Abstract
A study assessed the potential for using cumulative growing degree days (CGDD) to predict the weed emergence periodicity of three weed species: Argemone mexicana, Brassica tournefortii, and Rapistrum rugosum. Weed emergence was monitored regularly by placing 200 fresh seeds of each weed species on the soil surface. Weed emergence data was fit using a three-parameter sigmoidal Gompertz model. The CGDD required for 50% emergence of A. mexicana ranged from 3380 to 5302, depending upon the seasonal variation in temperature and rainfall. The majority of emergence appeared from March to June. The seeds of A. mexicana exhibited dormancy, as the majority of seeds germinated in the second season. The CGDD required for 50% emergence of B. tournefortii ranged from 824 to 2311, depending upon the seasonal variation in temperature and intensity of rainfall. Most cohorts of B. tournefortii appeared in the first season from February to June, indicating little dormancy in seeds. The CGDD required for 50% emergence of R. rugosum ranged from 2242 to 2699, depending upon weather parameters (temperature and rainfall). The main cohorts of R. rugosum appeared from February to June, and 60% of seeds germinated in the first season, while 40% germinated in the second season, indicating dormancy in seeds. The coefficients of determination for the model verification on the emergence pattern of three weeds were > 85%, suggesting that CGDD are good predictors for the emergence of these weeds. These results suggest that forecasting the emergence of three weed species on the basis of CGDD and rainfall patterns will help growers to make better weed management decisions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Knowledge of weed emergence time is very important for developing their control strategies, particularly for weeds that are established through seeds (Mohler 2001; Ryan et al. 2010). The timing of weed emergence is correlated with the biological attributes of the weed and the interaction of these attributes with environmental and management conditions (Cordeau et al. 2017). Therefore, improved knowledge of weed emergence periodicity may strengthen weed management programs (Norsworthy et al. 2012; Reinhardt-Piskackova et al. 2020). The timing of weed control, the sowing time of the crop, and seedbank depletion strategies (e.g., tillage) can be adjusted for the disfavour of weeds by having a thorough understanding of the weed emergence pattern (Sousa-Ortega et al. 2020; Batlla et al. 2020; Nordell and Nordell 2009).
The emergence of weeds varies with species, seed dormancy, pre-emergence growth habits, and prevailing environmental conditions (Batlla et al. 2020). Weeds with a wider emergence period are more difficult to control than weeds with a narrower emergence period. Modelling efforts are utilised to predict cumulative emergence and peak emergence time in relation to environmental conditions or growing degree days (GDD) using Weibull or Gompertz functions (Forcella et al. 1997; Royo-Esnal et al., 2020; Chauhan and Johnson, 2010). The peak emergence time of weeds could be helpful in deciding on timely weed management (Kumar et al. 2018).
In the northern grain region of eastern Australia, Argemone mexicana is considered one of the top 20 costly weeds (Llewellyn et al. 2016). This weed is a poor competitor in narrow-row crops, such as wheat (Triticum aestivum L.); however, it could cause substantial yield losses in chickpeas (Cicer arietinum L.) (Manalil and Chauhan 2019; Mahajan and Chauhan 2023). Seeds of A. mexicana have a resemblance to mustard seeds (Brassica nigra), and their mixing/adulteration in edible mustard oil could cause epidemic dropsy if consumed by humans in cooking (Verma et al. 2001). The yellow sap of the plant is poisonous to cattle due to the presence of berberine and potassium nitrate (Alam and Khan 2020; Vandebroek et al. 2020). In chickpea crops and under fallow conditions, A. mexicana could produce enough seeds to build up the seedbank in the soil due to its shattering behaviour (GRDC 2017). The fresh seeds of A. mexicana are dormant (Karlsson et al. 2003). The longevity of A. mexicana seeds is lower on the soil surface compared with buried seeds (Manalil and Chauhan 2021). The contamination of A. mexicana seeds in crops and fodder may reduce marketing quality. Moreover, the prickly nature of this weed may cause injury to humans and animals when in contact (GRDC 2017). Information on the emergence pattern of A. mexicana is limited in Australia, and an improved understanding of emergence periodicity may help in timely weed management.
Brassica tournefortii is a winter weed that is very prolific in seed production (Alemseged et al. 2001). The biological attributes, such as the ability to grow under a wide range of temperatures (15/5 to 35/15℃, day/night) and drought conditions, make this weed more invasive in Australia under different agro-ecological conditions (Mahajan et al. 2020; Singh et al. 2021). Under fallow conditions, this weed can produce up to 10,000 seeds/plant. The high seed-shattering tendency and ability to persist for a long time (> 2 years) in shallow soil layers could increase its infestation in the no-till cropping systems of Australia (Mahajan et al. 2020). Under Australian conditions, a 50% yield reduction in chickpeas could occur at a weed density of 10 plants m−2 (Mahajan and Chauhan 2023). However, this weed is a poor competitor in wheat crops, and its high infestation can cause only a 5–10% reduction in wheat yield (Gill et al. 2022; Chauhan et al. 2006). The evolved resistance of this weed to Group 2 herbicides in Australia necessitates its timely weed management (Alemseged et al. 2001). The filling of research gaps regarding the emergence periodicity of this weed may strengthen integrated weed management programs.
Rapistrum rugosum is widespread in Australia, and recently, infestations of this weed have become more prevalent in eastern Australia (Manalil et al. 2018). This weed in competition with wheat, could produce 32,000 seeds m−2 (Manalil and Chauhan 2019). Under fallow conditions, one single plant has the potential to produce 13,000 seeds (Mobli et al. 2020). The biological attributes, such as the ability to emerge under a wider range of temperatures (15/5 to 35/25 ℃; day/night temperatures), high seed production potential, fast growth (took 57 days to flower), and long persistence (> 3.5 years) of seeds in a shallow layer, are the reasons for its wider adaptation in Australia (Manalil et al. 2018; Mobli et al. 2020; Singh et al. 2021). In Australia, R. rugosum has evolved resistance to Group 2 herbicides and, therefore, requires strategic weed management programs based on an understanding of its emergence pattern (Hatami et al. 2016).
Knowledge gaps exist in relation to the emergence patterns of these three weeds, viz., A. mexicana, B. tournefortii, and R. rugosum, in eastern Australia. Information on weed emergence timing is important for developing suitable strategies for weed control and can strengthen decision-support systems for weed management (Chauhan et al. 2012; Mahajan and Chauhan 2021). It was hypothesised that populations of these three weeds collected from different environmental conditions might have adaptative traits for varied emergence patterns when tested under the same environmental conditions. Therefore, the objective of this study was to determine the emergence pattern of various populations of three important winter season weeds (A. mexicana, B. tournefortii, and R. rugosum) collected from different cropping regions of Australia.
Materials and Methods
The study comprised nine populations of A. mexicana, eight populations of B. tournefortii, and 10 populations of R. rugosum at the Gatton Research Farms (27.5514° S/152.3428° E) of the University of Queensland, Australia. Populations of different weeds selected for the study were collected from paddocks in the eastern region of Australia in October 2018. After collection, seeds were air-dried for 7 d in a screenhouse and then used in the study. Seeds were used immediately to coincide with the natural seed-shattering time of these weeds. The first and second experimental studies were commenced on December 2, 2018 and December 10, 2019, respectively.
The study was conducted by spreading 200 seeds in rings (made up of plastic) that were placed in the field (27.5514° S/152.3428° E). The diameter and height of the ring were 30 cm and 5 cm, respectively. Seeds in the rings were spread on the soil surface. Seedling emergence was counted regularly at 7 d intervals. Emerged seedlings were killed using spot herbicide (glyphosate at 740 g a.e. ha−1) applications each time seedlings were counted. Seedling emergence data were recorded for more than two years. The seeds for the second-year study were stored in a refrigerator at 4 ℃ to maintain the dormancy level of the seeds (Kumar et al. 2018), which was similar to the first year.
Weather Parameters
During the study, weather observations, viz., minimum and maximum air temperatures and rainfall, were recorded from the Bureau of Meteorology (BOM), Australia (http://www.bom.gov.au/climate/dwo/).
Statistical Analyses
Populations of three selected weeds showed a similar trend for emergence behaviour; therefore, all the tested populations for each species were pooled to calculate the emergence percentage of the maximum. Emergence pattern graphs were plotted using SigmaPlot 14.0 (Systat Software, San Jose, CA, USA). Daily emergence counts during the study were converted into cumulative percentages of total seeds, and the means of different populations were compared with standard errors.
Cumulative emergence was described as a function of GDD using Sigma Plot 14.0, and data were presented separately for each year as the year’s effect was significant. The data were subjected to a three-parameter sigmoidal Gompertz model:
where, y is the total cumulative emergence (%) at time x, b indicates the slope, a is the maximum emergence (%), and x0 is the estimated time (GDD) for 50% emergence (%). GDD was calculated using the formula:
where Tmax and Tmin are the maximum and minimum air temperatures, and Tb is the base temperature (5 ℃) for winter species (Vigil et al. 1997).
Results and discussion
Argemone mexicana
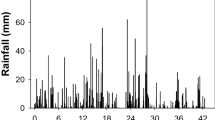
Emergence data in each experimental year were recorded for more than 24 months until emergence ceased. In the first experiment, multiple cohorts (eight cohorts) of A. mexicana appeared from March 2019 to June 2020 (Fig. 1; Tables 1 and 2). The emergence was 0.3% (of the maximum) on March 22, 2019 [2374 cumulative growing degree days (CGDD)], which increased to 5% on June 28, 2019 (2992 CGDD). This emergence in the second season further increased to 71 and 91% (of the maximum) on January 24, 2020 (7146 CGDD) and March 27, 2020 (8410 CGDD), respectively. The cumulative emergence of A. mexicana in the first-year experiment reached its maximum on June 19, 2020 (9577 CGDD; about 535 days after seed spread).
The emergence pattern of Argemone mexicana (means of nine populations) in relation to cumulative growing degree days a the first experiment starting from December 2018 and ending in June 2020; b the second experiment starting from December 2019 and ending in April 2021. Vertical bars represent daily rainfall. Error bars indicate standard error (±). The line represents a three-parameter sigmoidal Gompertz model fit to the data and y is the total cumulative emergence (%) at time x
In the second experiment, five cohorts of A. mexicana appeared from January 2020 to April 2021. The emergence was 3% on January 21, 2020 (906 CGDD), which increased to 51% on March 24, 2020 (2292 CGDD), and reached its maximum on April 2, 2021 (8157 CGDD). The regression analysis predicted that the CGDD required for 50% emergence of A. mexicana was 5302 and 3380 in the first and second-year experiments, respectively (Fig. 1 and Table 2).
In the first experiment, rain events of > 10 mm did not appear in January and February 2019 (the first season), while in the second experiment (the first season), there were three and five events of > 10 mm rainfall in January and February 2020, respectively (Table 3). High rainfall at the start of the second year experiment could be the reason for the lower CGDD for the 50% emergence of A. mexicana in the second experiment compared with the first experiment. These observations suggest that high rainfall (> 10 mm) in January and February (the start of the summer season) enhanced the emergence of A. mexicana and decreased the CGDD for emergence. If rainfall is low (< 10 mm: not effective rainfall) in the months of January and February, most seeds of A. mexicana remain dormant and germinate at a time when favourable environmental and soil moisture conditions are available. It has been suggested that 10 mm of rainfall (effective rainfall) could allow germination and emergence of winter plants such as wheat in most soil types in Australia (Clarke et al. 2019). Therefore, we have assumed that the three winter weeds require sufficient moisture (effective rainfall) in the surface layer of soil for germination.
In Australia, farmers keep their fields fallow after harvesting summer crops, such as sorghum [Sorghum bicolor (L.) Moench], maize (Zea mays L.), cotton (Gossypium hirsutum L.), etc. Argemone mexicana has a tendency for peak emergence in February and March if sufficient rainfall is available; therefore, this weed could emerge and grow luxuriantly in the winter seasons and reinfest and enrich the seedbank in the soil by producing more seeds. The infestation of A. mexicana in fallows has been observed in many parts of eastern Australia (GRDC 2017). One plant of A. mexicana could produce up to 30,000 seeds (https://portal.wiktrop.org/species/show/29); therefore, this weed could invade the fallow systems of Australia if it is not controlled. Further, A. mexicana is poisonous to humans and livestock (CottonInfo 2014), and its infestation in pasture land and Brassica/canola crops may injure humans and livestock (CottonInfo 2014).
The seeds of A. mexicana have innate dormancy, and the mechanism for its dormancy is not well understood (Manalil and Chauhan 2021). Dormant seeds of A. mexicana in the field are a concern and may increase weed seed persistence. However, this weed has poor competitive ability with closely spaced crops, such as wheat, barley (Hordeum vulgare L.), etc., and is unable to produce seeds in these crops (Manalil and Chauhan 2019). Therefore, an infestation of this weed in Australian fields could be reduced by exploiting crop competition using narrow-row crops and a high seeding rate. As this weed has a higher tendency to emerge before the start of winter seasons (evident from the data), pre-sowing tillage or the use of nonselective herbicides before winter crop sowing could eliminate this weed from the paddocks.
Brassica tournefortii
In both experimental years, multiple cohorts of B. tournefortii appeared. In the first experiment, emergence was 1% (of the maximum) on February 21, 2019 (1761 CGDD), which increased to 66 and became maximum on March 29, 2019 (2506 CGDD) and April 26, 2019 (2960 CGDD), respectively (Fig. 2; Tables 2 and 5). In the first experiment, cohorts of B. tournefortii did not appear in the second season, suggesting that seeds had low dormancy on the soil surface layer.
The emergence pattern of Brassica tournefortii (means of eight populations) in relation to cumulative growing degree days a the first experiment starting from December 2018 and ending in April 2020; b the second experiment starting from December 2019 and ending in April 2021. Vertical bars represent daily rainfall. Error bars indicate standard error (±). The line represents a three-parameter sigmoidal Gompertz model fit to the data and y is the total cumulative emergence (%) at time x
In the second experiment, the first cohort of B. tournefortii appeared on February 15, 2020 (1455 CGDD) with 71% emergence (Table 4). This emergence increased to 97% on June 14, 2020 (3352 CGDD). One cohort of B. tournefortii in the second experiment appeared on April 2, 2021 (8157 CGDD). The regression analysis predicted that the CGDD requirements for 50% emergence of B. tournefortii was 2311 and 824 in the first and second experiments, respectively.
These results suggest that most seeds of B. tournefortii germinate in the first season if sufficient rainfall is available. The rainfall at the experimental site from January to March 2019 was lower compared with 2020 (Table 3). Therefore, relatively higher rainfall from January to March 2020 could be the reason for less CGDD for the 50% emergence of B. tournefortii in 2020 compared with 2019. About 98% of seeds of B. tournefortii germinated in the first season, suggesting that seeds of B. tournefortii had lower dormancy compared with A. mexicana. Contrary to this, in our previous laboratory study, it was found that seeds of B. tournefortii had dormancy and germination increased in a dark environment or with NaOCl treatment (Mahajan et al. 2018). It is quite possible that under field conditions with the onset of sufficient rainfall, chemicals present in the soil are dissolved, which may result in the breaking of dormant seeds.
The little dormancy of B. tournefortii on the soil surface layer suggests that restricting seed production in the no-till field could eliminate this weed in a shorter time. However, the ability of this weed to produce a high number of seeds could increase its infestation in fallows (Mahajan et al. 2020). It has been observed that one plant of B. tournefortii could produce 5500 seeds, and the high shattering tendency of this weed could reinfest the area (Mahajan et al. 2020). Brassica tourneforii could grow under a wide range of temperatures, suggesting that in slow-growing winter crops, such as chickpeas, its infestation could increase due to less competition offered by the crop (Singh et al. 2021). Herbicide options for controlling broad-leaf weeds in crops, such as chickpeas, are limited (Shahbazi et al. 2019). Therefore, its infestation in such crops may build up the seedbank in the soil if not timely controlled. The ability of this weed to produce enough seeds in a drought environment could increase its invasion in fallows (Mahajan et al. 2020). The poor competitive ability of this weed in narrow row crops, such as wheat and barley, could reduce seed production for reinfestation in that area. It is essential to control this weed in winter fallows with the help of herbicides to reduce seedbank.
Rapistrum rugosum
In the first experiment, 14 cohorts of R. rugosum appeared from March 2019 to March 2020. Similarly, in the second experiment, 12 cohorts of R. rugosum appeared from February 2020 to April 2021 (Table 5).
In the first experiment, the first cohort of R. rugosum appeared on March 22, 2019 (2374 CGDD) with 16% (of the maximum) emergence (Fig. 3; Table 2). This emergence increased to 45, 60, and 62% on April 5 (2619 CGDD), June 28 (3700 CGDD), and July 12 (3854 CGDD) 2019, respectively. In the second season, this emergence increased to 97% on 14 February 2020 (7236 CGDD) and reached its maximum on March 27, 2020 (8057 CGDD).
The emergence pattern of Rapistrum rugosum (means of ten populations) in relation to cumulative growing degree days a the first experiment starting from December 2018 and ending in March 2020; b the second experiment starting from December 2019 and ending in April 2021. Vertical bars represent daily rainfall. Error bars indicate standard error (±). The line represents a three-parameter sigmoidal Gompertz model fit to the data and y is the total cumulative emergence (%) at time x
In the second experiment, the first cohort of R. rugosum appeared on February 7, 2020 (1288 CGDD) with 1% emergence (Fig. 3). This emergence increased to 41, 50, and 55% on April 5 (2005 CGDD), August 7 (3885 CGDD), and November 13 (5291 CGDD) 2019, respectively. In the second season, this emergence increased to 60% on March 19, 2021 (7907 CGDD) and became maximum on April 2, 2021 (8157 CGDD). The regression analysis predicted that the CGDD required for 50% emergence of R. rugosum was 2699 and 2242 in the first and second experiments, respectively (Fig. 3).
In both experiments, almost 40% of the seeds of R. rugosum germinated in the second season, suggesting that this weed also has a strong dormancy. These results also showed that the temperature conditions of February and March were ideal for the emergence of this weed, provided there was a sufficient amount of rainfall available.
Rapistrum rugosum produced multiple cohorts throughout the year due to its adaptability to a wide range of temperatures (15/5 C to 30/20 ℃) for emergence (Manalil et al. 2018). Previous studies have reported that this weed is highly competitive in winter crops, such as wheat and chickpea, and prolific in seed production (Mobli et al. 2020; Mahajan and Chauhan 2023). The high infestation of this weed could create problems in harvesting winter crops such as wheat, barley, chickpea, etc. However, the high seed retention ability of this weed provides an opportunity for harvest weed seed control (Manalil and Chauhan 2021). Early (April-May) cohorts of R. rugosum produced more seeds compared with late cohorts (June-July) (Mobli et al. 2020). Efforts should be made to control early cohorts of this weed in slow-growing winter crops, such as chickpeas. Late cohorts can also produce sufficient seeds in fallows; therefore, allowing surviving plants of R. rugosum in fallows may increase its seedbank in the soil. Late cohorts of R. rugosum in winter crops could escape from the early application of post-emergence herbicides, In such situations, integrated control of this weed using agronomic practices, such as narrow row spacing, adjusting planting time, and exploring weed competitive cultivars may reduce the seed production potential of this weed.
In conclusion, knowledge gained from the emergence patterns of A. mexicana, B. tournefortii, and R. rugosum may help in decision-making processes for timely weed control, enhance crop competition, expedite weed seedbank depletion, and strengthen sustainable weed management programs. Weed management decisions can be modified for improved weed control by having a thorough understanding of the season variation and weed emergence patterns of these weeds.
Data Availability
All relevant data are within the paper and its Supporting Information files.
References
Alam A, Khan AA (2020) Argemone mexicana L.: A weed with versatile medicinal and pharmacological applications. Ann Phytomed Int J: 218–223
Alemseged Y, Jones RE, Medd RW (2001) A farmer survey of weed management and herbicide resistance problems of winter crops in Australia. Plant Prot Q 16:21–25
Batlla D, Malavert C, Farnocchia RBF, Benech-Arnold R (2020) Modelling weed seedbank dormancy and germination. In: Chantre GR, González-Andújar JL (eds) Decision support systems for weed management. Springer, New York https://doi.org/10.1007/978-3-030-44402-0_4
Chauhan BS, Johnson DE (2010) The role of seed ecology in improving weed management strategies in the tropics. Adv Agron 105:221–262
Chauhan B, Gill G, Preston C (2006) African mustard (Brassica tournefortii) germination in southern Australia. Weed Sci 54:891–897
Chauhan BS, Singh RG, Mahajan G (2012) Ecology and management of weeds under conservation agriculture: a review. Crop Prot 38:57–65
Clarke G, Porker K, Hunt J, Angel K, Wallace A (2019) Management of early sown wheat: soil water requirements for establishment. In: Proceedings of the 2019 Agronomy Australia Conference, Wagga Wagga, 25–29 August 2019 (www.agronomyaustralia.org/conference-proceedings)
Cordeau S, Smith RG, Gallandt ER, Brown B, Salon P, Ditommaso A et al (2017) Timing of tillage as a driver of weed communities. Weed Sci 65:504–514
CottonInfo (2014) Weedpak weed Id Guide (Compiled by Charles Graham, Larsen David)
Forcella F, Wilson RG, Dekker J, Kremer RJ, John C, Randy LA et al (1997) Weed seed bank emergence across the corn belt. Weed Sci 45:67–76
Gill G, Borger C, Chauhan BS (2022) Ecology of Major Emerging Weeds. https://grdc.com.au/resources-and-publications/all-publications/publications/2021. Accessed: December 9, 2023
GRDC Weed management in chickpeas. https://grdc.com.au/resources-and-publications
Hatami ZM, Gherekhloo J, Rojano-Delgado AM, Osuna MD, Alcántara R, Fernández P, De Prado R (2016) Multiple mechanisms increase levels of resistance in Rapistrum rugosum to ALS herbicides. Front Plant Sci 7:169
Karlsson LM, Tamado T, Milberg PER (2003) Seed dormancy pattern of the annuals Argemone ochroleuca and A. mexicana (Papaveraceae). Flora Morphol Distrib Funct Ecol Plants 198:329–339
Kumar V, Jha P, Dille JA, Stahlman PW (2018) Emergence dynamics of kochia (Kochia scoparia) populations from the US Great Plains: a multi-site-year study. Weed Sci 66:25–35
Llewellyn R, Ronning D, Ouzman J, Walker S, Mayfield A, Clarke M (2016) Impact of weeds on Australian grain production: the cost of weeds to Australian grain growers and the adoption of weed management and tillage practices. Grains Research and Development Corporation and the Commonwealth Scientific and Industrial Research Organisation
Mahajan G, Chauhan BS (2021) Seed longevity and seedling emergence behavior of wild oat (Avena fatua) and sterile oat (Avena sterilis ssp. ludoviciana) in response to burial depth in eastern Australia. Weed Sci 69:362–371
Mahajan G, Chauhan BS (2023) Interference of Brassicaceae weeds (Brassica tournefortii, Rapistrum rugosum, and Sisymbrium thellungii) in chickpea. Weed Sci. https://doi.org/10.1017/wsc.2023.4
Mahajan G, Mutti NK, Jha P, Walsh M, Chauhan BS (2018) Evaluation of dormancy breaking methods for enhanced germination in four biotypes of Brassica tournefortii. Sci Rep 8:17103. https://doi.org/10.1038/s41598-018-35574-2
Mahajan G, Singh R, Chauhan BS (2020) Biology of Brassica tournefortii in the northern grains region of Australia. Crop Pasture Sci 71:268–277
Manalil S, Chauhan BS (2019) Interference of turnipweed (Rapistrum rugosum) and Mexican pricklepoppy (Argemone mexicana) in wheat. Weed Sci 67:666–672
Manalil S, Chauhan BS (2021) Seedbank persistence and emergence pattern of Argemone mexicana, Rapistrum rugosum and Sonchus oleraceus in the eastern grain region of Australia. Sci Rep 11:1–9
Manalil S, Ali HH, Chauhan BS (2018) Germination ecology of turnip weed (Rapistrum rugosum (L.) All.) in the northern regions of Australia. PLoS ONE 13(7):e201023
Mobli A, Manalil S, Khan AM, Jha P, Chauhan BS (2020) Effect of emergence time on growth and fecundity of Rapistrum rugosum and Brassica tournefortii in the northern region of Australia. Sci Rep 10:1–10
Mohler CL (2001) Weed life history: identifying vulnerabilities. In: Liebman M, Mohler CL, Staver CP (eds) Ecological management of agricultural weeds. Cambridge University Press, Cambridge, pp 40–98
Nordell A, Nordell E (2009) Weed the soil, not the crop. Acres Usa 40:21–28
Norsworthy JK, Ward SM, Shaw DR, Llewellyn RS, Nichols RL, Webster TM et al (2012) Reducing the risks of herbicide resistance: best management practices and recommendations. Weed Sci 60:31–62
Reinhardt Piskackova TA, Reberg-Horton C, Richardson RJ, Jennings KM, Leon RG (2020) Integrating emergence and phenology models to determine windows of action for weed control: a case study using senna obtusifolia. Field Crop Res 258:e107959. https://doi.org/10.1016/j.fcr.2020.107959
Royo-Esnal A, Torra J, Chantre GR (2020) Weed emergence models. In: Chantre GR, González-Andújar JL (eds) Decision support systems for weed management. Springer, Cham, pp 85–116
Ryan MR, Smith RG, Mirsky SB, Mortensen DA, Seidel R (2010) Management filters and species traits: weed community assembly in long-term organic and conventional systems. Weed Sci 58:265–277
Shahbazi S, Diyanat M, Mahdavi S, Samadi S (2019) Broadleaf weed control in rain-fed chickpea. Weed Technol 33:727–732
Singh S, Mahajan G, Singh R, Chauhan BS (2021) Germination ecology of four African mustard (Brassica tournefortii Gouan) populations in the eastern region of Australia. Weed Sci 69:461–467
Sousa-Ortega C, Royo-Esnal A, DiTommaso A, Izquierdo J, Loureiro I, Marı AI et al (2020) Modeling the emergence of north african Knapweed (Centaurea diluta), an increasingly troublesome weed in spain. Weed Sci 68:268–277. https://doi.org/10.1017/wsc.2020.22
Vandebroek I, Picking D, Vandebroek I, Picking D (2020) Argemone mexicana L.(Papaveraceae). In: Popular Medicinal Plants in Portland and Kingston, Jamaica, pp 45–54
Verma SK, Dev G, Tyagi AK, Goomber S, Jain GV (2001) Argemone mexicana poisoning: autopsy findings of two cases. Forensic Sci Int 115:135–141
Vigil MF, Anderson RL, Beard WE (1997) Base temperature and growing degree-hour requirements for the emergence of canola. Crop Sci 37:844–849
Funding
This research was funded by GRDC with grant number US00084.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions
Author information
Authors and Affiliations
Contributions
GM ran the experiment and recorded the data. BSC and GM designed the study. GM wrote the initial draft; BSC edited the manuscript; All authors read and approved the paper.
Corresponding author
Ethics declarations
Conflict of interest
G. Mahajan and B. Singh Chauhan declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mahajan, G., Singh Chauhan, B. Emergence Pattern of Argemone mexicana, Brassica tournefortii, and Rapistrum rugosum in Eastern Australia. Journal of Crop Health 76, 841–850 (2024). https://doi.org/10.1007/s10343-024-01003-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10343-024-01003-w