Abstract
Feces of wildlife species are commonly used indicators for species’ presence or relative abundance. These might however be biased due to inconspicuousness, due to rapid decay and disappearance, or due to high site-dependent variance in decay dynamics. Mapping of indirect signs is a frequently applied approach to study habitat use or distribution of grouse species. However, only a few studies addressed avian dropping decay up to now, and no study focused on dropping decay of European grouse species. Consequently, we conducted field surveys and greenhouse trials, studying time spans over which capercaillie (Tetrao urogallus) droppings persist and factors influencing the decay rates (i.e., habitat type, small mammal access, and precipitation regime). In the field survey, after an exposure of 98 days, only 6% of droppings (n = 156) decayed completely and most droppings remained nearly unchanged. The decay rate was influenced by microsite conditions (i.e., vegetation and location), with lowest decay rates in areas with little ground vegetation or on tree stubs. Destruents were not found to play a major role in affecting decay rate. The greenhouse trial revealed the impact of precipitation on the decay rate of droppings (n = 400): under high-amount and high-intensity precipitation regime droppings decayed faster compared to low-amount and low-intensity precipitation. The slow decay rate, and resulting long time period that they can be detected, therefore means that capercaillie droppings are a valid proxy for species’ occurrence.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Indirect signs of wildlife species like dens, tracks, roosts, or feces are commonly used indicators in wildlife ecology being a proxy for species’ presence or relative abundance (Bull 1981; Borchers et al. 2002). In general, such proxies frequently hold a higher degree of uncertainty than direct measures (Seber 2002), however, they are of particular interest for secretive species (Wallmo et al. 1962; Putman 1984; Laing et al. 2003). For those species, high variability in direct observation probabilities might distinctly constrain the acquisition of absolute data (Dawson 1981), whereas systematic surveys of indirect signs might provide more valuable estimates of elusive species’ presence (Kohn and Wayne 1997). Apart from being an indicator of species’ presence and habitat use (Zwickel and Bendell 2004), feces provide information on the diet of species and undigested remains may indicate presence of prey species in an area (Kohn and Wayne 1997; Bang and Dahlstrøm 2001).
Nonetheless, estimated data of presence deviates from absolute abundance due to biases that are difficult to define (Temple 1981). As droppings can persist for some time, mapping of the current distribution might also involve droppings, which had been deposited several months ago, thereby leading to a divergent distribution of recorded data and actual presence (Marques et al. 2001). Hence, for the pellet group count technique, additional studies are conducted to minimize these biases and to provide more reliable data (Neff 1968; Laing et al. 2003). The knowledge of how often a species defecates and how long the feces need to decompose simplifies projections on population size (Laing et al. 2003). However, these rates might be misleading due to seasonal and regional differences (Laing et al. 2003; Prugh and Krebs 2004). A variety of factors has been analyzed which affect decay rate of droppings (e.g., Harestad and Bunnell 1987; Iborra and Lumaret 1997; Fernandez-de-Simon et al. 2011) partially constraining the transfer of decay rates for one species at a given location to other circumstances (Prugh and Krebs 2004; Fernandez-de-Simon et al. 2011; Jones 2017). Hitherto, studies on feces decay strongly focused on mammalian species, including ungulates, lagomorphs, or elephants (e.g., Lehmkuhl et al. 1994; Marques et al. 2001; Fernandez-de-Simon et al. 2011; Vanleeuwe and Probert 2014). Contrary, only a few studies addressed avian dropping decay (i.e., ring-necked pheasants Phasianus colchicus, McClure 1945; wild geese species Anser sp. and Branta sp., Kear 1963; and Greater sage-grouse Centrocercus urophasianus, Wik 2002).
The Western capercaillie (Tetrao urogallus) is a large bird species. Due to its large distribution range, it is not globally endangered (BirdLife-International 2012). However, many populations are decreasing in Central Europe, resulting in endangered local populations (Storch 2007; Coppes et al. 2016) and making it a relevant species for species protection programs (Braunisch and Suchant 2013). Due to its elusive behavior, the main method to address capercaillie’s occurrence is searching for indirect signs, which include tracks, sand baths, feathers, and droppings (Klaus et al. 1989). Droppings are one of the most commonly used indirect signs, and it is assumed that they persist long enough to be used as indicator of year-round presence and habitat use (Storch 1999; Braunisch and Suchant 2007; Zohmann et al. 2014). This assumption however has not been validated up to now.
In general, decomposition of plant material bases on three main processes (Mason 1976; Swift et al. 1979): The first process is leaching, an abiotic process whereby rain or water runoff rapidly dissolves soluble matter from dead organic material. The second is weathering, in which physical factors like abrasion by wind break down organic material mechanically. The third main process is biological action: a step-by-step fragmentation and oxidation by living organisms (Mason 1976). All three decomposition processes are influenced by the physical-chemical environment, the resource quality (size, shape, soft/hard material, etc.), and decomposers’ community. Although these processes were described for litter decomposition, they might be transferred to decay processes (Jones 2017). Intestinal droppings of grouse species represent a conglomerate of hardly soluble compounds with high contents of coarse fibers (Watson and Moss 2008), thus resembling soil litter at the point of defecation.
Addressing these decomposition processes, we combined field surveys with greenhouse trials, studying the time capercaillie droppings persist as well as possible impacts on decay rates. Thereby, we addressed the following questions: (1) Do decay dynamics of T. urogallus droppings differ between different, naturally occurring microsite conditions? (2) Do specific destruent guilds (in particular small mammals) contribute to dropping decay in nature? (3) Are there any differences in decay dynamics for summer or winter droppings? (4) What effect has a winter season on dropping decay? (5) How do precipitation patterns (total amount and intensity) impact decay of summer and winter droppings under greenhouse conditions?
Material and methods
Study area of the field trial
In the field trial, we surveyed decay processes of intestinal capercaillie droppings at a west to northwest exposed mountain slope at the northern edge of the eastern central Alps. The experimental sites belong to the Austrian Federal Forests in Styria. The experimental sites lay at an altitude of 1488 m a.s.l. with a slight slope of 0°–25° (GIS Steiermark 2017), which has been shown to be preferred by capercaillie in German and Swiss studies (Storch 2002; Bollmann and Graf 2008). Bedrock is a silicate rock with different types of soil like podzolic brown earth or semi-podzols occurring. Forests are dominated by Norway spruce (Picea abies) with scattered occurrences of larch (Larix decidua).
The annual average precipitation varies from 1250 to 1500 mm, showing a maximum in July (Kilian et al. 1994). The relative sunshine duration in midsummer amounts 42–46% (actual sunshine duration July 6–8 h), in midwinter 40–45% (actual sunshine duration January 2–4 h), respectively. The area is characterized by cold winters (average temperature in January of − 3 to − 2 °C) and cool summers (average temperature in July around 12 °C). Snow cover starts between October 16 and 30 and ends in May (9 to 23). The duration of the vegetation period ≥ 5 °C is 155–185 days, whereas for ≥ 10 °C, it is only between 50 and 100 days (Prettenthaler et al. 2010). Capercaillie permanently lives in the study area (Zohmann et al. 2014).
Decay dynamics
Based on Laing et al. (2003), decay is defined as the disappearance of droppings without considering the mechanisms by which the process occurs. Decay also means that the dropping is broken down into single components (Mason 1976). In this study, decay is also related to the decrease in detectability of droppings, meaning that droppings cannot be found anymore, e.g., due to overgrow by mosses.
Capercaillie diet varies throughout the year (Heinemann 1989; Klaus et al. 1989; Summers et al. 2004): during winter, the birds feed almost exclusively on conifer needles, whereas during summer, a wide variety of plants are taken as food. Based on this difference in content and difference in seasonal appearance, we classified all droppings as either winter droppings (i.e., cylindrical, conifer needle dominated) or summer droppings (i.e., more variable shape and complex composition). In contrast to winter droppings, fresh summer droppings are partially coated with uric acid (Zwickel and Bendell 2004). We used both fresh summer droppings, collected during the vegetation period of the field trial, and also stored intestinal summer and winter droppings gained from preceding surveys on different study areas in Styria and Carinthia (covering the summer months of the years 2014–2016).
For the classification of decay, we defined five distinct stages after Vanleeuwe and Probert (2014) and additionally introduced transient stages between the five initial classes (Table 1, see also ESM). Based on long-term field experience, we assumed that droppings in decay stages A to tD are likely to be detected when mapping capercaillie droppings in a field survey and stages D, TE, and E are likely to be missed when mapping capercaillie signs.
Setting of the field trial
To study the impact of forest structure, ground vegetation, and the referring microclimate on dropping decay, we exposed droppings at seven different types of habitat patches potentially visited by capercaillie under natural conditions (Table 2). The main tree species on all habitat patches was Picea abies.
To separate potential impacts of invertebrate as well as vertebrate destruents on decay dynamics (Swift et al. 1979; Sanchez et al. 2004; Vanleeuwe and Probert 2014), we used two types of exclosures (10 cm high, 7–10 cm ø, see Nopp-Mayr et al. 2012): (1) a 12.7 × 12.7 mm wire mesh offering access only for invertebrates (mainly arthropods and gastropods) and (2) a 25 × 50 mm wire mesh allowing access for small vertebrates as well, but impeding a loss of experimental droppings due to gravity and protecting the droppings from trampling by other animals (Prugh and Krebs 2004). At each habitat patch (Table 2), 20 droppings were experimentally exposed at the beginning of July 2016 with ten summer and ten winter droppings, respectively. We placed five replicates of the two exclosure types at each habitat patch with a distance of approx. 1 m between the exclosures. At habitat patches “Vaccinium” and “Timber > 60%,” three more summer droppings were exposed (in total 26 samples each) as blueberry droppings, consisting of almost 100% of blueberries, were also tested. In total, 156 droppings were surveyed in the field trial. Droppings were monitored in the morning once per week (Laing et al. 2003) to avoid missing new signs of decay. We controlled fresh droppings from the recent year (stage A) every day at the beginning of the survey, as we expected them to decompose faster (see Swift et al. 1979) than older droppings. We conducted the last records in autumn on the 14 October 2016 and fixed the exclosures with tent pegs to the ground to avoid dislocation and loss of droppings due to snow coverage and snow movement on the 21 October 2016 after the first snow fall (approx. 20 cm). We recorded the final stage of droppings after winter season on the 8 June 2017. At each control date, we assigned each experimental dropping to a decay stage and took a photo. Precipitation data for the months July to October 2016 were provided by the Zentalanstalt für Meteorologie und Geodynamik (ZAMG).
Greenhouse trial
As precipitation is presumed to have the greatest influence on decomposition (Fernandez-de-Simon et al. 2011), we run a greenhouse experiment exploring dropping decay under controlled climatic conditions without impact of destruents and vegetation cover at a constant temperature of 23 °C. Both winter and summer droppings (50 each) were placed on tables that were covered with 1 m2 of commercial potting soil for each rain event. To simulate the natural acidic ground of coniferous forests, we chose potting soil containing peat as substrate. We classified all experimental droppings as stage B as we started the greenhouse trial in October, when fresh summer droppings were not available.
We simulated four precipitation regimes, using precipitation data (July–September) from two weather stations adjacent to the study area of the field trial (i.e., Hirschenkogel and Rax), varying the amount of rainfall in millimeters and the number of rainy days: precipitation patterns of 2014, representing a year with much rain (261 mm) and 2015 with less rain (179.5 mm); we further simulated two intensities of precipitation, i.e., light vs. heavy rainfall events. To simulate light rainfall events, we sprinkled the droppings with a watering can, and for heavy rainfall simulation, we used a shower-watering wand. For all four regimes, sprinkling of droppings was done at random intervals (ranging from three consecutive days up to 10-day intervals). We averaged the amount of recorded precipitation and the numbers of days with rain per month from the weather stations, calculated an amount of precipitation per day, and rounded up or down to centiliters (i.e., 2014, 4.2 l for 21 days, 2015, 5.3 l for 12 days). To approximate natural patterns of slight rainfall events, we split the sprinkling with the watering can into two distinct rounds per day. The break during both sprinkling events varied between 2 or 5 h. In total, we placed 400 droppings on greenhouse tables with 200 samples for each year and 100 samples for every rainfall event. The greenhouse trial was run for 165 days.
Statistical analyses
We calculated the progress of decay according to Wik (2002), referring the number of droppings still visible at a given visit to the initial number at the beginning of the trial. We analyzed these proportions with contingency analyses (Grafen and Hails 2002). We further analyzed the days passed from the initial stage of a dropping (at the time of artificial exposure) until the next stage (hereafter “total duration”) and the time that passed between the different stages (hereafter “transition time”). Due to non-normality of duration data (Karp 2010), we run non-parametric statistical tests (Kruskal-Wallis test and post hoc Mann-Whitney U test with Bonferroni-Holm correction, Sachs and Hedderich 2006). Simultaneously addressing effects of habitat types, season of defecation (summer vs. winter droppings), destruent guilds (small mammal access), and their interaction on the frequency of decay classes and on transition times, we run conditional inference trees (Hothorn et al. 2006). Conditional inference trees (CIT) conduct recursive binary partitioning based on the theory of permutation testing (Strasser and Weber 1999), allowing for an unbiased selection of covariates of different possible scales (Hothorn et al. 2006). Tests were done (1) for the summer months 2016 (July–October), simulating a decay scenario during regular field mapping in 1 year and (2) for an exposure time of approx. 1 year, simulating repeated surveys in consecutive years and thereby potentially encountering the same droppings in different years. We tested for correlation of decay processes with precipitation data during the vegetation period using non-parametric rank correlations (Kendall-τ-b, Sachs and Hedderich 2006).
We used the GNU R 3.4.1 (R Core Team 2017), RStudio 1.0.143 (RStudio Team 2016), and additional packages: tidyverse 1.1.1 (Wickham 2017), partykit 1.1-1 (Hothorn and Zeileis 2015), (Hothorn et al. 2006), openxlsx 4.0.17 (Walker 2017), knitr 1.16 (Xie 2016), and rmarkdown 1.6 (Allaire et al. 2017) as well as IBM SPSS Statistics 24 and Microsoft® Excel® for Mac 14.7.3 (MS 2011) for statistical analyses.
Results
Field trial
Proportion of decay stages
Of all droppings surveyed in the field trial (n = 156), 19 (12%) were initially categorized as stage A and the rest was categorized as stage B (88%). By the end of vegetation period 2016, after being exposed for 98 days, 6% (n = 9) of initially exposed droppings decayed completely to stage E (Table 3). Most droppings were still in stage B or tC. In total, 147 intestinal feces remained of which 43% had not even started to decay to a noticeable extent. After 98 days, the proportions of main decay stages (i.e., B, tC + C, tD + D, and tE + E) significantly differed within the habitat patches (Σχ2 = 275, df = 18, p < 0.001; Table 3). Significantly more droppings reached the final stages D and E on habitat patches mostly covered with Vaccinium or with herbs (Table 3) compared to those on other habitat patches. Vice versa, significantly more droppings remained more or less unchanged at the nudum site and on the tree stub (Table 3).
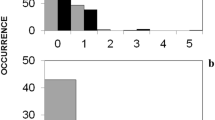
Classifying the frequency of decay stages after the vegetation period 2016 while accounting for hierarchical interactions between habitat patches, the season of defecation (i.e., summer vs. winter droppings), and the type of exclosure with CITs, location was ranked higher than the seasonal origin of droppings (Fig. 1).
Conditional inference trees, segregating the frequency of decay stages experimentally exposed droppings entered a after the vegetation period 2016 and b after the winter season 2016/17; input variables: season (summer vs. winter droppings), habitat patch, small mammal access 0/1; 19 droppings started at stage A
After an exposure time of 1 year, about one quarter of all droppings decayed completely to stage E, 4% of droppings remained fully intact (stage B), and the stages tC and C in total appeared at comparable proportions as observed before the winter season (Table 3). Accordingly, about two thirds of all initially exposed droppings were still in a stage allowing for detection in the course of potential field mappings (B to tD) after 1 year. Proportions of different decay stages significantly differed among habitat patches (Σχ2 = 256, df = 18, p < 0.001). In detail, the Vaccinium site, the herb site, and the open timber stand (with herb and grass cover of the ground) yielded significantly higher proportions of high or full decay, and droppings exposed on the nudum site or on the tree stub remained intact to a higher degree (Table 3). Simultaneously considering all potential classifiers for dropping decay frequencies for the 1-year exposure time, only the type of habitat patch remained in the CIT (Fig. 1). Thereby, the CIT yielded the following final ranking of classifiers, starting with the highest frequencies of low decay: nudum and tree stub > regeneration > herbs, timber stands, and Vaccinium site.
Transition time
Droppings initially categorized as stage A decayed to stage B within a median of 5 days. In all different habitat patches, transition time from B to tC was longest (Fig. 2) with a median value of 56 days. Regardless of the seasonal origin of droppings, only this transition time (B to tC) differed among habitat patches with a significantly longer duration on the regeneration patch compared to that among the Vaccinium patch and the patch situated under a timber stand with open canopy cover (i.e., Timber < 60%; Mann-Whitney U test: Z = − 2.852, p = 0.004 and Mann-Whitney U test: Z = − 3.443, p = 0.001, respectively).
Classifying transition times with CITs (incl. all independent variables), transition times of summer droppings differed from those of winter droppings. Furthermore, the types of habitat patches influenced the transition time of summer droppings with longer transition times under herbal vegetation, on the regeneration patch and on the tree stub (Fig. 3). For winter droppings, stage tC showed the longest transition time compared to higher stages of decay (i.e., C to E).
Conditional inference tree, segregating the number of days and experimentally exposed droppings needed for entering a subsequent stage of decay (= transition time) during the vegetation period 2016 (19 droppings started at stage A; input variables: season (summer vs. winter droppings), habitat patch, small mammal access 0/1
Total duration
By the end of the vegetation period 2016, not every dropping entered into new stages and some did not pass every stage, e.g., immediately changing from stages tC to tD or from C to E. As a consequence, the final stages (D to E) varied greatly in the number of preceding days (Fig. 4). Addressing the days that passed from the beginning of the trial till droppings entered a certain decay stage without considering their seasonal origin, droppings needed significantly different numbers of days between habitat patches to enter stage tC (Kruskal-Wallis test: χ2 = 13.11, df = 6, p = 0.041): Droppings deposited at the Vaccinium patch or at the timber patch with canopy cover below 60% entered this specific stage faster than droppings at the regeneration patch (Mann-Whitney U test: Z = − 2.852, p = 0.004 and Mann-Whitney U test: Z = − 3.453, p = 0.001, respectively).
Number of days and experimentally exposed summer and winter droppings needed for entering a stage of decay from the beginning of the field trial (= total duration) during the vegetation period 2016 at different habitat patches (19 droppings started at stage A); summer = summer droppings, winter = winter droppings
Classifying total duration (incl. all independent variables) comprised the decay stages and the seasonal origin of droppings, yielding the following sequence of nodes: the time to reach stage B built up the first node (representing only summer droppings that started at stage A) with the shortest duration; it was followed by the season of defecation, with summer droppings generally needing a slightly shorter time to reach the decay stages tC to E; finally, winter droppings took the longest total time to enter stages C to E (Fig. 5).
Conditional inference tree, segregating the number of days and experimentally exposed droppings needed for entering a stage of decay from the beginning of the field trial (= total duration) during the vegetation period 2016 (19 droppings started at stage A; input variables: season (summer vs. winter droppings), habitat patch, small mammal access 0/1
Destruents
In most cases, no differences in transition time were found between droppings with small mammal access and without access (i.e., exclusion experiment). There was only one exception, where the number of days of the transition between tC and C was significantly lower for those droppings, where small mammals had access (Mann-Whitney U test: Z = − 3.453, p = 0.001, see also Fig. 6). However, when simultaneously considering all independent variables, none of the resulting conditional inference trees contained the variable small mammal access (Figs. 1, 3, and 5).
Heat map of the number of days (median) and experimentally exposed summer and winter droppings needed for entering a subsequent stage of decay (= days in-between, left) or for entering a stage of decay from the beginning of the field trial (= days passed, right) during the vegetation period 2016 (19 droppings started at stage A); summer = summer droppings, winter = winter droppings
We observed mollusks or their trails of slime and gnaw marks on 20 droppings (Fig. 7). Most of directly observed mollusks were slugs of the family Arionidae. One species was a juvenile snail of the family Clausiliidae (R. Nordsieck, pers. comm.), found on a dropping within herbaceous vegetation. Slugs and their tracks were found in all habitat patches except nudum and open timber stand (< 60% canopy cover). Most slugs (n = 7) were found in Vaccinium, followed by the closed timber stand (three animals and tracks, respectively).
We also found springtails (Collembola) on some droppings as well without identifying the species. On the tree stub, we found drilling dust and a drill hole in summer dropping indicating arthropod species’ mining activity. Avoiding any manipulation of the subsequent decay process, we did not remove the dropping for species determination.
Summer droppings vs. winter droppings
As all winter droppings per definition started the field trial at stage B, only summer droppings could pass the first transition from A to B (Figs. 2 and 4). Only one winter dropping entered the decay class D and only one decayed completely (stage E) after 98 days. Summer droppings decayed to a higher degree, needing less time to decay in several cases. For reaching decay stage tC, summer and winter droppings did not differ significantly, whereas winter droppings needed a significantly longer time to enter stage C (Mann-Whitney U test: Z = − 2.289, p = 0.022, Fig. 5). Conditional inference trees for the frequencies of decay classes, for transition times (days in-between), and for total times (days passed) comprised a node representing the season of defecation (nodes 1 and 3, respectively; Figs. 3 and 5).
After passing the vegetation period 2016 and winter 2016/17, a comparable extent of summer and winter droppings entered the different stage classes. Only for decay stage C, we observed a significantly higher number of winter droppings and a lower number of summer droppings than expected (overall Σχ2 = 19.7, df = 5, p < 0.01).
Precipitation and decay
Addressing potential impacts of precipitation on decay dynamics, we contrasted the amount of precipitation during the months of the field trial in 2016 to two indicators of decay dynamics, i.e., the number of droppings that changed to a new stage per month (= no. of transitions) and the number of additional transitions compared to the preceding month (= starts of transitions). In 2016, precipitation was highest in July (169 mm) and lowest in September (72 mm) (Table 4). Nonetheless, most transitions from one stage to another took place in September. In this month, we recorded in total 94 droppings that changed stages with 64 droppings starting to decay, while in July and August, 25 and 24 droppings started to decay, respectively. In October, only two additional droppings started to show first signs of decay (Table 4).
Non-parametric rank correlations did not yield significant links between the number of transitions and the sum of precipitation of the same month or for the starts of transitions. The same applied for correlations of the number of transitions with the sum of precipitation of the preceding month and the starts of transitions. In contrast, accumulated amounts of precipitation and accumulated numbers of transitions (both iteratively accumulated per month) as well as accumulated transition starts correlated highly significantly (Kendall-τ-b = 1.0, n = 4, p < 0.001, respectively).
Greenhouse trial
Proportion of entered stages
In the greenhouse, 27% of all droppings reached stage E by the end of trial. None of initially exposed droppings (n = 400) remained at stage B (Table 5). Instead, all droppings reached or passed stage tC after 140 days. Whereas proportions of stages did not differ significantly between light and heavy rainfall patterns within the 2014 or the 2015 scenario, we found significant differences between the two scenarios (Σχ2 = 33.37, df = 5, p < 0.001): high-intensity precipitation (= heavy rainfall) resulted in higher proportions of decay stage E for the 2014 scenario and to lower proportions of the same stage for the 2015 scenario than expected (Table 5).
Classifying the decay stages by CIT while considering the precipitation scenario (2014 vs. 2015), the intensity of precipitation (light vs. heavy), and the seasonal origin of droppings, all three potential classifiers built significant nodes: Overall, the 2014 scenario yielded higher frequencies of full decay, particularly for the heavy rainfall simulation (Fig. 8). The light rainfall events in the same scenario lead to higher decay of summer droppings than it was the case for winter droppings. Contrary, within the 2015 scenario, the intensity of precipitation did not matter and winter droppings decayed to a higher degree to higher decay stages than summer droppings (Fig. 8).
Conditional inference trees, segregating the frequency of decay stages experimentally exposed droppings entered in the greenhouse trial; input variables: precipitation scenario (2014, 216 mm; 2015, 179.5 mm), precipitation intensity (light vs. heavy rainfall event), and season (summer vs. winter droppings)
Transition time
Similarly to the field trial, stage tC was again the stage that needed the significantly longest transition time per precipitation scenario and intensity (Kruskal-Wallis test for 2014: χ2 = 223.74, df = 5, p < 0.001; Kruskal-Wallis test for 2015: χ2 = 122.45, df = 5, p < 0.001; all post hoc Mann-Whitney U tests with p < 0.001; Fig. 9).
Number of days and experimentally exposed droppings needed for entering a subsequent stage of decay (= transition time) in the greenhouse trial for the 2014 scenario (261 mm, left) and the 2015 scenario (179.5 mm, right) and two rainfall intensities (light vs. heavy); all droppings started at stage B
Additionally, droppings remained at stage tD for a longer time in the 2014 scenario compared to that at the decay stages D to E. Overall, transition times significantly differed between rainfall intensities per scenario with longer duration in case of light intensities in the 2014 scenario (Mann-Whitney U test: Z = − 3.418, p = 0.001) and a shorter duration in the 2015 scenario (Mann-Whitney U test: Z = − 2.576, p = 0.010, Fig. 10).
Heat map of the number of days (median) and experimentally exposed droppings needed for entering a subsequent stage of decay (= days in-between, left) for the 2014 scenario (261 mm, left) and the 2015 scenario (179.5 mm, right) and two rainfall intensities (light vs. heavy); all droppings started at stage B; summer = summer droppings, winter = winter droppings
The heavy rainfall simulations in the 2014 scenario yielded the shortest transition times (Mann-Whitney U test: Z = − 6.063, p < 0.001, Fig. 8).
Total duration
The time droppings needed to reach a specific stage of decay from the beginning of the greenhouse trial till its end significantly differed between summer and winter droppings for the 2014 scenario (Kruskal-Wallis test: χ2 = 15.769, df = 1, p < 0.001). Specifically, summer droppings entered faster into stages tD, D, and tE than winter droppings (all three referring post hoc Mann-Whitney U tests with p < 0.001). For the 2015 scenario, passing times of summer and winter droppings did not differ significantly.
Summer droppings vs. winter droppings
Artificially exposed droppings from the summer and winter season showed a heterogeneous picture of decay dynamics. Contrasting proportions of droppings at different decay stages within the same precipitation regime (i.e., intensity and amount of precipitation) yielded significant differences between summer and winter droppings: In several cases, winter droppings decayed to a significantly higher proportion to classes tE and E than the summer droppings (Table 6).
Field trial vs. greenhouse trial
Addressing the total duration, droppings needed to reach a given stage of decay in the field trial and the greenhouse trial (standardized for a duration of 98 days each) highlighted the impact of vegetation and microclimate on decay dynamics (Fig. 11). Summer droppings decayed faster to the stages D and tE in the field trial compared to those in the greenhouse. Winter droppings reached the stages tD to E after a fixed period of time only within the greenhouse trial.
Discussion
Our results are the first studying the decay dynamics of capercaillie droppings. By combining field surveys with green house trials we demonstrate which processes affect droppings’ decay.
Field trial
Our field trial indicated that T. urogallus droppings may persist in nature for comparatively long time spans. After 3 months of exposure under natural conditions, nearly two thirds of all exposed droppings showed only low levels of decay (stages B to C) allowing for easy detection in the course of field surveys. Even after passing a winter season with snow cover and frosting-thawing cycles, only a quarter of droppings completely disappeared. Being in line with Wik (2002), who studied the decay of Greater sage-grouse (Centrocercus urophasianus) droppings, we showed that capercaillie droppings from winter can also be found during fieldwork in summer.
However, variation of decay patterns between different habitat patches, potentially visited by T. urogallus, differed markedly in our study. Chapman (2004) recognized high variation in decay of Reeves’ muntjac (Muntiacus reevesi) pellets at small spatial scales and attributed this to biotic and edaphic factors. In our study, sites with high cover of herbs or grasses yielded the highest rates of dropping decay, whereas droppings on nudum and on tree stubs remained nearly unchanged, indicating a major impact of microsite and small-scale environmental conditions (Geiger 1930; Potthoff 1984). Rainfall can scatter pellets (Harestad and Bunnell 1987), which might have also been assumed for capercaillie droppings deposited on tree stubs. Contrary, droppings on tree stubs, being highly exposed to mechanic impacts of rain, showed the lowest decay progress in our study. Obviously, these droppings without any contact to ground vegetation desiccated to a high degree and subsequently developed a comparatively high wetting resistance and a wetting hysteresis (Blume et al. 2010). This phenomenon was also shown by Breuer (2007) for elephant dung. Herbal vegetation and litter may in contrast provide a microclimate facilitating feces decay (Lehmkuhl et al. 1994). In our study, the second slowest decay rate was found for droppings on the nudum site which was stocked by a dense thicket (canopy closure = 100%). This might be due to the fact that the dense canopy highly intercepted precipitation, directly affecting droppings’ decay. Vice versa, Harestad and Bunnell (1987) found a longer lasting decay of black-tailed deer (Odocoileus hemionus columbianu) pellets in clear-cut areas and droppings of cottontail rabbits (Sylvilagus floridanus) decayed faster in woody areas than in open areas (Cochran and Stains 1961). Jung and Kukka (2016) also reported 70% of elk (Cervus canadensis) pellets remaining on grass meadows compared to 17% in wet conifer stands. Our results imply that visually striking droppings on open surfaces (forest litter or tree stub surface) might persist for longer times and may thus be overrepresented in the course of (repeated) field surveys. In our study, not all droppings passed every decay stage but some droppings skipped some stages between the control intervals (max. 1 week). Changes from stage C to stage E partially derived from overgrowing by vegetation (mainly mosses, see Lehmkuhl et al. 1994), leading to low detection probabilities of droppings in field surveys.
Fernandez-de-Simon et al. (2011) reported precipitation as the most important influencing factor, whereas Flinders and Crawford (1977) described the mean relative humidity to be more effective for decay than the monthly mean precipitation itself. This is in line with our results, where the lowest amount of precipitation occurred in September, but the humidity of the two preceding months had moistened the droppings, possibly accelerating the decay. White (1995) also showed that the preceding 2 months together with the rainfall of the current month predicted best the persistence of elephant dung.
Addressing the number of preceding days and droppings needed to enter final stages of decay, we observed high variation at the end of the vegetation period, with winter droppings needing the longest time to enter decay stages C to E. According to Flinders and Crawford (1977) and Bull (1981), animal’s diet affects the longevity of pellets besides weather conditions. As T. urogallus distinctly changes habitat preferences as well as food sources among seasons (Gremmels 1986), droppings contain differing components with high content of lignified, crude fibers in winter droppings (Bergmann et al. 2003; Watson and Moss 2008). Consequently, due to the higher content of lignin, which can only be broken down by a few organisms (Actinomyceta and Streptomyceta, Fuchs 2014), winter droppings are more difficult to decompose (Bolger et al. 2000) and might thus not be as attractive to decomposers. Compared to dung, which might be colonized and manipulated by dung beetles within short time spans (Breuer 2007), intestinal droppings of T. urogallus underlie a lower degree of digestion (Watson and Moss 2008). Due to a frequently assumed faster decay of summer droppings and a resulting assumed quicker disappearance, summer habitats could be misclassified as non-habitat, as described by Murray et al. (2005) for snowshoe hares (Lepus americanus). However, our results showed that field surveys during the summer months are not a priori biased towards higher proportions of summer droppings, as winter droppings may pass weeks or months of exposure without decaying to a high degree. Although summer droppings entered higher stages of decay in several cases and also needed less time to reach these stages, most of them could still be found within our study period. After an exposure time of 1 year, the difference between decay patterns of summer and winter droppings at higher decay stages (> C) disappeared.
Transition time, i.e., the time span droppings needed to enter a new stage of decay, was only influenced by habitat types for the change from stages B to tC. In our study, early-stage transitions from A to B were passed rapidly (within approx. 5 days). As stages A and B only differ in the presence of uric acid which is easy to solve (Fuchs 2014), this very first step in dropping decomposition is fast. Contrary, the next stages from B to tC and from tC to C took the longest time span compared to subsequent stages of decay. This result seems to be opposite to Swift et al. (1979), who described the early phase of (litter) decomposition as a rapid process. Thereby, easily solubilizing carbon compounds are broken down, leaving a higher proportion of hardly soluble compounds which in turn need more time to decompose (Fuchs 2014). As shown by Breuer (2007) for elephant (Loxodonta africana) dung, the stages A to C were reached quickly within 1 or 2 days whereas stage D needed more time to be passed. However, differences in digestive tracts, in relating digesting mechanisms, and in the digestibility of food yield different food remains in excrements (Putman 1984) and differences in the persistence of feces. Herbivore feces contain moderately digested plant material and microbial tissues (Swift et al. 1979; Jones 2017). Thus, being only marginally changed compared to original fed vegetation, dung of herbivores is still a useful substrate for invertebrates (Putman 1983). Intestinal droppings of grouse species represent a conglomerate of hardly soluble compounds with high contents of coarse fibers (Watson and Moss 2008), thus again resembling soil litter at the point of defecation. With its complex digestive system (i.e., extreme long caeca, Gremmels 1986), even the breakdown of cellulose has been found in capercaillie (Suomalainen and Arhimo 1945; Klaus et al. 1989). Our results indicate that these droppings only provide marginal food sources for other destruents, resulting in a long decay times.
Even though small mammals and birds have been found to feed on mammal feces (Sanchez et al. 2004; Livingston et al. 2005; Jones 2017), we did not find evidence for significant impact of small mammals in decay dynamics in our study. However, the exclosures provided some shelter for exposed droppings, preventing of being moved and trampled by other, larger animals. Such impacts might accelerate decay under natural conditions as shown by Wik (2002) for Greater sage-grouse.
Greenhouse trial
Apart from the fact that all droppings showed signs of decay under greenhouse conditions, results of the greenhouse trial corresponded to some extend to the results of the field trial: Stage tC was again the stage that needed the significantly longest transition time. Moreover, the time spans to reach different stages showed high variation as well, and summer and winter droppings decayed at different speeds within the different setups. However, decay rates and transition times were not a simple function of precipitation amounts and intensities, but were affected by interactions of both factors. This was reflected by contingency analyses and CITs: The high-amount and high-intensity precipitation setup yielded higher frequencies of high decay (stage E), than all other settings. A faster decay due to heavy rainfall events was described by Wallmo et al. (1962) for deer pellets. Iborra and Lumaret (1997) also observed that pellet decay was rather linked to the intensity of precipitation than to its quantity. Thereby, the daily amount of precipitation might be of minor importance compared to the number of days with rain, influencing the drying of droppings and the resulting decay speed (Wallmo et al. 1962; Iborra and Lumaret 1997). Accordingly, our trial indicated that a combination of amount, frequency, and intensity of precipitation has impact on dropping decay.
The decay progress of the first 98 days of the greenhouse trial generally resembled that of the field trial. However, for the remaining 70 days, decay accelerated, supporting the assumption that a certain amount of moisture is necessary to speed up decay. Warm and moist conditions favor decomposition (Schowalter 2000), which might also apply for dropping decay. The higher temperature of 23 °C in the greenhouse (compared to the field trial) might have accelerated the decay process, as temperature is the second most important factor triggering decay (Lehmkuhl et al. 1994). However, contrasting the results of the greenhouse trial against results of the field trial based on comparable time spans, pure effects of precipitation in the greenhouse did not produce stringently lower or higher decay of droppings compared to natural conditions, but again revealed differences between summer and winter droppings and different decay stages.
Conclusions
Our study showed that—similarly to mammal feces—the decay of capercaillie droppings was influenced by microsite conditions (i.e., vegetation and location) and precipitation. Both have impact on the desiccation status of feces and on further decay rates due to wetting resistance and potential colonization by decomposing fungi, bacteria, and animals. In our study, droppings decayed faster on sites with dense ground vegetation compared to those on areas with little ground vegetation or on stubs. Accordingly, presence-absence modeling based on feces counts might be biased towards preferences of open habitats, depending on structural composition of feces of the target species. Surprisingly, we found very little destruents affecting the decay rate, with small mammals playing no significant role and even though snails were found to feed on the droppings, they only took small pieces, and the dropping decay rate was only slightly influenced. Precipitation played a role in decay dynamics with fastest decay under high-amount and high-intensity precipitation. Summer droppings decayed faster compared to droppings secreted in winter, most likely due to different diets between seasons. This could affect their detectability or cause biases when using indirect signs as predictors in habitat models. When mapping during the summer season, both droppings as well as feathers can be used as indicators of capercaillie presence. We thus assume that a possible “decay-rate bias” will be balanced out and the best time to investigate year-round habitat use of western capercaillie is the summer months. Since droppings generally remained at early decomposition stages in all habitats for a long period of time, they represent a valid proxy for species’ occurrence and an accurate source of data for presence-absence modeling. To avoid pseudo-replication in presence-absence data, we recommend removing T. urogallus droppings in case of repeated field surveys.
References
Allaire JJ, Xie Y, McPherson J, et al (2017) rmarkdown: Dynamic Documents for R. R package version 1.8. https://CRAN.R-project.org/package=rmarkdown. Accessed 2 Sept 2017
Bang P, Dahlstrøm P (2001) Animal tracks and signs. Oxford University Press, Viborg
Bergmann H-H, Klaus S, Suchant R (2003) Auerhühner. G Braun Buchverlag, Karlsruhe
BirdLife-International (2012) Tetrao urogallus. IUCN Red List of Threatened Species. https://doi.org/10.2305/IUCN.UK.2016-3.RLTS.T22679487A85942729.en
Blume H-P, Brümmer GW, Horn R et al (2010) Scheffer/Schachtschabel: Lehrbuch der Bodenkunde. Springer, Berlin
Bolger T, Heneghan L, Neville P (2000) Invertebrates and nutrient cycling in coniferous Forest ecosystems: spatial heterogeneity and conditionality. In: Coleman D, Hendrix P (eds) Invertebrates as webmasters in ecosystems. CABI Publishing, Wallingford, pp 225–269
Bollmann K, Graf RF (2008) Wie beeinflussen Lebensraumangebot und-fragmentierung die Verbreitung von Lokalpopulationen beim Auerhuhn. Ornithol Beob 105:45–52
Borchers D, Buckland S, Zucchini W (2002) Estimating animal abundance—closed populations. D.L. Borchers, Springer, London
Braunisch V, Suchant R (2007) A model for evaluating the ‘habitat potential’ of a landscape for capercaillie Tetrao urogallus: a tool for conservation planning. Wildl Biol 13:21–33. https://doi.org/10.2981/0909-6396(2007)13[21:AMFETH]2.0.CO;2
Braunisch V, Suchant R (2013) The capercaillie Tetrao urogallus action plan in the Black Forest: an integrative concept for the conservation of a viable population. Vogelwelt 134:29–41
Breuer T (2007) Forest elephant dung decay in Ndoki Forest, northern Congo. Pachyderm 43:43–51
Bull EL (1981) Indirect estimates of abundance of birds. Stud Avian Biol 6:76–80
Chapman N (2004) Faecal pellets of Reeves muntjac, Muntiacus reevesi: defecation rate, decomposition period, size and weight. Eur J Wildl Res. https://doi.org/10.1007/s10344-004-0053-0
Cochran GA, Stains HJ (1961) Deposition and decomposition of fecal pellets by cottontails. J Wildl Manag 25:432–435. https://doi.org/10.2307/3798835
Coppes J, Ehrlacher J, Müller G, et al (2016) Rückgang von Bestand und Verbreitung des Auerhuhns Tetrao urogallus im Schwarzwald. Ornithologischer Beobachter 113:235-248
Dawson D (1981) Counting birds for a relative measure (index) of density. In: Estimating numbers of terrestrial birds. Cooper Ornithological Society, Camarillo, pp 12–16
Fernandez-de-Simon J, Díaz-Ruiz F, Villafuerte R, Delibes-Mateos M, Ferreras P (2011) Assessing predictors of pellet persistence in European rabbits Oryctolagus cuniculus: towards reliable population estimates from pellet counts. Wildl Biol 17:317–325. https://doi.org/10.2981/10-001
Flinders JT, Crawford JA (1977) Composition and degradation of jackrabbit and cottontail fecal pellets, Texas High Plains. J Range Manag 30:217–220
Fuchs G (2014) Allgemeine Mikrobiologie, 9th edn. Thieme Verlag, Stuttgart
Geiger R (1930) Mikroklima und Pflanzenklima. Gebrüder Berntraeger, Berlin
Grafen A, Hails R (2002) Modern Statistics for the Life Sciences. Oxford University Press, New York
GIS Steiermark (2017) Digitaler Atlas der Steiermark. http://gis2.stmk.gv.at/atlas/ (S(0ixwtmsv24e2wv3zwwsterm3))/init.aspx?karte=grenzen&ks=das&cms=da&massstab=800000&darste llungsvariante=verwaltgre
Gremmels H-D (1986) Das Verdauungssystem der Rauhfußhühner—Eine Übersicht zur Physiologie und Mikroanatomie dieses Organsystems. Z Für Jagdwiss 32:96–104. https://doi.org/10.1007/BF02241243
Harestad AS, Bunnell FL (1987) Persistence of black-tailed deer fecal pellets in coastal habitats. J Wildl Manag 51:33–37. https://doi.org/10.2307/3801624
Heinemann U (1989) Zur Winternahrung des Auerhuhns (Tetrao urogallus L.) im Harz (Niedersachsen). Z Für Jagdwiss 35:35–40. https://doi.org/10.1007/BF02244354
Hothorn T, Zeileis A (2015) partykit: a modular toolkit for recursive partitioning in R. J Mach Learn Res 16:3905–3909
Hothorn T, Hornik K, Zeileis A (2006) Unbiased recursive partitioning: a conditional inference framework. J Comput Graph Stat 15:651–674
Iborra O, Lumaret J-P (1997) Validity limits of the pellet group counts in wild rabbit (Oryctolagus cuniculus). Mammalia 61. https://doi.org/10.1515/mamm.1997.61.2.205
Jones R (2017) Call of nature. The secret life of dung. Pelagic Publishing, Exeter
Jung TS, Kukka PM (2016) Influence of habitat type on the decay and disappearance of elk Cervus canadensis pellets in boreal forest of northwestern Canada. Wildl Biol 22:160–166. https://doi.org/10.2981/wlb.00186
Karp NA (2010) R commander an Introduction. http://cran.r-project.org/doc/contrib/Karp-Rcommander-intro.pdf. Accessed 11 Feb 2017
Kear J (1963) The agricultural importance of wild goose droppings. Wild 14:6
Kilian W, Müller F, Starlinger F (1994) Das forstliche Wuchsgebiet Österreichs. Eine Naturraumgliederung nach waldökologischen Gesichtspunkten. Bundesministerium für Land- und Forstwirtschaft, Wien
Klaus S, Andreev V, Bergmann H et al (1989) Die Auerhühner. Die Neue Brehm-Bücherei, Magdeburg
Kohn MH, Wayne RK (1997) Facts from feces revisited. Trends Ecol Evol 12:223–227. https://doi.org/10.1016/S0169-5347(97)01050-1
Laing SE, Buckland ST, Burn RW, Lambie D, Amphlett A (2003) Dung and nest surveys: estimating decay rates. J Appl Ecol 40:1102–1111. https://doi.org/10.1111/j.1365-2664.2003.00861.x
Lehmkuhl JF, Hansen CA, Sloan K (1994) Elk pellet-group decomposition and detectability in coastal forests of Washington. J Wildl Manag 58:664–669. https://doi.org/10.2307/3809679
Livingston TR, Gipson PS, Ballard WB, Sanchez DM, Krausman PR (2005) Scat removal: a source of bias in feces-related studies. Wildl Soc Bull 33:172–178. https://doi.org/10.2193/0091-7648(2005)33[172:SRASOB]2.0.CO;2
Marques FFC, Buckland ST, Goffin D, Dixon CE, Borchers DL, Mayle BA, Peace AJ (2001) Estimating deer abundance from line transect surveys of dung: sika deer in southern Scotland. J Appl Ecol 38:349–363. https://doi.org/10.1046/j.1365-2664.2001.00584.x
Mason C (1976) Decomposition. Edward Arnold, Southampton
McClure HE (1945) Comparison of census methods for pheasants in Nebraska. J Wildl Manag 9:38–45
Murray D, Ellsworth E, Zack A (2005) Assessment of potential bias with snowshoe hare fecal pellet-plot counts. J Wildl Manag 69:385–395. https://doi.org/10.2193/0022-541X(2005)069<0385:AOPBWS>2.0.CO;2
Neff DJ (1968) The pellet-group count technique for big game trend, census, and distribution: a review. J Wildl Manag 32:597–614. https://doi.org/10.2307/3798941
Nopp-Mayr U, Kempter I, Muralt G, Gratzer G (2012) Seed survival on experimental dishes in a central European old-growth mixed-species forest—effects of predator guilds, tree masting and small mammal population dynamics. Oikos 121:337–346. https://doi.org/10.1111/j.1600-0706.2011.19099.x
Potthoff H (1984) Ökologische-kleinklimatische Messungen in Bonn unter besonderer Berücksichtigung der Wirkungen der Vegetation. Rheinische Friedrich-Wilhelms-Universität Bonn
Prettenthaler F, Podesser A, Pilger H (2010) Klimaatlas Steiermark. Periode 1971–2000. In: www.umwelt.steiermark.at. http://www.umwelt.steiermark.at/cms/ziel/16178332/DE/. Accessed 14 Feb 2017
Prugh LR, Krebs CJ (2004) Snowshoe hare pellet-decay rates and aging in different habitats. Wildl Soc Bull 32:386–393. https://doi.org/10.2193/0091-7648(2004)32[386:SHPRAA]2.0.CO;2
Putman R (1983) Carrion and dung: the decomposition of animal wastes. The Camelot Press Ltd., Southampton
Putman RJ (1984) Facts from faeces. Mammal Rev 14:79–97. https://doi.org/10.1111/j.1365-2907.1984.tb00341.x
R Core Team (2017) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
RStudio Team (2016) RStudio: integrated development environment for R. RStudio, Inc., Boston
Sachs L, Hedderich J (2006) Angewandte Statistik - Methodensammlung mit R, 12th edn. Springer, Berlin
Sanchez DM, Krausman PR, Livingston TR, Gipson PS (2004) Persistence of carnivore scat in the Sonoran Desert. Wildl Soc Bull 32:366–372. https://doi.org/10.2193/0091-7648(2004)32[366:POCSIT]2.0.CO;2
Schowalter T (2000) Insect ecology. An ecosystem approach. Academic Press, San Diego
Seber G (2002) The estimation of animal abundance, 2nd edn. The Blackburn Press, New Jersey
Storch I (1999) Auerhuhnschutz im Bergwald: Methoden und Konzepte. Wildbiologische Gesellschaft, München
Storch I (2002) On spatial resolution in habitat models: can small-scale forest structure explain capercaillie numbers? Conserv Ecol 6:1–25
Storch I (2007) Grouse: status survey and conservation action plan 2006–2010. IUCN, World Pheasant Assoc., Gland, Fordingbridge
Strasser H, Weber C (1999) On the asymptotic theory of permutation statistics. Mathematical Methods of Statistics 8:220-250
Summers RW, Proctor R, Thorton M, Avey G (2004) Habitat selection and diet of the capercaillie Tetrao urogallus in Abernethy Forest, Strathspey, Scotland. Bird Study 51:58–68. https://doi.org/10.1080/00063650409461333
Suomalainen H, Arhimo E (1945) On the microbial decomposition of cellulose by wild gallinaceous birds (family Tetraonidae). Ornis Fennica 22:21–23
Swift M, Heal O, Anderson J (1979) Decomposition in terrestrial ecosystems. University of California Press, Berkeley
Temple S (1981) Summarizing remarks: estimating relative abundance. Studies in Avian Biology 6:112
Vanleeuwe H, Probert J (2014) Decay rate of elephant dung in Conkouati-Douli National Park, Republic of Congo. Pachyderm 55:89–91
Walker A (2017) openxlsx: read, write and edit XLSX Files. https://cran.r-project.org/web/packages/openxlsx/openxlsx.pdf. Accessed 2 September 2017
Wallmo OC, Jackson AW, Hailey TL, Carlisle RL (1962) Influence of rain on the count of deer pellet groups. J Wildl Manag 26:50–55. https://doi.org/10.2307/3798167
Watson A, Moss R (2008) Grouse: the natural history of British and Irish species. Happer Collins Publisher, UK
White LJT (1995) Factors affecting the duration of elephant dung piles in rain forest in the Lopé Reserve, Gabon. Afr J Ecol 33:142–150. https://doi.org/10.1111/j.1365-2028.1995.tb00789.x
Wickham H (2017) tidyverse: easily install and load “Tidyverse” packages. http://cran.r-project.org/web/packages/tidyverse/index.html. Accessed 2 September 2017
Wik P (2002) Ecology of greater sage-grouse in south-central Owyhee county, Idaho. Master Thesis, University of Idaho
Xie Y (2016) knitr: a general-purpose package for dynamic report generation in R. https://cran.r-project.org/web/packages/knitr/index.html. Accessed 2 September 2017
Zohmann M, Immitzer M, Wöss M et al (2014) Modelling habitat use of Tetrao urogallus L. in Austria for conservation issues. J Nat Conserv. https://doi.org/10.1016/j.jnc.2014.01.002
Zöfel P (1992) Statistik in der Praxis. Fischer, Jena
Zwickel F, Bendell J (2004) Blue grouse: their biology and natural history. National Research Council of Canada, Canada
Acknowledgements
We are grateful to Veronika Grünschachner-Berger for the help with the fieldwork, as well as valuable discussions on capercaillie dropping decay. This study was only possible due to the support and permission of Michaela Peer, forester of the local forestry department of the Austrian Federal Forests. We cordially thank Thomas Kranabitl, who provided fresh blueberry droppings for the field and the greenhouse trial, as well as Jasmin Barl, who prepared paintings of decay stages. We further thank Florian Kunz and Stephanie Forstner for support in sorting droppings and in the field surveys. Margit Zohmann-Neuberger provided valuable comments on the manuscript. Evelyne Holub, head of the research greenhouse at the University of Natural Resources and Life Sciences, Vienna, provided facilities and equipment for the greenhouse trial, Prof. Herbert Formayer from the Institute of Meteorology, University of Natural Resources and Life Sciences, Vienna, compiled the weather data form from the ZAMG, Vienna. Dr. Karin Tremetsberger from the Institute of Botany, University of Natural Resources and Life Sciences, Vienna, supervised the identification of grass species in the field trial. Finally, we thank all fieldworkers who collected fresh capercaillie droppings to be used in this study.
Funding
Open access funding provided by University of Natural Resources and Life Sciences Vienna (BOKU).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with animals performed by any of the authors.
Additional information
Ursula Nopp-Mayr is equally contributing first author.
Electronic supplementary material
ESM 1
(PDF 6.40 MB)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Poggenburg, C., Nopp-Mayr, U., Coppes, J. et al. Shit happens … and persists: decay dynamics of capercaillie (Tetrao urogallus L.) droppings under natural and artificial conditions. Eur J Wildl Res 64, 29 (2018). https://doi.org/10.1007/s10344-018-1187-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10344-018-1187-9