Abstract
In forest ecosystems, interception of rainwater on foliar and woody surfaces and the subsequent partitioning into stemflow is largely controlled by physical and hydrological properties of bark. Few forest ecohydrology studies have explored the role of bark properties (e.g., thickness, density) on bark water storage capacity and stemflow production. Even fewer have explored how different phases of water (e.g., liquid, vapor) may affect bark through bark swelling properties across the stem and how the degree of swelling affects tradeoffs between bark water storage and stemflow generation. Thus, the objective of this study was to analyze changes in a bark swelling index (BSI) vertically along stems of Picea abies (Norway spruce) after exposure to both water vapor and liquid water, as a function of tree age and bark moisture content. We found that tree age influenced BSI and bark moisture content, wherein BSI was ∼ 6.5% lower in older trees (70 years) compared to younger trees (35 and 50 years), and average moisture content was 10.4–13.2% lower. BSI increased when bark was exposed to hygroscopic water vapor and reached maximum swelling after 1 day of water saturation. BSI also increased from the base of the tree to 20–30% of total tree height, beyond which BSI remained relatively stable across all age classes. Enhanced understanding of bark swelling mechanisms as a result of stem position, age, and moisture content and exposure provide stronger foundations for understanding canopy hydrologic partitioning and the fate of rainwater moving through forest canopies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In forest ecosystems, rainwater may be intercepted by foliar or woody surfaces of trees. The characteristic of those surfaces then determines whether the rainwater is shed as throughfall or stemflow or retained as interception to be evaporated back to the atmosphere (Van Stan et al. 2016; Campellone et al. 2020). In general, the forest ecohydrological research community has focused mainly on leaf-water interactions, with bark-water interactions remaining an understudied process in forest ecosystems (Van Stan et al. 2021). Specifically for woody surfaces, bark physical and hydrologic properties determine the proportion of rainfall partitioned into stemflow. Tree bark has a complex chemical structure rich in extractives, polyphenolics and inorganic materials (Baptista et al. 2013; Tonello et al. 2021a), as well as differences in physical and mechanical properties including density, hygroscopicity, and dimensional stability (e.g., shrinkage and swelling). These properties are highly variable—not only among different tree species but also within individual species and even within individual trees (Ilek et al. 2021). Bark morphology, chemical composition and microscopic structure of bark determine its moisture absorption properties (Klügl and Di Pietro 2020). Bark porosity, as well as pore size distribution, plays a fundamental role in the absorption of moisture and rain. While large pores may absorb water quickly, water tends to be restricted to these pores. On the other hand, small pores absorb water through capillary action and can redistribute water within bark tissues. Under both of these scenarios, bark absorbs rainwater or environmental moisture, which leads to bark swelling (Raczkowski 1979) and change the stem diameter (Oberhuber et al. 2020).
Our current understanding of the role of bark in forest hydrology comes primarily from studies on bark surface texture and thickness. For example, species with thin, smooth bark have lower water holding capacities and generate stemflow more quickly compared to species with thicker bark that absorb more water and generate very little stemflow (Alexander and Arthur 2010; Siegert and Levia 2014; Tonello et al. 2021a). Additional bark properties such as hygroscopicity and wettability have come under consideration in recent years as key determinants in canopy water partitioning. In species with highly wettable bark there is a strong and positive correlation between bark absorbability and stemflow generation. In contrast, species with non-wettable bark displayed inverse relationships between absorbability and stemflow generation (Tonello et al. 2021b). While these studies provide preliminary insight into the role of bark properties on bark water storage capacity (Valovà and Bieleszovà 2008; Ilek et al. 2021) and the stemflow production (Livesley et al. 2014; Tonello et al. 2021a, b limited amount of research exists as to how rainwater or water vapor affect bark swelling and further, how the degree of swelling or shrinkage of bark can subsequently affect tradeoffs between bark water storage and stemflow generation. Most studies concerning the sorption behavior of bark focused on the influence of the addition of bark material to the properties of wooden particleboards (e.g., Standke and Schneider 1981; Holmberg et al. 2016) or the influence of shrinkage/swelling of the inner bark and xylem on daily stem diameter variations (e.g., Sevanto et al. 2011; Zweifel 2016).
Moreover, little work has focused on determining vertical variability in bark’s physical and hydrological properties (e.g., Levia and Wubbena 2006; Ilek et al. 2021), and to the best of our knowledge, no study to date has been conducted on the swelling properties of bark depending on bark location on the stem, tree age and bark moisture content. Therefore, in our research, we focused on the potential ability of Norway spruce bark (containing both outer and inner bark) to swell under the influence of both water vapor from saturated air and liquid water, expressed in terms of the bark swelling index. Consideration of outer bark, inner bark, and both components as a whole are important to our understanding of bark water relations. While the outer bark serves primarily as a barrier between the living tree and the external environment (Biggs 1992; Klügl and Di Pietro 2020), it has greater water storage capacity and more variable structural characteristics (Ilek et al. 2021) compared to living inner bark whose primary function in translocation of photosynethic products. Together, the outer bark and inner bark provide a complex and multifunctional system (Eberhardt 2013). As such, our research objectives are (1) to determine the vertical variation of bark swelling index at different levels of bark moisture content and (2) to determine the influence of tree age on the vertical variation of bark swelling index-bark moisture content relations. Better recognition of the bark swelling phenomenon will enhance the current understanding of species-specific behavior in response to contact with rainwater or water vapor and may be helpful in ecohydrological and physiological studies. Understanding the swelling and shrinking of the bark also has practical importance when measuring the diameter at breast height of the trees and in fact, importance for estimating wood volume that could be extracted from trees or logs.
Materials and methods
Study site and bark samples collection
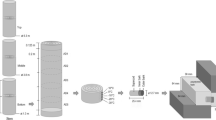
Bark samples including outer and inner bark were collected during the 2020 growing season from three Norway spruce stands differing in age, i.e. 35, 50 and 70 years old, located in western Poland within the State Forests (Lwówek Śląski District, Gradówek Subdistrict), elevation ∼ 355 m asl (Fig. 1a; Table 1). The study site is situated in a temperate climate area, where the average annual temperature is 8℃, and the average annual precipitation is ∼ 600 mm (www.bdl.lasy.gov.pl/portal/mapy). In each stand, we randomly selected three representative trees (Table 1) that were free of lichen and beetles, both of which can influence bark hydrology. We felled each tree and segmented the stem into 11 equally divided sections based on 0.0, 0.1, 0.2, 0.3, 0.4, 0.5, 0.6, 0.7, 0.8, 0.9 and 1.0 of tree height, where 0.0 and 1.0 are the bottom and the top of given tree, respectively (Fig. 1b). Rectangular bark samples were collected using a chisel, knife and hammer from each location along the stem (11 samples per tree, 33 samples per stand, and 99 bark samples in total).
Bark swelling index measurements
A bark-swelling index was calculated to assess the temporal degree of change in bark area relative to bark water sorption as follows. Bark samples were first air-dried in the laboratory and then measured for the thickness of outer and total bark using a digital calliper and calculated the outer to total bark thickness ratio. Next, bark samples were cut into segments 1.0 cm wide using a band saw. The cross-sections of each segment were smoothed with sandpaper and cleaned of sawdust with a brush, and then we used two bark pieces per location on the stem to analyze bark swelling properties after different times of water contact with bark (22 bark samples per tree, 66 samples per stand, and 198 samples in total). The prepared, air-dried samples were first weighed with an accuracy of 0.01 g, and then scans of cross-sections of the bark samples were made at a resolution of 600 dpi using SigmaScan Pro.5 software (FLATBED SCANNER A3 2400 S). For each sample, a scan of one cross-section was completed. Next, all bark samples were placed in desiccators partially filled with water, where relative air humidity was close to 100%. We kept bark samples in desiccators, recording change in mass every two days until reaching a constant mass and maximum hygroscopicity (Ilek et al. 2016). Next, we rescanned the bark samples (Fig. 2). Then bark samples were placed in containers with water and entirely immersed by covering the samples of the bark floating on the water surface with cotton material. The samples were taken out of the water after 1 h, 1 day, 2 days, and 7 days from their immersion in water, and each time the samples were weighed, and their cross-sections were scanned (Fig. 2). Excess water was removed from bark surfaces using a damp paper towel before measuring sample mass and scanning.
We calculated the bark moisture content MC (%) after initial water vapor absorption and then after each soaking time according to equation:
where M is the mass of bark sample, and x is the given time of contact of the bark with water (1 h, 1d, 2d or 7d) or the state of filling the bark with water vapor (SH).
The swelling of each bark sample was computed using a bark swelling index BSI which considers the irregular plate–fissure bark morphology, i.e., cracks and cavities. The BSI determination consisted of (1) measuring the initial cross-section area for individual dry bark samples (A0), (2) measuring the cross-section area for individual bark samples after the absorption of water vapor from saturated air (ASH) and after the given soaking time (A1h, A1d, A2d, A7d), and (3) calculating the BSI according to the formula:
The minimum value that BSI can reach is 1.0, indicating bark did not swell and that the cross-sectional area measured after contact with water or water vapor was equal to the initial dry area A0. The greater the value of BSI, the greater the swelling of the bark in relation to the initial dry area A0 (Fig. 2).
In total, we measured 1188 cross sectional areas of Norway spruce bark samples (198 measurements for dry bark and 990 measurements after the contact of bark samples with water vapor or liquid water).
3-stages scheme of bark swelling index (BSI) determination. Stage I shows scans of the cross-section of bark sample in different states of bark hydration, i.e., air-dried bark (0), after saturation of bark with hygroscopic water (SH), and after immersion of bark in water by 1 h, 1 day, 2 days, and 7 days. Stage II is the cross-section area measurements (A) in the given state of bark hydration. Stage III is the bark swelling index calculation procedure based on the cross-section area, where x is the given time of contact of the bark with water (1 h, 1d, 2d, 7d) or the state of filling the bark with water vapor (SH).
Statistical analysis
We performed the statistical analysis and associated graphics using Statistica 13.3 PL software (StatSoft Inc.). Data were checked for normality using the Shapiro-Wilk test and were found to be non-parametric. Therefore, we used Kruskal-Wallis to test for significant differences in thickness, the ratio of outer to total bark thickness, bark swelling index and moisture content between stem locations, tree ages, and bark hydration as a function of tree age, stem height, and time exposure to moisture. We adopt a general linear model (GLM) to investigate the effect of the location on the stem, tree age, and bark hydration on bark swelling index. All tests were performed at a significance level of 0.05.
Results
Both bark thickness and the ratio of outer to total bark thickness decreased with stem height (Table 2). The thickness of Norway spruce bark ranged from 0.05 to 0.98 cm (on average 0.23 ± 0.01 cm), while the outer to total bark thickness ratio ranged from 0.10 to 0.70 (on average 0.43 ± 0.01). The bark thickness of 70 year old trees was 58.2 and 45.2% higher than 35 and 50 years old trees (p < 0.001), respectively. Similarly, the outer to total bark thickness ratio of oldest trees was 29.1% higher than younger trees (p < 0.001) (Fig. 3). Although bark thickness differed significantly between 35 and 50 years old trees (p < 0.001), we did not observe differences in the ratio of outer to total bark thickness between these two age groups (p > 0.05, Fig. 3). Across all ages of trees, bark thickness decreased from the bottom (0.0) to the top (1.0) of trees (Table 2). Outer to total bark thickness exhibited a consistent decline with height across all age trees with an average decline of ∼ 30%, but the most distinct variation between stem sections were observed in the oldest trees (Table 1).
Regardless of tree age and location on the stem, BSI of all examined bark samples ranged from 1.00 to 2.03 (on average 1.44 ± 0.01), while the bark moisture content ranged from 21.7 to 270.0% (on average 117.1 ± 2.0%). The BSI of the oldest trees was ∼ 6.5% lower than that of younger trees, i.e., 35 and 50 years old (p < 0.001) (Fig. 4a). Similarly, the average moisture content of bark collected from oldest trees was 13.2% and 10.4% lower than those collected from 35 (p = 0.003) and 50 years old trees (p = 0.009), respectively (Fig. 4b).
The contact of bark with water vapor or liquid water significantly affected the BSI (Fig. 5a) and bark moisture content (Fig. 5b). During initial exposure to water vapor, bark hygroscopicity ranged from 21.7 to 40.4% (on average 28.5 ± 0.3%). BSI in this bark moisture content range was 1.20 ± 0.01 (Fig. 5). The greatest changes in BSI were observed after 1 h and 1 day of bark saturation, beyond this time frame BSI did not change (Fig. 5a), although bark moisture content continued to increase significantly with each time interval, reaching a maximum after 7 days of immersion (Fig. 5b). In combination, BSI and bark moisture content exhibited a strong positive relationship (Fig. 5c).
We observed some influence of tree age on the BSI and MC in each state of bark hydration. In most states of bark hydration, BSI and MC from the 70 year old trees were significantly lower than BSI and MC of bark collected from younger trees, i.e., 35 and 50 years old (Fig. 6a and b).
Regardless of the time of bark saturation and tree age, average BSI was significantly lower in the bottom (0.0) and at 0.1 stem height than in the upper parts of the trees (Fig. 7a). The moisture content of bark collected from the lowest tree section was 36.4% lower than in the upper parts of the trees (Fig. 7b). Marginally more variability was observed in BSI compared to moisture content in upper tree sections, with BSI displaying a slight decrease in the uppermost tree section (Fig. 7a and b). Similar trends were also observed for BSI when taking into account age of trees and bark hydration (Fig. 8). The GLM analysis confirmed the influence of tree age and stem location on the BSI in the particular state of bark hydration (Table 3).
BSI was inversely correlated with bark thickness, where thicker bark resulted in lower BSI values. This relationship was stronger after 7 days of submersion (BSI7d) compared to after initial hygroscopic wetting (BSISH) (Fig. 9). Interestingly, in the case of the thickest bark with a large proportion of outer bark at lower stem heights (Table 2), the differences between BSISH and BSI7d were not so clear. The greatest differences between these indices were observed in stem sections with thinner bark which also had a smaller ratio of outer to total bark thickness(Fig. 9; Table 2).
Variability of bark thickness and outer to total bark thickness ratio for all examined bark samples (regardless of bark location on the stem) depends on Norway spruce trees’ age. Different letters indicate significant differences in bark thickness (red letters) and outer to total bark thickness ratio (black letters) between tree ages determined using the Kruskal-Wallis test (p < 0.05)
(a) Variability of bark swelling index (BSI) and (b) bark moisture content (MC) reached after bark saturation with hygroscopic water (SH) and liquid water after immersion bark samples in water from 1 h to 7 days, and (c) correlation between the bark swelling index and bark moisture content. Different letters indicate significant differences in BSI and MC between the time of bark saturation determined using the Kruskal-Wallis test (p < 0.05)
(a) Variability of bark swelling index (BSI) and (b) bark moisture content (MC) reached after bark saturation with hygroscopic water (SH) and liquid water after immersion bark samples in water from 1 h to 7 days, depending on tree age (mean ± SE). Different letters indicate significant differences in BSI and MC between tree ages in the particular state of bark hydration determined using the Kruskal-Wallis test (p < 0.05)
Discussion
In environmental research, high-resolution dendrometers are frequently applied to determine environmental factors of stem radial increment and daily stem diameter variations (Oberhuber et al. 2020). According to Fernández and Cuevas (2010) when transpiration begins at the morning, a tension is created in the xylem from the evaporative surface of the leaves to every organ of the plant, and part of the water stored in the plant tissues during the night is then lost, allowing the plant to respond rapidly to changes in atmospheric demand, and diurnal diameter changes occur in every water-storing organ of the plant. However, some studies indicate that daily growth may be obscured by changes caused by elastic bark and xylem shrinkage/swelling during the day and night (Chan et al. 2015; Zweifel 2016). Moreover, since the outer bark is a highly hygroscopic tissue (Ilek et al. 2016), diurnal diameter changes are likely also responding to relative air humidity. For Norway spruce trees, Gall et al. (2002) found that diurnal changes in bark thickness corresponded with relative air humidity but not with changes in tree water status. Thus, the knowledge of the bark swelling index and bark moisture content relation may be helpful in ecophysiological studies.
In this study, we demonstrated that hygroscopic water contributed to an increase of BSI. This confirms that the bark is sensitive to water vapor and relatively high BSI values obtained in contact with hygroscopic water indicate that changes in air humidity may affect the daily stem diameter variations, caused by the swelling and shrinking of the bark. Interestingly, we observed a slight decrease in BSI at higher levels of the tree stem. This height coincided with sections where the ratio of outer bark thickness to total thickness was greatest, suggesting that outer bark may have a lower swelling capacity compared to sections of bark with proportionally more inner bark. Outer bark has been shown to have a higher density than inner bark (Meyer et al. 1981), leading to lower total pore volume and a smaller capacity to hold water. Chemical properties between outer bark and inner bark also differ. For example, Standke and Schneider (1981) found that the outer bark of birch and poplar was less hygroscopic than the inner bark, probably due to a high suberin content, thus leading to a reduced ability to absorb water and water vapor from external sources.
With regards to temporal changes, we observed rapid changes in BSI during the first 24 h of bark saturation (especially during the first hour).with no significant changes in BSI occurring after this time. Raczkowski (1979) found that pine bark in water reaches its maximum swelling after about 100 h of soaking indicating that temporal bark swelling properties may be a tree species dependent phenomena.
With regards to vertical variation in BSI, we observed an increase in BSI with stem height in lower stem sections. This may be attributed to the rapid decrease in bark thickness at the base of the tree up to approximately breast height, especially in 35 and 50 years old trees. BSI remained relatively constant in the middle section of the stem of younger trees then decreased in the highest sections of trees, reaching the minimum at the top of the tree. Interestingly, in the top part of the trees, BSI had similar values regardless of age. Tree bark is very thin at the top of trees (Musić et al. 2019), so variability in bark thickness across ages was minimal and as a result so was BSI. The greatest differences in BSI between younger and older (70 years old) trees was observed throughout the middle sections of the stems. These differences probably were caused by the overall greater bark thickness and the higher ratio of outer to total bark thickness in 70 years old trees than in younger trees. The decreasing trend in BSI reached after contact of bark with liquid water when bark thickness and outer to total bark thickness ratio increase (Fig. 9) indicates that those two factors have a strong influence on bark swelling properties. Butler et al. (2005) investigated bark swelling – temperature relationship for four tree species and showed that for Douglas-fir swelling is dependent on bark structure and age. However, according to Browning (1963), diversity of bark density and presence of hydrophobic extractives are probably the most important and decisive factors which influence on bark swelling properties, which likely manifest in older trees. This is supported by findings of Cardoso et al. (2018), who found greater concentrations of polar extractives in bark with trunk height of Pseudotsuga menziesii, as well as high variability of suberin content with bark age. Fritsch et al. (2022) observed interspecific variability in extractives, lignin, suberin, holocellulose, α-cellulose, hemicelluloses, and ash contents of bark between Abies alba, Pseudotsuga menziesii, and Picea abies. They also observed intraspecific variability within some species according to the position of the sample in the stem. For spruce bark, they found that the extractives content of bark ranged from 8.6 to 17.4% and tended to increase from the bottom to the top of the stem. However, bark sampled in the middle of tree trunks presented more variable values, while suberin, lignin, and holocellulose content decreased with increasing tree height. Taken together, the results of this study and others suggest that the variability in the chemical composition of bark along with the variation in bark thickness properties, leads to variation in BSI across stem position and tree age.
Bark swelling is a direction-depending phenomenon that is influenced by the structural properties of the bark. Bark swelling can occur in three different directions (longitudinal, radial, and tangential), and each one has its physical features (Raczkowski 1979). Longitudinal swelling occurs in an up/down direction, radial swelling occurs perpendicular to the growth of tree rings, and tangential swelling occurs parallel to the growth of tree rings. We measured the potential swelling ability of Norway spruce bark under the influence of liquid water and water vapor depending on the bark location on the stem and tree age. We measured these abilities using the bark swelling index, which takes into account cracks and cavities of bark and measures the radial and tangential dimensions of swelling by measuring changes in the cross-sectional area of the bark. For the bark of six tree species (Pinus sylvestris, Larix decidua, Robinia pseudoacacia, Populus alba, Salix alba, Quercus robur, and Betula pendula), Raczkowski (1979) found the highest values of swelling pressure appear in a tangential direction, while lowest ones in a longitudinal direction. Swelling pressure in a radial direction was intermediate. Despite this, our measurements may have some errors as the swelling was measured on samples of bark detached from adjacent bark and wood in natural conditions. When the bark is intact on the tree, expansion and contraction with varying moisture content may be restrained by the bark fibers’ interwoven and ‘interlocked’ nature (Burgan 1971). In fact, our measurements may differ from the natural swelling properties of bark intact on the tree. However, our approach to measuring bark swelling ex situ enabled us to consider bark swelling at varying heights along the tree stem and control for environmental variation in the laboratory setting.
Conclusion
This study evaluated the vertical variation in the bark swelling index at different bark moisture content along the stems of Norway spruce trees differing in age. Results revealed the following:
-
(1)
Bark swelling is initiated after contact with both saturated air or liquid water.
-
(2)
Hygroscopic water may cause an increase of bark swelling index, on average to 1.20 indicating that bark is sensitive to water vapor and changes in air humidity may affect the daily stem diameter variations caused by the swelling and shrinking of the bark.
-
(3)
Bark swelling index decreased as bark thickness increased and as the ratio of outer bark to total bark thickness increased.
-
(4)
Bark swelling index showed variation with stem height, rapidly increasing from the bottom of the stem to the 0.2–0.3 tree height, remaining consistent through middle portions of the stem, and marginally decreasing in the top uppermost reaches of the stem.
-
(5)
Tree age influenced the bark swelling index. In general, bark swelling index was smallest in the oldest trees and can be attributed to greater bark thickness and ratio of outer bark to total bark thickness that are correlated with tree age.
-
(6)
To better understand the swelling properties of the bark, further research is needed that considers specific mechanisms of bark responsible for bark swelling including the vertical variation in both physical and chemical bark properties along tree stems.
References
Alexander HD, Arthur MA (2010) Implications of a predicted shift from upland oaks to red maple on forest hydrology and nutrient availability. Can J for Res 40(4):716–726. https://doi.org/10.1139/X10-029
Baptista I, Miranda I, Quilhó T, Gominho J, Pereira H (2013) Characterisation and fractioning of Tectona grandis bark in view of its valorisation as a biorefinery raw-material. Ind Crops Prod 50:166–175. https://doi.org/10.1016/j.indcrop.2013.07.004
Biggs AR (1992) Anatomical and physiological responses of bark tissues to mechanical injury. Defense mechanisms of woody plants against fungi. Springer, Berlin, Heidelberg, pp 13–40. https://doi.org/10.1007/978-3-662-01642-8_2
Browning BL (1963) The chemistry of wood. Interscience Publ, New York - Toronto
Burgan RE (1971) Variations in diameter measurements of robusta eucalyptus due to swelling and shrinking of bark, vol 244. Forest Service, US Department of Agriculture, Pacific Southwest Forest and Range Experiment Station
Butler BW, Webb BW, Jimenez D, Reardon JA, Jones JL (2005) Thermally induced bark swelling in four north American tree species. Can J for Res 35(2):452–460. https://doi.org/10.1139/x04-194
Campellone SV, Levia DF, Montalto FA (2020) Differences in submillimetre surface morphology and canopy interception storage capacities of Gleditsia triacanthos L. (Honey Locust) in relation to canopy phenophase and position. Ecohydrology 13(3):1–11. https://doi.org/10.1002/eco.2192
Cardoso S, Ferreira J, Miranda I, Pereira H (2018) Age variation of Pseudotsuga menziesii – fir bark chemical composition. J Wood Chem Technol 38(5):385–396. https://doi.org/10.1080/02773813.2018.1513036
Chan T, Hölttä T, Berninger F, Mäkinen H, Nöjd P, Mencuccini M, Nikinmaa E (2015) Separating water-potential induced swelling and shrinking from measured radial stem variations reveals a cambial growth and osmotic concentration signal. Plant Cell Environ 39:233–244. https://doi.org/10.1111/pce.12541
Eberhardt TL (2013) Longleaf pine inner bark and outer bark thicknesses: measurement and relevance. South J Appl for 37(3):177–180. https://doi.org/10.5849/sjaf.12-023
Fernández JE, Cuevas MV (2010) Irrigation scheduling from stem diameter variations: a review. Agric for Meteorol 150(2):135–151. https://doi.org/10.1016/j.agrformet.2009.11.006
Fritsch C, Dumarçay S, Colin F, Gérardin P (2022) Bark composition changes along the trunk of three softwood species: Picea abies, Abies alba Mill. And Pseudotsuga menziesii. Scand J for Res 1–6. https://doi.org/10.1080/02827581.2022.2132283
Gall R, Landolt W, Schleppi P, Michellod V, Bucher JB (2002) Water content and bark thickness of Norway spruce (Picea abies) stems: phloem water capacitance and xylem sap flow. Tree Physiol 22(9):613–623. https://doi.org/10.1093/treephys/22.9.613
Holmberg A, Wadsö L, Stenström S (2016) Water vapor sorption and diffusivity in bark. Drying Technol 34(2):150–160. https://doi.org/10.1080/07373937.2015.1023310
Ilek A, Kucza J, Morkisz K (2016) Hygroscopicity of the bark of selected forest tree species. iForest-Biogeosciences Forestry 10(1):220. https://doi.org/10.3832/ifor1979-009
Ilek A, Van Stan JT, Morkisz K, Kucza J (2021) Vertical Variability in Bark Hydrology for two coniferous tree species. Front Forests Global Change 4:687907. https://doi.org/10.3389/ffgc.2021.687907
Klügl J, Di Pietro G (2020) The interaction of water with archeological birch bark and its effects on swelling, shrinking and deformations. 24 August 2020 PREPRINT (Version 1) available. https://doi.org/10.21203/rs.3.rs-58064/v1. at Research Square
Levia DF, Wubbena NP (2006) Vertical variation of bark water storage capacity of Pinus strobus L.(Eastern White pine) in southern Illinois. Northeastern Naturalist 13(1):131–137. https://doi.org/10.1656/1092-6194(2006)13[131:VVOBWS]2.0.CO;2
Livesley SJ, Baudinette B, Glover D (2014) Rainfall interception and stem flow by eucalypt street trees–the impacts of canopy density and bark type. Urban Forestry Urban Green 13(1):192–197. https://doi.org/10.1016/j.ufug.2013.09.001
Meyer RW, Kellogg RM, Warren WG (1981) Relative density, equilibrium moisture content, and dimensional stability of western hemlock bark. Wood Fiber Sci 13:86–96
Musić J, Lojo A, Balić B, Ibrahimspahić A, Avdagić A, Knežević J, Halilović V (2019) Modelling bark thickness of Norway Spruce (Picea abies Karst). South-east Eur Forestry: SEEFOR 10(2):125–135. https://doi.org/10.15177/seefor.19-17
Oberhuber W, Sehrt M, Kitz F (2020) Hygroscopic properties of thin dead outer bark layers strongly influence stem diameter variations on short and long time scales in scots pine (Pinus sylvestris L). Agric for Meteorol 290:108026. https://doi.org/10.1016/j.agrformet.2020.108026
Raczkowski J (1979) Swelling properties of bark. Wood Sci Technol 13(3):187–196. https://doi.org/10.1007/BF00350223
Sevanto S, Hölttä T, Holbrook NM (2011) Effects of the hydraulic coupling between xylem and phloem on diurnal phloem diameter variation. Plant Cell Environ 34(4):690–703. https://doi.org/10.1111/j.1365-3040.2011.02275.x
Siegert CM, Levia DF (2014) Seasonal and meteorological effects on differential stemflow funneling ratios for two deciduous tree species. J Hydrol 519:446–454. https://doi.org/10.1016/j.jhydrol.2014.07.038
Standke W, Schneider A (1981) Untersuchungen über das Sorptionsverhalten des Bast-Und Borkeanteils Verschiedener Baumrinden. Holz als Roh-und Werkst 39(12):489–493
Tonello K, Van Stan J, Rosa A, Balbinot L, Pereira L, Bramorski J (2021a) Stemflow variability across tree stem and canopy traits in the Brazilian Cerrado. Agric for Meteorol 308:108551. https://doi.org/10.1016/j.agrformet.2021.108551
Tonello KC, Campos SD, de Menezes AJ, Bramorski J, Mathias SL, Lima MT (2021b) How is bark absorbability and wettability related to stemflow yield? Observations from isolated trees in the Brazilian Cerrado. Front Forests Global Change 4:650665. https://doi.org/10.3389/ffgc.2021.650665
Valovà M, Bieleszovà S (2008) Interspecifc variations of bark’s water storage capacity of chosen types of trees and the dependence on occurrence of epiphytic mosses. GeoSci Eng LIV 4:45–51. http://hdl.handle.net/10084/84249
Van Stan JT, Lewis ES, Hildebrandt A, Rebmann C, Friesen J (2016) Impact of interacting bark structure and rainfall conditions on stemflow variability in a temperate beech-oak forest, central Germany. Hydrol Sci J 61(11):2071–2083. https://doi.org/10.1080/02626667.2015.1083104
Van Stan JT, Dymond SF, Klamerus-Iwan A (2021) Bark-water interactions across ecosystem states and fluxes. Front Forests Global Change 4:660662. https://doi.org/10.3389/ffgc.2021.660662
Zweifel R (2016) Radial stem variations—a source of tree physiological information not fully exploited yet. Plant Cell Environ 39:231–232. https://doi.org/10.1111/pce.12613
Acknowledgements
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) - Finance Code 001” and Grant 2022/07550-5, São Paulo Research Foundation (FAPESP).
Author information
Authors and Affiliations
Contributions
AI: conceptualization. AI and AP: samples collection, preparation for analysis. AI, AP, KCT: laboratory research. AI, KCT: methodology. AI, CS, SDC, KCT: analysis and interpretation of data. AI: writing – original draft preparation. AI: visualization. AI, CS, SDC, MS, KCT: resources. AI, CS, SDC, MS, KCT: writing – review and editing. CS: writing – language review and editing. All authors contributed to the manuscript revision and read and approved the submitted version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Communicated by Andreas Rais.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ilek, A., Płachta, A., Siegert, C. et al. Vertical variation in swelling properties of Norway spruce bark depending on tree age and bark moisture content. Eur J Forest Res 143, 1225–1235 (2024). https://doi.org/10.1007/s10342-024-01686-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10342-024-01686-w