio amadryas ursinus) living in a human-modified habitat. Int J Primatol 33:872–887. https://doi.org/10.1007/s10764-012-9620-5
Peckarsky BL, Abrams PA, Bolnick DI, Dill LM, Grabowski J, Luttbeg B, Orrock JL, Peacor SD, Preisser EL, Schmitz O, Trussell G (2008) Revisiting the classics: considering nonconsumptive effects in textbook examples of predator–prey interactions. Ecology 89:2416–2425. https://doi.org/10.1890/07-1131.1
Pedersen ÅØ, Yoccoz NG, Ims RA (2009) Spatial and temporal patterns of artificial nest predation in mountain birch forests fragmented by spruce plantations. Eur J Wildl Res 55:371–384. https://doi.org/10.1007/s10344-009-0253-8
Poole KG (1995) Spatial organization of a lynx population. Can J Zool 73:632–641. https://doi.org/10.1139/z95-074
Powell RA, Zimmerman JW, Seaman DE, Powell C (1996) Ecology and behaviour of North American black bears: home ranges, habitat and social organization. Springer, Netherlands
Prange S, Gehrt SD, Wiggers EP (2004) Influences of anthropogenic resources on raccoon (Procyon lotor) movements and spatial distribution. J Mammal 85:483–490. https://doi.org/10.1644/BOS-121
R Core Team (2017) A language and environment for statistical computing
Robinson RA, Sutherland WJ (2002) Post-war changes in arable farming and biodiversity in Great Britain. J Appl Ecol 39:157–176. https://doi.org/10.1046/j.1365-2664.2002.00695.x
Roos S, Smart J, Gibbons DW, Wilson JD (2018) A review of predation as a limiting factor for bird populations in mesopredator-rich landscapes: a case study of the UK. Biol Rev 93:1915–1937. https://doi.org/10.1111/brv.12426
Rudolph B (2005) Das Europäische Vogelschutzgebiet (SPA) Mittlere Havelniederung. Natursch Landschaftspfl Brandenburg 3(4):130–133
Rushton SP, Luff ML, Eyre MD (1989) Effects of pasture improvement and management on the ground beetle and spider communities of upland grasslands. J Appl Ecol 26:489–503. https://doi.org/10.2307/2404076
Salo P, Korpimäki E, Banks PB, Nordström M, Dickman C (2007) Alien predators are more dangerous than native predators to prey populations. Proc R Soc Lond B Biol Sci 274:1237–1243. https://doi.org/10.1098/rspb.2006.0444
Sanders MD, Maloney RF (2002) Causes of mortality at nests of ground-nesting birds in the upper Waitaki Basin, South Island, New Zealand: a 5-year video study. Biol Conserv 106:225–236. https://doi.org/10.1016/S0006-3207(01)00248-8
Sargeant AB, Greenwood RJ, Sovada MA, Shaffer TL (1993) Distribution and abundance of predators that affect duck production—prairie pothole region. U.S. Fish and Wildlife Service, Washington, D.C.
Schai-Braun SC, Weber D, Hackländer K (2013) Spring and autumn habitat preferences of active European hares (Lepus europaeus) in an agricultural area with low hare density. Eur J Wildl Res 59:387–397. https://doi.org/10.1007/s10344-012-0684-5
Schmidt KA (2003) Nest predation and population declines in Illinois songbirds: a case for mesopredator effects. Conserv Biol 17:1141–1150. https://doi.org/10.1046/j.1523-1739.2003.02316.x
Sherfy FC, Chapman JA (1980) Seasonal home range and habitat utilization of raccoons in Maryland. Carnivore 3:8–18
Sikes RS (2016) 2016 guidelines of the American Society of Mammalogists for the use of wild mammals in research and education. J Mammal 97:663–688. https://doi.org/10.1093/jmammal/gyw078
Simon CA (1975) The influence of food abundance on territory size in the iguanid lizard Sceloporus jarrovi. Ecology 56:993–998. https://doi.org/10.2307/1936311
Smith RK, Vaughan Jennings N, Harris S (2005) A quantitative analysis of the abundance and demography of European hares Lepus europaeus in relation to habitat type, intensity of agriculture and climate. Mammal Rev 35:1–24. https://doi.org/10.1111/j.1365-2907.2005.00057.x
Sørensen T (1948) A method of establishing group of equal amplitude in plant sociobiology based on similarity of species content and its application to analyses of the vegetation on Danish commons. Biol Skrifter 5:1–34
Sotherton N, Self MJ (2000) Changes in plant and arthropod diversity on lowland farmland: an overview: in the ecology and conservation of lowland farmland birds. British Ornithologists’ Union 26–35
Starik N (2016) Fledermäuse als Bioindikatoren für die ökologischen Auswirkungen verschiedener Landnutzungsformen auf Biodiversität. Dissertation, Humboldt-Universität zu Berlin, Germany
Stiebling U (2000) Untersuchungen zur Habitatnutzung des Rotfuchses, Vulpes vulpes (L., 1758), in der Agrarlandschaft als Grundlage für die Entwicklung von Strategien des Natur- und Artenschutzes sowie der Tierseuchenbekämpfung. Dissertation, Humboldt-Universität zu Berlin, Germany
Stubbe M (1989) Buch der Hege. Band 1. Haarwild. Landwirtschaftsverlag, Berlin
Südbeck P, Krüger T (2004) Erhaltungssituation und erforderliche Schutzmaßnahmen für Wiesenvögel in Niedersachsen - Bilanz und Ausblick. Natursch Landschaftspfl Niedersachsen 41:106–123
Teubner J, Teubner J, Zscheile K (2015) Nachweise des Goldschakals (Canis aureus) in Nordostdeutschland. Beitr Jagd- u Wildforsch 40:452–455
Timm R, Cuarón AD, Reid F, Helgen K, Gonzáles-Maya JF (2015) Procyon lotor: the IUCN red list of threatened species 2016
Tolhurst B, Grogan A, Hughes H, Scott D (2016) Effects of temporary captivity on ranging behaviour in urban red foxes (Vulpes vulpes). Appl Anim Behav Sci 181:182–190. https://doi.org/10.1016/j.applanim.2016.05.004
Travaini A, Ferreras P, Delibes M, Aldama JJ (1992) Xylazine hydrochloride-ketamine hydrochloride immobilization of free-living red foxes (Vulpes vulpes) in Spain. J Wildl Dis 28:507–509. https://doi.org/10.7589/0090-3558-28.3.507
Tschanz B, Hegglin D, Gloor S, Bontadina F (2011) Hunters and non-hunters: skewed predation rate by domestic cats in a rural village. Eur J Wildl Res 57:597–602. https://doi.org/10.1007/s10344-010-0470-1
Tufto J, Andersen R, Linnell J (1996) Habitat use and ecological correlates of home range size in a small cervid: the roe deer. J Anim Ecol 65:715–724. https://doi.org/10.2307/5670
Van Winkle W (1975) Comparison of several probabilistic home-range models. J Wildl Manag 39:118–123. https://doi.org/10.2307/3800474
Vorndran M (2012) Home range size and habitat choice of the raccoon (Procyon lotor)—a GPS tracking study in the north of Brandenburg. Johannes Gutenberg Universität Mainz, Germany
Wandeler AI, Lüps P (1993) Vulpes vulpes - Rotfuchs. In: Stubbe, M. und Krapp, F. (Eds.): Handbuch der Säugetiere Europas, 1st edn. AULA-Verlag, Wiesbaden, pp 139–193
Webb JB (1975) Food of the otter (Lutra lutra) on the Somerset levels. J Zool 177:486–491. https://doi.org/10.1111/j.1469-7998.1975.tb02249.x
Weber J-M, Meia J-S (1996) Habitat use by the red fox Vulpes vulpes in a mountainous area. Ethol Ecol Evol 8:223–232. https://doi.org/10.1080/08927014.1996.9522914
White GC, Garrott RA (1990) Analysis of wildlife radio-tracking data. Academic Press Inc, San Diego
White PCL, Saunders G, Harris S (1996) Spatio-temporal patterns of home range use by foxes (Vulpes vulpes) in urban environments. J Anim Ecol 65:121–125. https://doi.org/10.2307/5705
Wicke M (2014) Auswirkungen der Schutzmaßnahmen für bodenbrütende Vögel auf das Raumnutzungsverhalten ihrer wichtigsten Prädatoren im NSG Havelländisches Luch. Dissertation, Humboldt-Universität zu Berlin, Germany
Wiesner H, von Hegel G (1985) Praktische Hinweise zu Immobilisation von Wild- und Zootieren. Tierarztl Prax:113–127
Winter M, Stubbe M, Heidecke D (2005) Zur Ökologie des Waschbären (Procyon lotor L., 1758) in Sachsen-Anhalt. Beitr Jagd- u Wildforsch 30:303–322
Woods M, Mcdonald R, Harris S (2003) Predation of wildlife by domestic cats Felis catus in Great Britain. Mammal Review Blackwell Science 33:174–188. https://doi.org/10.1046/j.1365-2907.2003.00017.x
Worton BJ (1989) Kernel methods for estimating the utilization distribution in home-range studies. Ecology 70:164–168. https://doi.org/10.2307/1938423
Worton BJ (1995) Using Monte Carlo simulation to evaluate kernel-based home range estimators. J Wildl Manag 59:794–800. https://doi.org/10.2307/3801959
Zeller U, Starik N, Göttert T (2017) Biodiversity, land use and ecosystem services—an organismic and comparative approach to different geographical regions. Glob Ecol Conserv 10:114–125. https://doi.org/10.1016/j.gecco.2017.03.001
Acknowledgments
We thank the agricultural cooperative of the village of Gortz and the hunting societies of Gortz, Ketzür, Bagow, Linde, and Lünow for providing access to the study areas and for supporting this study by sharing their experiences. Special thank go to R. Falke, P. Bengsch, D. Wagener, S. Jurischka, O. Schröder, R. Mattes, M. Hasselbach, and H. Kühne. Furthermore, we thank Dr. T. Langgemach from the ornithological station in Buckow and Dr. M. Wicke from the research station Linde as well as Dr. N. Starik and T. Rottstock for supporting this study with their advice. Special thanks go to the Foundation Zwillenberg-Tietz Stiftung for funding this project and to the research station Linde for providing accommodation and various materials. This study was conducted under the permit of the Ministry of Environment, Health and Consumer Protection (file no. 2347-26-2015).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 9945 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Fiderer, C., Göttert, T. & Zeller, U. Spatial interrelations between raccoons (Procyon lotor), red foxes (Vulpes vulpes), and ground-nesting birds in a Special Protection Area of Germany. Eur J Wildl Res 65, 14 (2019). https://doi.org/10.1007/s10344-018-1249-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10344-018-1249-z