Abstract
The transmission of pestiviruses between domestic and wild ruminants has not been documented in communal alpine pastures shared between wildlife and livestock. The aim of this study was to investigate the role of domestic and wild ungulates species from Varaita Valley (SW Italian Alps) in the epidemiology of Pestivirus infections. Sera from free-ranging alpine chamois (Rupicapra rupicapra) and roe deer (Capreolus capreolus) were collected from 1994 to 2009 and 2001 to 2009, respectively. Also, sera from cattle and sheep sampled in 2009 were studied. Sera were tested for the presence of antibodies against pestivirus with an ELISA assay. Sera from positive animals were subsequently tested with a comparative virus neutralisation test using the BVDV-NADL and BDV-137/4 strains. Sera were tested for the presence of pestiviral antigen and the presence of viral RNA with a commercial ELISA assay and RT-PCR. Antibodies against Pestivirus were detected in 132 out of 312 (42%) chamois, in 30 out of 175 (17%) cattle and 6 out of 24 (25%) sheep. No antibodies were found in roe deer. No Pestivirus antigen or RNA was detected in any of the samples. Results indicate circulation of pestiviruses among the studied chamois, cattle and sheep populations. However the role of wild ungulates in the dynamics of Pestivirus infection is still unknown and monitoring the presence of these viruses in wild ungulates would be of importance, especially in the chamois population, where pestiviruses seem to circulate extensively.
Similar content being viewed by others
Introduction
Pestiviruses (family Flaviviridae) are single-stranded, positive-sense RNA viruses, which have the ability to cross species barriers and to infect a wide range of artiodactyls. Thus, bovine viral diarrhoea virus (BVDV) and border disease virus (BDV) are not strictly host specific and antibodies against these viruses have been reported in several species of domestic and wild ruminants (Nettleton and Entrican 1995; Loken 1995; Nettleton et al. 1998). Pestiviruses have also been isolated from different artiodactyls such as camelids (Evermann 2006), cervids (Frolich and Hofmann 1995) and in different Bovidae species (Vilcek and Nettleton 2006). In Pyrenean chamois, important disease outbreaks with high mortality rates have been described, associated with a BDV-4 strain (Marco et al. 2007).
Pestiviruses cause in livestock a wide range of reproductive clinical manifestations entailing severe economic losses worldwide. Acute BVDV infection in cattle produces enteric disease consisted in diarrhoea, pyrexia and mild depression with high morbidity and low mortality, and, in some cases, acute fatal haemorrhagic syndrome has been described (Carman et al. 1998). BDV infection among sheep causes acute infections characterized by short-period viraemia (Nettleton et al. 1998). However some outbreaks of fatal disease associated to BDV have been described in sheep (Chappuis et al. 1984). After acute BVDV and BDV infections, neutralising antibodies appear in serum. However, the success of pestiviruses is based in their capacity to be transmitted congenitally to foetus. This congenital infection can lead to the birth of persistently infected (PI) animals characterized by the immunotolerance to the virus and continuous replication and excretion of it.
BVDV and BDV infections in free-ranging wild ruminants have been relatively poorly studied in Europe. There is a lack of knowledge about the epidemiology of Pestivirus infection between livestock and wildlife. Pestivirus investigations in domestic and wild ruminants from Alps have been performed in Switzerland (Holzwarth et al. 2011), Austria (Krametter-Froetscher et al. 2010), France (Martin et al. 2011) and Italy (Olde Riekerink et al. 2005). Also, studies on Pestivirus epidemiology in alpine areas have been reported (Krametter-Froetscher et al. 2007) describing a transmission of pathogens between domestic livestock and wildlife, especially during the sharing of communal alpine pastures along the summer.
Varaita Valley is an alpine valley located in the Piedmont region in Italy. This region recently has started a BVDV pilot eradication programme in cattle. In Varaita Valley, use of communal alpine pastures during the summer is common, but Pestivirus infections in wild ungulates have not been studied in this area. Because of this, data about the transmission of pestiviruses between domestic and wild ruminants from this valley are unknown. The aim of this study was to investigate Pestivirus infections in ungulates from Varaita Valley and to assess the role of domestic and wild ungulates species in the epidemiology of Pestivirus infections.
Materials and methods
Study area
The Varaita Valley (44°49′0″ N, 7°36′0″ E) is an alpine valley of south-western Piedmont (NW Italy, Fig. 1). The valley is approximately 60 km long, from the city of Verzuolo to the 2,748-m-high Colle dell'Agnello, which connects the valley to the French Vallée du Guil. Several wild Artiodactyla species inhabit this area such as alpine ibex (Capra ibex), Alpine chamois (Rupicapra rupicapra), roe deer (Capreolus capreolus), red deer (Cervus elaphus) and wild boar (Sus scrofa). Domestic livestock (cattle, sheep and goats) share the pastures with wild ungulates during the summer. Approximately 8,550 cattle and 4,000 sheep and goats ascend each year from the neighbouring regions to the alpine meadows of Valle Varaita.
Animals and samples
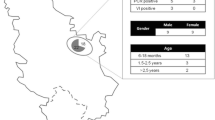
Blood samples from free-ranging Alpine chamois (n = 312) and roe deer (n = 213) were collected from 1994 to 2009 and 2001 to 2009, respectively (Table 1). These ungulates are major game species in Varaita Valley, and samples were collected during the hunting season (September–November) following the established hunting programmes. Blood samples were collected whenever possible after being shot by hunters themselves. Sex, age and location of shot were given. The age of the animals varied between 0 and 16 years old in the chamois and between 0 and 8 years old in the roe deer.
Also, sera from cattle (n = 175) and sheep (n = 24) sampled in 2009 were studied (Table 1). Cattle and sheep came from different small herds (16 and 5, respectively) breed in the valley and that spend the summer months in the alpine pastures. Sampling of these animals was performed in autumn, at the returning from the summer pastures. The sampling of the cattle was designed and balanced in order to cover all the high mountain summer grazing area of the valley. In addition the cattle came from non-BVD-vaccinated herds.
Blood samples from wild ungulates were obtained by intracardiac venipuncture from dead animals after being hunted. Blood samples from domestic ungulates were obtained by venipuncture of the tail vein in cattle and of the jugular vein in sheep. Samples were placed into sterile serum separator tubes and centrifuged at 1,200×g for 15 min. Sera were stored at −20°C until processed.
Laboratorial analysis
Serological tests
Sera were tested for the presence of Pestivirus-specific antibodies against NS3 protein using a commercial blocking ELISA assay (Pourquier, Montpellier, France). Sera from positive animals were subsequently tested with a comparative virus neutralisation test (VNT) for neutralising antibodies against BVDV-1 strain NADL (Collett et al. 1988; Gen Bank accession number M31182) and BDV-1 strain 137/4 (Vilček et al. 1997; Gen Bank accession number U65052). Since there is no information about which Pestivirus strains are circulating in the study area, we choose two reference strains to perform the VNT test. Our results should allow us to differentiate if the animals were infected with a BVDV or with a BDV. VNT was performed under procedure described in the “Manual of diagnostic tests and vaccines for terrestrial animals” (OIE 2008) using Madin–Darby bovine kidney cells. Neutralising antibody titres were expressed as the reciprocal of the highest dilution that neutralised 100 tissue culture infective doses (100 TCID50) in all cultures, calculated according to the method of Reed and Muench (1938). Titres of 1:10 and higher were considered positive. Viral replication was monitored by the immuno-peroxidase monolayer assay with polyclonal home-made pestivirus-specific serum. We considered that the antibodies were specific of a Pestivirus strain if the titre against this strain was at least three times higher when compared to the titre against the other strain (OIE 2008).
Virus detection
Chamois, cattle and sheep sera were tested for the presence of pestiviral antigen with a commercial sandwich ELISA assay (Synbiotics, Lyon, France) according to the manufacturer’s procedure. Reverse transcription polymerase chain reaction (RT-PCR) was performed in sera with a positive or inconclusive result in the antigen ELISA test. Total viral RNA was extracted directly from 150 μl of sera by a commercial kit (Macherey Nagel Nucleospin Viral RNA Isolation; Düren, Germany) according to the manufacturer’s procedure. The RT-PCR was performed using previously described panpestivirus primers 324 (5′-ATGCCCWTAGTAGGACTAGCA–3′; W = A or T) and 326 (5′-TCAACTCCATGTGCCATGTAC-3′, Vilcek et al. 1994) and a commercial kit (One-Step PCR kit; Qiagen Inc., Hilden Germany).
Statistical analysis
For data analysis we used the functions for analysing epidemiological data included in the library epiR (http://cran.r-project.org/web/packages/epiR/epiR.pdf/) by using the free statistical software R (http://www.r-project.org/). In order to test for differences in the observed proportions of positive animals by sex, we computed a chi-squared test stratified by age and reported the odds ratio values with their confidence interval. The same test was computed to test for differences by class of age (animals were separated in two groups: animals under or with 2 years and animals over 2 years).Within this R package point estimates and confidence intervals are based on formulae provided by Rothman (2002). A p value for the chi-squared statistic below 0.05 was interpreted as a lack of homogeneity between the proportions and therefore as a statistical significant difference between groups. Confidence intervals for the proportion of positive animals by year were calculated by using the confidence interval for a single proportion calculation (exact binomial method) implemented in the epiR package (Altman et al. 2000). An analysis of variance was applied to test for differences between virus titres.
Results
Serological results are reported in Table 1. Antibodies against pestivirus were detected by ELISA in 132 out of 312 (42%) chamois, in 30 out of 175 (17%) cattle and in 6 out of 24 (25%) sheep. No antibodies were found in the 213 samples of roe deer. The VNT confirmed the ELISA results in all positive animals except 11 chamois that had negative titres against both pestivirus strains.
Seroprevalence was significantly higher in chamois over 2 years (OR > 2 years = 2.98; 95% CI 1.78–4.99). The antibody prevalence was significantly higher in females than in males in the chamois over 2 years (OR, female = 2.13; 95% CI 1.22–3.73), but no significant differences were found in the prevalence between males and females in the ≤2 years old group.
The VNT results indicate that 24 chamois were infected with a BDV (titre range 10–2,560). However, seven chamois had significant higher titres against the BVDV strain (titre range 20–10,240). In 101 chamois, the specificity of the antibodies could not be determined. No significant differences were found between the titres against BD-137/4 and BVD-NADL in the chamois and in the sheep group. Cattle had significant higher antibody titres against the BVDV-NADL strain.
Twelve chamois had positive results in the ELISA of antigen detection test, and 13 presented inconclusive results. They were all negative to RT-PCR.
Discussion
The results of the present study indicate high exposure of Alpine chamois to Pestivirus in the Varaita Valley. Serosurveys of antibodies to Pestivirus previously performed in Alpine chamois populations also described high prevalence, ranging from 25.5% to 45.9% (Martin et al. 2011; Olde Riekerink et al. 2005). These studies associated the high seroprevalence with the transmission of the infection from domestic ruminants to chamois in alpine pasture areas. The global prevalence (42% CI 37–47) detected in chamois in the present study is similar to the one described by Martin et al. in 2005 in the French South Alps. During our study period, the prevalence in chamois populations showed fluctuations. Interestingly, high rates of antibodies were found in samples from 1994 and 1995 (apparent prevalence of 61% CI 45–74 and 57% CI 42–72, respectively). This is the highest seroprevalence against Pestivirus described in an Alpine chamois population. Moreover, the antibody titres during this period were higher compared to other years, suggesting that it was an epidemic phase of the infection. However, no reduction has been described in the chamois population during this period. In addition, although pestivirus has demonstrated to cause disease in Pyrenean chamois (Marco et al. 2009b), detection of abortions or deaths of adult animals due to Pestivirus infection has not been described in Italian Alpine chamois populations. The reproduction rate (kids/females) showed fluctuations during the study period, but it did not show significant decreases (Dematteis, unpublished data).
The statistically significant differences observed in the seroprevalence between sexes (higher seroprevalence in females than in males) may be explained by the different social behaviour of both sexes: the males lead a more solitary life while the females form groups, where there are more social interactions between animals and the transmission of pestiviruses is more probable. In addition, no sex differences were observed in the group of ≤2 years old animals. This can be explained by the fact that the young males and females live together with the adult females, and both sexes of this group have the same probability of becoming infected with the virus. Other studies have described these sex differences in Pestivirus antibody rates (Martin et al. 2011; Olde Riekerink et al. 2005). Also, the results of the present study showed that the oldness increases the risk of seroconversion in chamois, as described by other authors (Martin et al. 2011). The increasing proportion of seropositive chamois with age could be explained by the fact that older animals had more probabilities of contacting the virus during their life when compared to younger animals.
The VNT results of chamois sera indicate that 24 chamois were infected with a BDV. However, seven chamois had higher titres against the BVDV strain, suggesting that they probably were infected with a virus from bovine origin. Nevertheless, in a high number of chamois (101), the specificity of the antibodies could not be determined, and no significant differences were found when the titres between BD-137/4 and BVDV-NADL of the chamois group were compared. Different hypotheses could explain these results. The most probable is that the chamois became infected with a BDV, since the mean antibody titre of the chamois was higher against the BDV strain when compared to the BVDV strain. The lack of major differences between the titres against BDV-137/4 and BVDV-NADL could be explained by the fact that chamois were infected with a BDV strain other than BDV-137/4. Due to the BD strain used in the VNT test was the BD-137/4, a strain of type 1, there is the possibility that a different type of BDV was circulating in these chamois populations. Cross-reactivity of antibodies to Pestivirus is high, and this would have contributed to make difficult the differentiation between the BVDV and BDV strains. The extent of cross-reactivity depends on the strain of Pestivirus involved and the interval between infection and time of sampling (Terpstra et al. 1984; Terpstra and Wensvoort 1988). A less likely hypothesis could be the infection with both BDV and BVDV in most of the chamois, preventing us from establishing differences between the titres against the two Pestivirus species (Olde Riekerink et al. 2005).
There are two possible explanations for the chamois that had negative results in the VNT but were positive to ELISA antibody test. They could have a low antibody titre to a different Pestivirus strain, or they could be false positives in the ELISA test. We consider that the first explanation is more probable. We have used extensively this ELISA kit, and it has a high sensitivity, since it detects very low titers of antibodies (<1/10). In our laboratory we have tested ovine sera with negative titers against several Pestivirus strains and a very low titer against the circulating strain in the area of origin.
Although the prevalence rate among chamois suggests a high circulation of pestiviruses, no viruses were found in the analysed samples. Pestiviruses are most easily detected in PI animals, but the existence of PI in chamois has not been proved. If they existed, they would have a poor survival rate. In this study only 20 of the 312 sampled chamois were under 1 year old. This could have decreased the chance of detecting possible PI animals and therefore the virus.
Twelve chamois showed positive antigen ELISA results, but they were negative to RT-PCR. There are two possible explanations for these ELISA-positive but RT-PCR-negative animals. The first one is that sensitivity and specificity of the commercial test used are known for domestic animals only. In addition, sera samples of this study were collected in dead animals and then specificity and sensitivity values are lower than in live animals (Olde Riekerink et al. 2005). Actually, the presence of false positives using this ELISA commercial kit in chamois samples has already been described (Marco et al. 2009a), suggesting a low specificity of this ELISA kit when used in sera from hunted animals. This emphasizes the problems of commercial kits’ use in species and conditions different from those recommended by the manufacturer. The second explanation is that these animals are not false positive but real positives, and that degradation of viral RNA during storage at −20°C has caused failure to detect pestiviral RNA by RT-PCR. We consider this second explanation less probable, since in retrospective studies performed in our laboratory, we have detected pestiviral RNA in samples stored at −20°C during 15 years.
The negative results observed in roe deer concur with other studies performed in roe deer (Olde Riekerink et al. 2005; Marco et al. 2009a). Seronegativity in roe deer could be explained by the different habitat and different social behaviour in this species compared to chamois. Roe deer are leading a more solitary life and prefer a more bush-containing habitat. Because of fewer inter-animal contacts, infectious diseases like BD or BVD may be expected to be less prevalent.
Summer communal pasturing is a common practice in Varaita Valley. This farming system frequently has been associated with the transmission of pathogens from domestic to wild ruminants (Loken 1995). The low seroprevalence of antibodies detected in cattle does not suggest a high circulation of pestiviruses among this species during the sampling period (2009), but there is a lack of information relating the frequency of antibodies in cattle in previous years. Unfortunately a very little number of sheep could be sampled, but the proportion of positive sheep was similar to the proportion of positive chamois in the same year. However, due to this lack of samples, these results should be interpreted with caution.
Regarding VNT results from livestock, cattle showed higher specific titres against the BVDV-NADL strain. Taking into account that sampled cattle came from nonvaccinated herds, the results of the present study indicate that BVDV are circulating in the herds from the valley. However, no differences were found in the titres between the two pestivirus strains in sheep. This strongly suggests the circulation of a different type of BDV, since sheep are more likely to be infected with BDV rather than with BVDV. Although seroprevalence and VNT results from sheep and chamois from our study suggest that pestiviruses are shared between these two species, we cannot dismiss the possibility of specific Pestivirus cycle into chamois populations. The higher proportion of seropositive females supports this hypothesis since it indicates that pestiviruses are transmitted between chamois in the group of females, and the maintenance of the virus in the population could be possible. However this study has some limitations like the relatively small sample size. Therefore, more studies with a bigger sample size should be performed.
The occurrence of BVDV-specific antibodies and isolation of virus from wild animals has lead to the speculation that free-living ungulates may be a reservoir of virus for transmission to cattle and sheep. This would be especially important in countries which are applying BVD eradication programmes (Vilcek and Nettleton 2006). In Italy voluntary BVDV control programmes for cattle have been described (Ferrari et al. 1999). The Region Piedmont, where Varaita Valley is located, started recently an eradication pilot programme for BVDV (Conterbia et al. 2010). Interestingly, results of our study indicate high exposure to pestiviruses in the chamois population of this valley, suggesting that this species could act as a Pestivirus reservoir. But our findings do not allow us to dismiss the possibility that the chamois were infected by domestic ruminants, playing a “victim” role in the dynamics of Pestivirus infection. For this reason, monitoring the presence of these viruses in wild ungulates would be of importance and this surveillance would be crucial in the chamois population, where pestiviruses seem to circulate extensively.
References
Altman DG, Machin D, Bryant TN, Gardner MJ (2000) Statistics with confidence, 2nd edn. British Medical Journal, London, pp 116–118
Carman S, van Dreumel T, Ridpath J, Hazlett M, Alves D, Dubovi E, Tremblay R, Bolin S, Godkin A, Anderson N (1998) Severe acute bovine viral diarrhea in Ontario, 1993–1995. J Vet Diagn Invest 10:27–35
Chappuis G, Brun A, Kato F, Duffour R, Durant M (1984) Isolement et caractérisation d'un pestivirus dans un foyer d'entérocolite leucopénie chez des moutons e l'Aveyron. Epidémiol Santé Anim 6:117–1180
Collett MS, Larson R, Gold C, Strick D, Anderson DK, Purchio AF (1988) Molecular cloning and nucleotide sequence of the pestivirus bovine viral diarrhea virus. Virology 165:191–199
Conterbia M, Pitti M, Brosio A, Franchini R, Beccaria D, Soncin AR, Ariello D, Bertola G, Cava P, Tinelli F, Dondo A, Gennero S, Masoero L, Chiavacci L, Bergagna S, Canale G, Guglielmetti C, Vitale N, Rossi F, Rubinetti F (2010) Definizione di un protocollo per la diagnosi ed il controllo della diarrea virale bovina in allevamenti da riproduzione ad alta produzione in Piemonte. Med Vet Prev 32:35–41
Evermann JF (2006) Pestiviral infection of llamas and alpacas. Small Ruminant Res 61:201–206
Ferrari G, Scicluna MT, Bonvicini D, Gobbi C, Della Verita F, Valentini A, Autorino GL (1999) Bovine virus diarrhoea (BVD) control programme in an area in the Rome province (Italy). Vet Microbiol 64:237–245
Frolich K, Hofmann M (1995) Isolation of bovine viral diarrhea virus-like pestiviruses from roe deer (Capreolus capreolus). J Wildl Dis 31:243–246
Holzwarth N, Pospischil A, Mavrot F, Vilei EM, Hilbe M, Zlinszky K, Regenscheit N, Pewsner M, Thoma R, Borel N (2011) Occurrence of Chlamydiaceae, Mycoplasma conjunctivae, and pestiviruses in Alpine chamois (Rupicapra r. rupicapra) of Grisons, Switzerland. J Vet Diagn Invest 23:333–337
Krametter-Froetscher R, Kohler H, Benetka V, Moestl K, Golja F, Vilcek S, Baumgartner W (2007) Influence of communal alpine pasturing on the spread of pestiviruses among sheep and goats in Austria: first identification of border disease virus in Austria. Zoonoses Public Health 54:209–213
Krametter-Froetscher R, Duenser M, Preyler B, Theiner A, Benetka V, Moestl K, Baumgartner W (2010) Pestivirus infection in sheep and goats in West Austria. Vet J 186:342–346
Loken T (1995) Ruminant pestivirus infections in animals other than cattle and sheep. Vet Clin North Am Food Anim Pract 11:597–614
Marco I, Lopez-Olvera JR, Rosell R, Vidal E, Hurtado A, Juste R, Pumarola M, Lavin S (2007) Severe outbreak of disease in the southern chamois (Rupicapra pyrenaica) associated with border disease virus infection. Vet Microbiol 120:33–41
Marco I, Rosell R, Cabezon O, Beneria M, Mentaberre G, Casas E, Hurtado A, Lopez-Olvera JR, Lavin S (2009a) Serologic and virologic investigations into pestivirus infection in wild and domestic ruminants in the Pyrenees (NE Spain). Res Vet Sci 87:149–153
Marco I, Rosell R, Cabezon O, Mentaberre G, Casas E, Velarde R, Lavin S (2009b) Border disease virus among chamois, Spain. Emerg Infect Dis 15:448–451
Martin C, Letellier C, Caij B, Gauthier D, Jean N, Shaffii A, Saegerman C (2011) Epidemiology of Pestivirus infection in wild ungulates of the French South Alps. Vet Microbiol 147:320–328
Nettleton PF, Entrican G (1995) Ruminant pestiviruses. Br Vet J 151:615–642
Nettleton PF, Gilray JA, Russo P, Dlissi E (1998) Border disease of sheep and goats. Vet Res 29:327–340
OIE (2008) Border disease. In Manual of diagnostic tests and vaccines for terrestrial animals. www.oie.int/manual-of-diagnostic-tests-and-vaccines-for-terrestrial-animals. Accessed 20 Jul 2011
Olde Riekerink RG, Dominici A, Barkema HW, de Smit AJ (2005) Seroprevalence of pestivirus in four species of alpine wild ungulates in the High Valley of Susa, Italy. Vet Microbiol 108:297–303
Reed LJ, Muench H (1938) A simple method for estimating fifty percent endpoints. Am J Hyg 27:493–497
Rothman KJ (2002) Epidemiology. An introduction. Oxford University Press, London, pp 130–143
Terpstra C, Wensvoort G (1988) The protective value of vaccine-induced neutralising antibody titres in swine fever. Vet Microbiol 16:123–128
Terpstra C, Bloemraad M, Gielkens AJL (1984) The neutralising peroxidase-linked assay for detection of antibody against swine fever virus. Vet Microbiol 9:113–120
Vilcek S, Nettleton PF (2006) Pestiviruses in wild animals. Vet Microbiol 116:1–12
Vilcek S, Herring AJ, Herring JA, Nettleton PF, Lowings JP, Paton DJ (1994) Pestiviruses isolated from pigs, cattle and sheep can be allocated into at least three genogroups using polymerase chain reaction and restriction endonuclease analysis. Arch Virol 136:309–323
Vilček S, Nettleton PF, Paton DJ, Belak S (1997) Molecular characterization of ovine pestiviruses. J Gen Virol 78:725–735
Acknowledgements
The authors would like to give special thanks to the staff and the hunters of the Comprensorio Alpino di Caccia CN2 in Varaita Valley for the collection of the samples and their support to this study. They are also very grateful to the veterinaries of the valley for their enthusiastic collaboration. Ph.D. studies of L. Fernández-Sirera are funded by a Formación de Profesorado Universitario grant of Ministerio de Educación of Spain.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by C. Gortázar
Rights and permissions
About this article
Cite this article
Fernández-Sirera, L., Cabezón, O., Dematteis, A. et al. Survey of Pestivirus infection in wild and domestic ungulates from south-western Italian Alps. Eur J Wildl Res 58, 425–431 (2012). https://doi.org/10.1007/s10344-011-0591-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10344-011-0591-1