Abstract
Dead wood is a key substrate of forests that plays an important role in fertility and productivity. However, dead wood is scarce in traditionally managed forests like Mediterranean dehesas. The chemical composition of downed dead wood in Quercus pyrenaica dehesas was analysed in different physical decomposition stages as a proxy of dead wood decay length. We also assessed the contribution of fungal activity, by quantifying ergosterol, to the chemical composition of deadwood. Chemical analyses included elemental composition determination, thermogravimetry and infrared spectroscopy. Our results showed that both the physical decomposition stage and ergosterol content extensively predicted the chemical composition of Q. pyrenaica dead wood decay processes under field conditions. The physical stage was a better predictor of the C/P ratio and polysaccharides proportion, while ergosterol better predicted P content and the N/P ratio. In other cases like lignin, the relation between ergosterol content and chemical composition varied depending on the physical stage. In addition, environmental local factors differentially affected chemical composition across physical decomposition stages. We conclude that the physical decomposition stage and ergosterol content complementarily contribute to estimate the temporal behaviour of the chemical composition of dead wood in Mediterranean areas. Moreover, we recommend using the FT-IR analysis to assess the nature of temporal chemical changes in downed dead wood. Finally, our study claims to consider the potential impact of local environmental factors, such as air temperature and relative humidity, on dead wood decay processes in traditionally managed forests in the current global change scenario.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Downed dead wood (DDW), defined as wood detrital forest component (Woodall et al. 2019), plays an important role for nutrient availability in forest ecosystems for being involved in soil development and the biogeochemical dynamics of key elements, such as carbon (C), nitrogen (N) and phosphorous (P) (Strukelj et al. 2013; Rajala et al. 2015; van der Wal et al. 2015; Shannon et al. 2022). In addition, woody substrates are a key structural element of forests and act as reservoirs of C (IPCC 2003, and subsequent) and N in the medium to long term by improving hydrogeological efficiency and, ultimately, contributing to increase forest productivity and maintain biodiversity (Grove 2002; Stokland et al. 2012; Herrmann and Bauhus 2018). However, the abundance of this key forest structural component (DDW) decreases from unmanaged to managed forests, which adversely affects both system productivity and diversity (Keren and Diaci 2018; Parisi et al. 2018; Hmäläinen et al. 2018; Öder et al. 2021).
In agrosilvopastoral systems like Spanish dehesas (also named montados in Portugal) [savanna-like open woodland landscapes resulting from long-term traditional forest management (Campos et al. 2013)], DDW on soil can form an important gap due to low tree density and the local population’s uses (Micó et al. 2022). In this particular cultural landscape, DDW supply mainly comes from the senescence process of old scattered trees, or from non-harvested pruning, and is normally used by local people as firewood (Moreno and Pulido 2009).
The importance of DDW quantity in forest productivity and diversity is widely documented (Økland et al. 1996; Siitonen 2001; Gossner et al. 2013; Hmäläinen et al. 2018; Seibold and Thorn 2018), but DDW quality also has a special relevance (Abrego and Salcedo 2013). However, very few studies cover the chemistry of dead wood degradation in nature, especially in agrosilvopastoral systems where wood and its decomposition are key for maintaining ecosystem services (Moreno and Pulido 2009; Micó et al. 2022).
Dead wood is primarily composed of polysaccharides and lignin, and is poor in N or P content (Pettersen 1984; Filipiak and Weiner 2014). It is noteworthy that P is one of the most limiting nutrients in virtually all natural ecosystem types (Du et al. 2020; Hou et al. 2020). Polysaccharides like celluloses or hemicelluloses are made up of different types of sugars linked by carbohydrates chains, which provide a profuse and easily available source of energy for saproxylic decomposers (Stokland et al. 2012; Ferro 2018). In contrast, lignin comprises more complex compounds and a few organisms (e.g. white-rot fungi) that have the proper enzymes to degrade them (Eriksson et al. 1990; Ferro 2018). Therefore, depending on both the peculiarities of each decomposition process and the organisms involved in its decay, DDW quality can vary (Laiho and Prescott 2004; Strukelj et al. 2013). In addition, the chemical composition of wood also varies between tree species (Noll et al. 2016 Błońska et al. 2017; Jomura et al. 2022). Abiotic factors, such as humidity and temperature, in turn determine the speed of the decomposition process (Woodall and Liknes 2008; Fravolini et al. 2018). Moreover, biotic factors like the activity of saproxylic organisms may also influence decomposition dynamics and determine dead wood quality (Purahong et al. 2022). Strukelj et al. (2013) found non-selective degradation of all chemical compounds in early decomposition stages. In more advanced stages, degradation was more selective to carbohydrates and lignin. In this way, the organic composition of dead wood (polysaccharides and lignin) and its inorganic composition (C, N and P, and their ratios), vary with the decomposition stage due to the balance between nutrient demand and supply promoted by the activity of wood-decomposing organisms (Lonsdale et al. 2008; Clinton et al. 2009; Filipiak 2018).
Fungi are one of the major groups of decomposer organisms across terrestrial ecosystems (Harmon et al. 1986; Stokland et al. 2012; Bani et al. 2018). Fungal activity has the potential to change the dead wood structure both chemically and physically (Pandey and Pitman 2003; Liers et al. 2011); i.e., fungi are able to translocate soil elements by enhancing the nutritional composition of dead wood (Filipiak et al. 2016). Ergosterol content in dead wood is one of the most dependable and specific estimators of fungal activity (Seitz et al. 1979; Montgomery et al. 2000; Mille-Lindblom et al. 2004), and can help to predict dead wood decomposition overtime to some extent (Větrovský et al. 2011; Baldrian et al. 2016; Noll et al. 2016). The study of fungal activity and its relation to different wood decomposition stages under field conditions is especially relevant in the current climate change context of where the diversity of decomposing communities is threatened and could be even more altered as a consequence of the rapid changes that are taking place (Brennan et al. 2009; Walter et al. 2013).
Despite the importance of dead wood in nature, many studies about the decomposition dynamics of DDW have been carried out in boreal and temperate forests (Harmon et al. 1986; Siitonen 2001), with very little research to date on the composition and degradation of wood in Mediterranean forests (Lombardi et al. 2013; Fravolini et al. 2016, 2018) where climate change may acutely affect the decomposition processes and biotic elements that act on them. Furthermore, knowledge about the quality of long-decaying woods like Quercus pyrenaica Willd, is scarce (Fernández de Simón et al. 2006).
In this study, we assessed DDW quantity and quality in dehesa-like Mediterranean Q. pyrenaica forests in the western Iberian Peninsula. We aimed to discern the relation among the physical decomposition stages, chemical composition and fungal activity (quantified as ergosterol content) in Q. pyrenaica DDW under natural conditions. Moreover, we aimed to test the influence of local environmental factors on DDW decomposition.
We assumed a small amount of DDW in traditionally dehesa-like managed forests, and that the proportion of different DDW decomposition stages is not balanced (Micó et al. 2022). We hypothesized that: (I) the distinct physical stages of dead wood decomposition differ in organic compounds and elemental composition (Ferro 2018) which are also differentially affected by environmental factors (Seibold et al. 2021); (II) the ergosterol content is a proxy of the chemical composition of DDW (Seibold et al. 2021; Jomura et al. 2022) and provides complementary information to that provided by physical decomposition; (III) the use of the FT-IR technique likely provides accurate results about polysaccharides and lignin decomposition (Sánchez et al. 2017) Assessing the decomposition process of dead wood with chemical composition and its relation to fungal activity under field conditions can help to better predict the ecosystem services provided by dead wood.
Materials and methods
Study area and sample collection
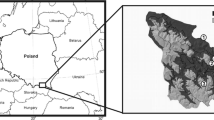
The study was carried out in the southern part of the province of Salamanca, Spain (Fig. 1). The study area is one of the largest continuous areas of Quercus pyrenaica Willd. forests in the Iberian Peninsula (174,236 ha). It includes three subareas with different degrees of protection: the El Rebollar Natural Area (40°22′55″N 6°37′34″W) (R: first replicate or forest unit); the Sierra de las Quilamas Natural Area and the Batuecas-Sierra de Francia Natural Park (F + Q: second replicate) (40°33′37″N 5°59′10″W); the Sierra de Béjar UNESCO Biosphere Reserve (40°30′46″N 5°52′30″W) (B: third replicate) (Fig. 1). The dominant tree species is Q. pyrenaica, whereas the undergrowth includes heterogeneous combinations of shrubs species, such as Genista spp., Cytisus spp., Cistus spp., Erica spp., Pteridium aquilinum (L.) Kuhn in Kersten and Crataegus monogyna Jacq., as the most representative ones. The predominant climate is continental Mediterranean, with dry summers and wet and cool winters (Blondel and Aronson 1999). The mean annual rainfall is about 1,000 mm (Oliver-Moscardó and Luis-Calabuig 1979; García-Rodriguez et al. 1984; Llamas et al. 2011).
Thirteen sampling sites (Fig. 1; Table 1) ranging from 600 to 1100 m a.s.l. were selected to represent the heterogeneity of old managed Q. pyrenaica forests in the studied areas, in which the mean trunk diameter was always greater than 20 cm. The selected sites involve some kinds of traditional management practices (extensive livestock, grazing tree pollarding, firewood collection or forest thinning) to different degrees, or have been managed in the past. Consequently, forest structure differs in tree density and DDW availability among sites, from dehesas with fewer than 20 trees per hectare to others with 300 trees per hectare (Table 1) (Micó et al. 2022). Similar site heterogeneity was considered in each area.
Environmental characterisation and dead wood selection
We quantified tree density, DDW volume and abiotic factors (air temperature and humidity) per sampling site. Tree density affects not only the supply of types and volume of DDW, but also insolation conditions, and the diversity and activity of wood-dependent insects involved in the decomposition process through wood fragmentation and symbiotic relationships with fungi and microorganisms (Sverdrup-Thygeson and Birkemoe 2009; Horák 2017; Micó et al. 2022). In addition, the mean air temperature and humidity can also directly and indirectly affect the provision of ecosystem services in forests related to nutrient cycling (Morán-Ordóñez et al. 2021; Micó et al. 2022).
To quantify such variables in the study area, based on methodology proposed by Micó et al. (2022), at all 13 sampling sites three plots of an 18 m-radio, placing them 20 to 113 m (mean = 58 m) away from one another, were established. Each plot was established around a tree on which a HOBO U23 Pro v2 Temperature/Relative Humidity Data Logger with a U23-001 sensor was attached to the trunk at about two meters high above the ground. They were programmed to record temperature and RH values every 8 h from a whole year (March 2017 to March 2018). The tree density of each plot was estimated as the total number of trees, expressed as the number of trees per ha (Table 1). DDW amount per plot was calculated as the total volume (dm3) of fallen dead wood (all pieces ≥ 7 cm diameter were included) (Öder et al. 2021). The DDW data were extrapolated to obtain values per hectare (Table 1). All the site-level variables were the result of averaging the three plot values per site.
For chemical analyses, five to eleven dead wood pieces (always ≥ 7 cm diameter) were selected from each sampling site (except sampling site 2, which lacked DDW). Each DDW piece was classified into one of three physical decomposition stages (Table 1) depending on the wood hardness and bark adherence combination as so: 1 – hard stage: hard wood impenetrable with a knife and intact bark; 2 – medium stage: wood penetrable to a few centimetres with a knife and partly present bark; 3 – soft stage: soft wood completely penetrable with a knife without resistance and loose bark (Franc et al. 2007; Baldrian et al. 2016; García-López et al. 2016). In no case was more than one decaying stage established for the same selected DDW piece. Altogether, 76 DDW pieces were selected at the 12 possible sites (Table 1). From each selected DDW piece, sawdust samples were collected using an electric drill (DeWalt® DCD995P2) with an 8-mm diameter auger, which was sterilised with a blowtorch flame after drilling any DDW to avoid fungal contamination. Four to seven perforations were made along each piece. The sawdust samples of each piece were taken from the field in April 2017 and stored in plastic bags under freezing and dark conditions until analyses were performed.
Chemical composition and ergosterol analysis
We analysed the decomposition processes of Q. pyrenaica DDW in nature by chemically characterising decomposition stages and relating them to fungal activity through ergosterol content (Young 1995; Jomura et al. 2022). To do so, we examined the chemistry of dead wood by analysing variation in elemental composition (C, N and P, and their ratios), and the changes produced in organic compounds (polysaccharides and lignin) by Fourier-Transform Infrared (FT-IR) spectroscopy. This technique has been used to monitor chemical changes in living wood (see Martín et al. 2005; Conrad et al. 2014), but there are very few reports of its application to analyse decaying dead wood in nature (Sánchez et al. 2017).
For chemical analyses, a portion of all the 76 sawdust samples (see above) was ground and dried at 60ºC. C and N were analysed by a combustion procedure in a Carlo Erba CHNS-O EA1108 elemental analyser, while P was determined by phospho-molybdovanadate colorimetry at 460 nm using a JASCO V-630 spectrophotometer (Kitson and Mellon 1944).
The thermogravimetric analysis was carried out on a Mettler Toledo TGA/SDTA851e/SF/1100 to determine the primary and secondary thermal decompositions of macromolecules (lignin and polysaccharides). A linear heating rate of 10ºC min-1 within the 25–600ºC temperature range was performed for the thermal tests (Peuravuori et al. 1999). Infrared spectroscopy was used to determine lignin and polysaccharides decomposition (Pandey and Pitman 2003). The infrared spectra for the Q. pyrenaica DDW samples defined in the region from 1,860 to 780 cm-1 were recorded with a Bruker IFS 66 FT-IR spectrophotometer by means of direct measurement with ATR units within the 4,000-600 cm-1 range. The FT-IR spectra were baseline-corrected and normalised. This allows to make comparisons of the intensities of the bands of different spectra. Band heights were measured from a baseline established in the fingerprint region of wood from 1,860 to 780 cm-1 (Fackler et al. 2007; Sánchez et al. 2017) (Fig. 2).
FT-IR spectrum of a Quercus pyrenaica downed dead wood (DDW) sample defined in the region from 1,860 to 780 cm-1 (fingerprint). Intensities of bands are associated with lignin [5, 6 (C = C of aromatic skeletal)], polysaccharides [3 (unconjugated C = O in xylans), 9, 13 (C–O–C vibration in cellulose and hemicellulose) and 17 (C–H deformation in cellulose)], and with different groups of lignin and polysaccharides [7, 8 (C–H deformation in lignin and carbohydrates), 10 (C–H vibration in cellulose and C1–O vibration in syrigyl derivates), 12 (syringyl ring and C–O stretch in lignin and xylan), 14 and 16 (C–O and C–C stretching ring in cellulose and hemicelluloses)] (Mononen et al. 2005; Pandey et al. 2003; Popescu et al. 2007, 2010)
Ergosterol content, which is widely considered a surrogate of fungal activity (Gessner et al. 1991; Gessner and Schmitt 1996; Ruzicka et al. 2000), was estimated by the microwave-assisted ergosterol extraction (MAE) method adapted from Young (1995). Samples were previously lyophilised and ground in liquid N2, followed by the suspension of 150 mg of samples in 2 mL of methanol and 0.5 mL 2 M sodium hydroxide. Tubes were tightly sealed with a Teflon-lined screw inside a plastic bottle and heated in a consumer microwave at 450 W for 20 s. Once cooled, the suspension was neutralised with 1 mL of 1 M aqueous hydrochloric acid and extracted with hexane (3 x ca. 2 mL). The solution was mixed for 120 s by a manual vortex and the hexane fraction was dried by evaporation. The residue was dissolved in 2 mL methanol and ergosterol was quantified by high performance liquid chromatography (HPLC) inside a Poroshell 120 EC-C18 (3.0 × 50 mm − 2.7 Micron) column and eluted with methanol (0.5 mL min-1, 20ºC). Ergosterol was detected at 282 nm and was estimated by a comparison to a standard curve of pure ergosterol (purity > 95%, Sigma-Aldrich) (Pascoal et al. 2010).
Statistical analyses
We used the statistical program R version 4.2.0 for the analyses (R Core Team 2021). We firstly performed univariate general (or generalised when error distribution was non-normal) linear mixed models (GLMMs) to test the effects of physical decomposition stage (hard, medium or soft) and ergosterol content on the different chemical variables: elemental composition and thermogravimetric and FT-IR data. Area of origin was included in GLMMs as a random factor. GLMMs were firstly performed with each predictor variable separately, and later in combination, to discern any potential interactions between both predictor variables. The goodness of fit of the different models was based on Akaike’s information criterion (AIC). When the physical decomposition stage effect was significant, we made Tukey’s HSD pairwise post hoc comparisons to determine significant differences between physical stages. In addition to GLMMs, the multivariate method discriminant analysis of principal components (DAPC) was utilised to explore the clustering patterns of the different analysed chemical variables according to the physical decomposition stages. Package lme4 (Bates et al. 2015), package emmeans (Lenth et al. 2022) and package adegenet (Jombart et al. 2008) were used to perform GLMMs, post hoc tests and DAPCs, respectively.
Lastly, we performed univariate general or generalised linear models (GLMs) to test the effects of the site environmental variables (total DDW, tree density, mean temperature and relative humidity) on the elemental and organic compound composition variables according to the physical decomposition stages. GLMs were performed using package lme4 following the same error distribution fitting as in GLMMs.
Results
Downed dead wood quantity and characterisation of the physical decomposition stages
The dead wood in the study areas ranged from 0 to 18 m3/ha (medium values were around 3 m3/ha) (Table 1). The distribution of the physical decomposition stages was not homogeneous throughout the study areas, and the mean composition skewed towards the hard physical stages at all the 12 sampling sites.
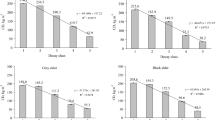
The physical decomposition stage of DDW influenced P content and the C/P ratio, which were higher and lower, respectively, in the most advanced decomposition stage compared to the most preliminary one (Table 2; Fig. 3). In contrast, C and N contents were not influenced by the decomposition physical stage, whereas the C/N and N/P ratios were only marginally affected (Table 2; Online Resource 1). The polysaccharides proportion was higher in the soft stage than in the hard and medium ones, while the lignin proportion was lower in the soft stage than in the hard stage (Table 2; Fig. 3).
Boxplots (mean ± SD) of the physical decomposition stage effect on fungal activity (ergosterol content), elemental composition (P content, C/P ratio) and organic compound composition (polysaccharides and lignin content), and on specific groups of polysaccharides and lignin compounds (I3, I8, I16 and I17) of the Q. pyrenaica downed dead wood (DDW). Different uppercase letters indicate significant post hoc differences (p < 0.05) based on the GLMM results, tested using Tukey’s HSD pairwise comparisons
Regarding the FR-IR data, the relative intensities of four groups of polysaccharides and lignin compounds showed significant differences among the physical decomposition stages. The relative content of polysaccharides I3 and I17 (assigned to hemicellulose and cellulose, respectively) was lower in the soft stage than in the hard and medium stages (Table 2; Fig. 3). Conversely, I8 content (allocated to lignin and carbohydrates) was higher in the soft stage compared to the hard and medium stages, while I16 content (corresponding to celluloses and hemicelluloses) was higher in the soft stage than the hard stage.
When the DAPC clustering patterns of the elemental composition and thermogravimetric and FT-IR data were analysed according to the physical decomposition stage, the elemental composition and thermogravimetric data gave similar results. Soft DDW only was slightly different from the hard and medium stages, which were, in turn, similar (Fig. 4). The FT-IR data indicated more delimited clusters according to the physical stage.
Clustering patterns based on the discriminant analysis of principal components (DAPC) of the physical decomposition stage (h – hard, m – medium, s – soft) according to the Q. pyrenaica downed dead wood (DDW) elemental composition, and the thermogravimetric and FT-IR data. Data are centred and scaled. On the upper and right sides of each DAPC, the contributions of each variable to the discriminant function axes are shown, but for FT-IR, only those variables (compounds) that significantly contributed to each axis appear
Ergosterol content and effect on DDW chemical composition
The ergosterol content in the Q. pyrenaica DDW (mean ± SD = 47.42 ± 45.33, range: 2 to 266 µg g− 1) showed significant differences in the physical decomposition stages, and was the highest in the soft stage (Table 2; Fig. 3). P content increased as ergosterol content rose, while the C/P, C/N and N/P ratios lowered with increasing ergosterol content (Table 2; Fig. 5). Similar patterns were found for polysaccharides and lignin content, which respectively increased and decreased in relation to ergosterol content. I3, I8, I16 and I17 increased or decreased in relation to ergosterol similarly to that observed for the physical decomposition stage (Table 2; Fig. 5).
Linear regressions of the variables significantly influenced by ergosterol content in the Quercus pyrenaica downed dead wood (DDW) based on the GLMM results. The variables related to elemental composition (P content, and C/N, C/P and N/P ratios), organic compound composition (proportion of polysaccharides and lignin) and specific groups of polysaccharides and lignin compounds (I3, I8, I16 and I17), are shown
Physical decomposition stage and ergosterol content were predictors of Q. pyrenaica chemical composition, but were not completely collinear. Based on ∆AIC, the physical decomposition stage was a better predictor of the C/P ratio and polysaccharides content, while ergosterol better predicted P content and the N/P ratio (Table 2). Considering both variables simultaneously improved the goodness of fit of some models (e.g. C/P ratio; Online Resource 2). When both variables were considered jointly in GLMMs, changes in P, C/P, N/P and I17 were mainly due to ergosterol content, which were consistently related to the response variable regardless of physical condition, whereas, for example, variations in I16 were better predicted by the physical stage. In other cases (e.g. lignin, I5 and I7), the relation between ergosterol and the response variable varied depending on the physical decomposition stage (Online Resource 2; Fig. 6). By way of example, when ergosterol was low, the physical stage did not matter for lignin, whose values were always high. With increasing ergosterol content, a relation with lignin degradation was observed, but mainly in the hard physical stage (Online Resource 2; Fig. 6).
Projections of variation in elemental composition (P content, C/P and N/P ratios), organic compound composition (proportion of polysaccharides and lignin) and specific groups of polysaccharides and lignin compounds (I3, I5, I7, I8, I13, I16 and I17) of the Q. pyrenaica downed dead wood (DDW) based on the joint consideration of the physical decomposition stage and ergosterol content
Effect of local scale environmental factors on DDW chemical composition
The 12 sites from which DDW was collected were heterogeneous to one another, and such heterogeneity was reproduced at each of the three sampling areas. However, sampling areas differed in relative humidity terms, and were moister those from El Rebollar (F2,9 = 10.04, p = 0.005, Table 1). Site environmental variables had no significant effects on DDW sample composition when the physical decomposition stage was not considered. C content marginally decreased with mean relative humidity (F1,74 = 3.64, p = 0.06) and polysaccharides content marginally increased with tree density (F1,74 = 2.91, p = 0.09).
When the physical decomposition stage was considered, several relations were observed between environmental variables and DDW chemical composition. Total DDW lowered C (F1,39 = 6.89, p = 0.012) and P (χ2 = 4.78, p = 0.028) contents in the least advanced (hard) decomposition stage (Fig. 7). Tree density lowered ergosterol content (χ2 = 5.93, p = 0.014) in the least advanced decomposition stage, increased the N/P ratio (χ2 = 5.05, p = 0.024) and lignin content (F1,20 = 4.88, p = 0.038) in the medium advanced one and marginally increased polysaccharides content (F1,11 = 4.16, p = 0.06) in the most advanced decomposition stage (Fig. 7). Mean temperature only affected the soft stage composition by marginally lowering P (χ2 = 3.58, p = 0.06) and polysaccharides contents (F1,11 = 4.57, p = 0.05), increasing lignin content (F1,11 = 9.42, p = 0.010), and marginally increasing the C/P ratio (F1,11 = 3.78, p = 0.07) (Fig. 7). Mean relative humidity had an effect on not only the composition of the medium decomposition stage by increasing the N/P ratio (χ2 = 5.37, p = 0.020) and lowering ergosterol content (χ2 = 5.50, p = 0.018), but also on the composition of advanced decomposition stage by increasing P (χ2 = 7.70, p = 0.005) and polysaccharides (F1,11 = 17.29, p = 0.001) contents, and by lowering lignin content (F1,11 = 6.23, p = 0.029) (Fig. 7).
Linear regressions of the Quercus pyrenaica downed dead wood (DDW) elemental composition (C and P content, C/P and N/P ratios), organic compound composition (polysaccharides and lignin content) and ergosterol content variables per physical decomposition stage influenced by site environmental variables (total DDW, tree density, mean temperature and mean relative humidity)
Discussion
Despite the maturity and relatively high productivity of the studied forests (mean tree diameter > 20 cm) (Micó et al. 2021), dehesa-like Q. pyrenaica forests show smaller mean DDW volumes on the ground than those recommended for maintaining an adequate diversity of saproxylic organisms (Micó et al. 2022). In light of this, both the total dead wood volume on the ground and saproxylic organisms’ diversity are necessary to ensure correct nutrients flow in forest ecosystems (Hämäläinen et al. 2018; Přívětivý et al. 2018). The study of the dead wood decay in dehesa-like Q. pyrenaica forests evidenced differences in both inorganic and organic compositions that depended on fungal action and local environmental factors to different extents.
Physical decomposition stage and ergosterol content predict the chemical composition of Q. Pyrenaica
The physical decomposition stage of the Q. pyrenaica DDW predicted changes in chemical composition, such as P content and the C/P ratio (the highest and lowest values, respectively, were obtained in the most advanced decomposition stage) (Table 2; Fig. 3). The loss of mass resulting from the decomposition process may be more than enough to justify the increased P content (Herrmann and Bauhus 2018), although the biotic action of saproxylic organisms can also contribute to a real increase of P in DDW in advanced decomposition stages. Indeed certain saproxylic fungi can translocate nutrients, such as P and N, from external sources, which would explain P enrichment as wood decomposition progresses (Lonsdale et al. 2008; Clinton et al. 2009). In this way, ergosterol content (surrogate of fungal activity) was also predicted by the decomposition stage, and was higher in the last stage, as previously reported by Jomura et al. (2022) and Seibold et al. (2022). In addition, microorganisms and insects can affect the wood degradation process (Ulyshen 2016). For example, the effect of insects may account for 29% of the carbon flux from dead wood (Siebold et al. 2021). Moreover, some saproxylic beetle larvae can chemically modify dead wood by producing residue that is poor in C and rich in N and P (Micó et al. 2011), which could also lead to a lower C/P ratio, combined with CO2 losses by respiration (Boddy and Watkinson 1995; Filipiak and Weiner 2014). However, our results showed that the decomposition stage only marginally affected the C/N and N/P ratios (Table 2; Online Resource 1), with lower values in soft DDW that are presumably due to C loss and increased N. The lower C/N ratio is a rough indicator of changes in organic matter decomposability, and support the evidence for different decomposition rates among decay classes (Weedan et al. 2009; Strukelj et al. 2013; Pastorelli et al. 2021). In addition to changes in elemental composition, the assessment of organic compounds in each decomposition stage provided valuable information about the nutrient availability of dead wood under natural conditions for decomposers. The Quercus pyrenaica DDW is known to be a richer woody substrate in polysaccharides than in lignin (Micó et al. 2011; Sánchez et al. 2017), as reported here. With this study, we revealed that the overall dead wood organic composition is also driven by the physical decomposition stage (as in Strukelj et al. 2013). A higher polysaccharides proportion was found in the soft DDW than in the hard and medium stages, and the soft DDW also presented a lower proportion of lignin than the hard DDW, which likely indicates lesser polysaccharide degradation versus lignin. This means that the most advanced decomposition stages may proportionally offer more easily degradable compounds to saproxylic organisms, such as celluloses and hemicelluloses (Spaccini and Piccolo 2009).
Saproxylic fungi (especially white rot and brown rot) are presumably the primary agents of dead wood decomposition (Boddy 2000; Liers et al. 2011; Crowther and Bradford 2013; Arnstadt et al. 2016). They degrade cellulose and lignin, and remove hemicellulose from wood during decomposition (Strukelj et al. 2013; Hu et al. 2021). So their action should explain most of the changes found in polysaccharides and lignin across physical stages. Saproxylic insects and bacteria may contribute to the degradation of less complex organic molecules (celluloses and hemicelluloses), but lignin degradation is driven mostly by fungal communities. In line with this, the selective action of fungi on lignin can explain the proportional increase in polysaccharides with ergosterol content (as a proxy of fungal activity) (Fig. 5). By depicting the macromolecule identity of each decomposition stage, we found that ergosterol content was positively related to relative I16 intensity (Figs. 2 and 5). This band corresponded to the highest peak of the Q. pyrenaica spectrum produced by the presence of C-O and C-C of cellulose and hemicellulose (Popescu et al. 2010). The increase in this band might reflect the general relative rise in polysaccharides in detriment to lignin, which also likely occurred with I8. Conversely, I16 (cellulose and hemicellulose) seemed to be better predicted by the physical stage than by ergosterol (Fig. 6). The behaviour of bands, such as I3 and I17 (see Fig. 5), in turn reflects the possible action of some fungi kind on xylans (I3), as well as other hemicelluloses and celluloses (I17), because different fungal families exhibit distinct enzymatic activity (Hoppe et al. 2016). Overall, employing FT-IR data provided more properly delimited clusters according to the physical decomposition stage than inorganic and organic contents (Fig. 4) and, thus, serves as a helpful tool to better understand the nature of chemical changes. However, specific studies addressing the complexity and interactive effects of biotic factors under field conditions, as well as the possible relation between fungal composition and dead wood chemistry, are necessary to fully understand wood decay processes in forest ecosystems.
Physical decomposition stage and ergosterol content were predictors of Q. pyrenaica DDW chemical composition under field conditions, but they were not completely collinear (Fig. 6). Ergosterol content was by far the best predictor of the P-related variables. Regarding molecule composition, one noteworthy aspect of this work was that the interactive effects between the ergosterol content and degradation of some molecules varied across physical decomposition stages. This was the case of the relation between ergosterol with lignin and I7, which reinforced the differential involvement of fungi in the chemical transformation of dead wood during the decomposition process, along with the digestion, translocation and retention of nutrients in dehesa-like forest habitats.
Local environmental factors may differentially affect each decomposition stage
Local environmental factors are known to affect dead wood decomposition both directly [i.e. weather conditions (Fravolini et al. 2018)] and indirectly [by driving saproxylic organisms diversity (i.e. amount of dead wood) or affecting microclimatic conditions (i.e. forest openness) (Sverdrup-Thygeson and Birkemoe 2009; Horák 2017)]. In the studied Mediterranean region, environmental local factors showed contrasting effects on the chemical composition across the different decomposition stages of Q. pyrenaica DDW. Forest structure (i.e., total DDW volume, tree density) was the main determining factor of the chemical composition of DDW in the earlier decomposition stage (note that these variables can affect indirectly DDW decomposition by, for example, driving saproxylic organisms diversity) whereas abiotic factors (mean air temperatures and relative humidity) better determined the chemical composition in the advanced DDW decomposition stage. However, results showed opposing patterns: mean air temperature lowered P content and the polysaccharides proportion, but increased the C/P ratio and lignin. Relative humidity, in turn, increased P and polysaccharides, but decreased lignin. Saproxylic organisms, including fungi, depend not only on the availability of woody resources, but also on the moisture content of dead wood (Cornelissen et al. 2012; Fukasawa and Matsuoka 2015). Unlike what occurs in tropical and subtropical areas, where combined high air temperatures and high relative humidity favour dead wood decomposition (Pietsch et al. 2019), in dehesa-like forests, as in most Mediterranean ecosystems, high temperatures generally entail low moisture and slow down organic matter decay (Fravolini et al. 2018). So although several studies support positive effects of increasing mean air temperatures on nutrient turnover through wood decomposition (Pietsch et al. 2019; Seibold et al. 2022), our results suggest that increasing global temperatures and decreasing precipitation in the Mediterranean region as a result of climate change (Lozano-García et al. 2017) may jeopardise dead wood decomposition processes, especially in agricultural and cultural landscapes like dehesa forests, which are characterised by scattered trees (high canopy openness) and low dead wood inputs.
Conclusions
We conclude that physical decomposition stage and ergosterol content as a proxy of decay length and fungal activity can, respectively, help to understand the temporal behaviour of the dead wood chemical composition in Mediterranean areas, and both are closely related, but not completely collinear. We recommend using FT-IR analyses to better assess the nature of the temporal chemical changes of DDW. Additionally, environmental factors, such as temperature and humidity, should be taken as potential modulators of dead wood degradation to make better quality predictions of dead wood ecosystem services in managed forests in today’s global change scenery.
Data availability
All data will be made available on request.
References
Abrego N, Salcedo I (2013) Variety of Woody debris as the factor influencing wood-inhabiting fungal richness and assemblages: is it a question of quantity or quality? Ecol Manag 291:377–385. https://doi.org/10.1016/j.foreco.2012.11.025
Arnstadt T, Hoppe B, Kahl T, Kellner H, Krüger D, Bässler C, Bauhus J, Hofrichter M (2016) Patterns of laccase and peroxidases in coarse woody debris of fagus sylvatica picea abies and pinus sylvestris and their relation to different wood parameters. Eur J For Res 135(1):109–124. https://doi.org/10.1007/s10342-015-0920-0
Baldrian P, Zrůstová P, Tláskal V, Davidová A, Merhautová V, Vrška T (2016) Fungi associated with decomposing deadwood in a natural beech-dominated forest. Fungal Ecol 23:109–122. https://doi.org/10.1016/j.funeco.2016.07.001
Bani A, Pioli S, Ventura M, Panzacchi P, Borruso L, Tognetti R, Tonon G, Brusetti L (2018) The role of microbial community in the decomposition of leaf litter and deadwood. Appl Soil Ecol 126:75–84. https://doi.org/10.1016/j.apsoil.2018.02.017
Bates D, Maechler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48. https://doi.org/10.18637/jss.v067.i01
Blondel J, Aronson J (1999) Biology and Wildlife of the Mediterranean Region. Oxford University Press
Błońska E, Kacprzyk M, Spólnik A (2017) Effect of deadwood of different tree species in various stages of decomposition on biochemical soil properties and carbon storage. Ecol Res 32:193–203. https://doi.org/10.1007/s11284-016-1430-3
Boddy L (2000) Interspecific combative interactions between wood-decaying basidiomycetes. FEMS Microbiol Ecol 31:185–194. https://doi.org/10.1111/j.1574-6941.2000.tb00683.x
Boddy L, Watkinson SC (1995) Wood decomposition, higher fungi, and their role in nutrient redistribution. Can J Bot 73:1377–1383. https://doi.org/10.1139/b95-400
Brennan KEC, Christie FJ, York A (2009) Global climate change and litter decomposition: more frequent fire slows decomposition and increases the functional importance of invertebrates. Glob Chang Biol 15:2958–2971. https://doi.org/10.1111/j.1365-2486.2009.02011.x
Campos P, Huntsinger L, Oviedo JL, Starrs PF, Díaz M, Standiford RB, Montero G (2013) Mediterranean oak woodland working landscapes. Landsc Ser 16. https://doi.org/10.1007/978-94-0076707-2
Clinton PW, Buchanan PK, Wilkie JP, Smaill SJ, Kimberley MO (2009) Decomposition of Nothofagus wood in vitro and nutrient mobilization by fungi. Can J Res 39:2193–2202. https://doi.org/10.1139/X09-134
Conrad AO, Rodriguez-Saona LE, McPherson BA, Wood DL, Bonello P (2014) Identification of Quercus agrifolia (coast live oak) resistant to the invasive pathogen Phytophthora Ramorum in native stands using Fourier-transform infrared (FT-IR) spectroscopy. Front Plant Sci 5:521. https://doi.org/10.3389/fpls.2014.00521
Cornelissen JHC, Sass-Klaassen U, Poorter L, van Geffen K, van Logtestijn RSP, van Hal J, Goudzwaard L, Sterck FJ, Klaassen RKWM, van der Freschet GT, Eshuis H, Zuo J, de Boer W, Lamers T, Weemstra M, Cretin V, Martin R, den Ouden J, Berg MP, Aerts R, Mohren GMJ, Hefting MM (2012) Controls on coarse wood decay in temperate tree species: birth of the LOGLIFE experiment. Ambio 41:231–245
Crowther TW, Bradford MA (2013) Thermal acclimation in widespread heterotrophic soil microbes. Ecol Lett 16:469–477
Du E, Terrer C, Pellegrini AFA, Ahlström A, van Lissa CJ, Zhao X, Xia N, Wu X, Jackson RB (2020) Global patterns of terrestrial nitrogen and phosphorus limitation. Nat Geosci 13:221226. https://doi.org/10.1038/s41561-019-0530-4
Eriksson KEL, Blanchette RA, Ander P (1990) Morphological aspects of wood degradation by fungi and bacteria. In: Eriksson KEL, Blanchette RA, Ander P (eds) Microbial and enzymatic degradation of wood and wood components. Springer Berlin, Berlin, pp 1–87. https://doi.org/10.1007/978-3-642-46687-8_1
Fackler K, Schwanninger M, Gradinger C, Hinterstoisser B, Messner K (2007) FEMS Microbiol Lett 271(2):162–169. https://doi.org/10.1111/j.1574-6968.2007.00712.x. Qualitative and quantitative changes of beech wood degraded by wood-rotting basidiomycetes monitored by Fourier transform infrared spectroscopic methods and multivariate data analysis
Fernandez de Simón B, Sanz M, Cadahía E, Poveda P, Broto M (2006) Chemical characterization of oak heartwood from Spanish forests of Quercus Pyrenaica (Wild.). Ellagitannins, low molecular weight phenolic, and volatile compounds. J Agric Food Chem 54:8314–8321. https://doi.org/10.1021/jf061546t
Ferro ML (2018) It’s the end of wood as we know it: insects in veteris (highly decomposed) wood. In: Ulyshen MD (ed) Saproxylic insects, zoological monographs 1. Springer, Switzerland. https://doi.org/10.1007/978-3-319-75937-1_22
Filipiak M (2018) Nutrient dynamics in decomposing dead wood in the context of wood eater requirements: the ecological stoichiometry of saproxylophagous insects. In: Ulyshen M. (ed) Saproxylic insects. Zool Monographs, vol 1. Springer, Cham. https://doi.org/10.1007/978-3-319-75937-1_13
Filipiak M, Weiner J (2014) How to make a beetle out of wood: multi-elemental stoichiometry of wood decay, xylophagy and fungivory. PLOS ONE 9:e115104. https://doi.org/10.1371/journal.pone.0115104
Filipiak M, Sobczyk Ł, Weiner J (2016) Fungal transformation of tree stumps into a suitable resource for xylophagous beetles via changes in elemental ratios. Insects 7:13. https://doi.org/10.3390/insects7020013
Franc N, Götmark F, Økland B, Nordén B, Paltto H (2007) Factors and scales potentially important for saproxylic beetles in temperate mixed oak forest. Biol Conserv 135:86–98. https://doi.org/10.1016/j.biocon.2006.09.021
Fravolini G, Egli M, Derungs C, Cherubini P, Ascher-Jenull J, Gómez-Brandón M, Bardelli T, Tognetti R, Lombardi F, Marchetti M (2016) Soil attributes and microclimate are important drivers of initial deadwood decay in sub-alpine Norway spruce forests. Sci Total Environ 569–570:1064–1076. https://doi.org/10.1016/j.scitotenv.2016.06.167
Fravolini G, Tognetti R, Lombardi F, Egli M, Ascher-Jenull J, Arfaioli P, Bardelli T, Cherubini P, Marchetti M (2018) Quantifying decay progression of deadwood in Mediterranean mountain forests. Ecol Manag 408:228–237. https://doi.org/10.1016/j.foreco.2017.10.031
Fukasawa Y, Matsuoka S (2015) Communities of wood-inhabiting fungi in dead pine logs along a geographical gradient in Japan. Fungal Ecol 18:75–82. https://doi.org/10.1016/j.funeco.2015.09.008
García Rodríguez JA, Puerto Martín A, Rodríguez González R (1984) Aspectos ecológicos de la provincia de Salamanca (España). Centro Edafología Y Biología aplicada. Diputación provincial de Salamanca, Spain
García-López A, Galante E, Micó E (2016) Saproxylic beetle assemblage selection as determining factor of species distributional patterns: implications for conservation. J Insect Sci 16:45. https://doi.org/10.1093/jisesa/iew030
Gessner MO, Schmitt AL (1996) Use of solid-phase extraction to determine ergosterol concentrations in plant tissue colonized by fungi. Appl Env Microbiol 62:415–419. https://doi.org/10.1128/aem.62.2.415-419.1996
Gessner MO, Bauchrowitz MA, Escautier M (1991) Extraction and quantification of ergosterol as a measure of fungal biomass in a leaf litter. Microb Ecologu 22:285–291. https://doi.org/10.1007/BF02540230
Gossner MM, Lachat T, Brunet J, Isacsson G, Bouget C, Brustel H, Brandl R, Weisser WW, Müller J (2013) Current near-to-nature forest management effects on functional trait composition of saproxylic beetles in beech forests. Conserv Biol 27:605–614. https://doi.org/10.1111/cobi.12023
Grove S (2002) Saproxylic insect ecology and the sustainable management of forests. Ann Rev Ecol Syst 33:1–23. https://doi.org/10.1146/annurev.ecolsys.33.010802.150507
Hämäläinen A, Strengbom J, Ranius T (2018) Conservation value of low-productivity forests measured as the amount and diversity of dead wood and saproxylic beetles. Ecol Appl 28:1011–1019. https://doi.org/10.1002/eap.1705
Harmon ME, Franklin JF, Swanson FJ, Sollins P, Gregory SV, Lattin JD, Anderson NH, Cline SP, Aumen NG, Sedell J, Lienkaemper GW, Cromack K Jr, Cummins KW (1986) Ecology of coarse woody debris in temperate ecosystems. Adv Ecol Res 15:133–302. https://doi.org/10.1016/S0065-2504(03)34002-4
Herrmann S, Bauhus J (2018) Nutrient retention and release in coarse woody debris of three important central European tree species and the use of NIRS to determine deadwood chemical properties. Ecosyst 5:22. https://doi.org/10.1186/s40663-018-0140-4
Hoppe B, Purahong W, Wubet T, Kahl T, Bauhus J, Arnstadt T, Hofrichter M, Buscot F, Krüger D (2016) Linking molecular deadwood-inhabiting fungal diversity and community dynamics to ecosystem functions and processes in central European forests. Fungal Divers 77:367–379. https://doi.org/10.1007/s13225-015-0341-x
Horák J (2017) Insect ecology and veteran trees. J Insect Conserv 21:1–5. https://doi.org/10.1007/s10841-017-9953-7
Hou E, Luo Y, Kuang Y, Chen C, Lu X, Jiang L, Luo X, Wen D (2020) Global meta-analysis shows pervasive phosphorus limitation of aboveground plant production in natural terrestrial ecosystems. Nat Commun 11:637. https://doi.org/10.1038/s41467-020-14492-w
Hu Y, Yesilonis I, Szlavecz K (2021) Microbial and environmental controls on wood decomposition in deciduous forests of different ages. Appl Soil Ecol 166:103986. https://doi.org/10.1016/j.apsoil.2021.103986
IPCC (2003) Good practice guidance for land use, land-use change and forestry. Institute for Global Environmental Strategies (IGES), Kanagawa, Japan
Jombart T (2008) Adegenet: a R package for the multivariate analysis of genetic markers. Bioinformatics 24:1403–1405. https://doi.org/10.1093/bioinformatics/btn129
Jomura M, Yoshida R, Michalcíková L, Tláskal V, Baldrian P (2022) Factors controlling dead wood decomposition in an old-growth temperate forest in central Europe. J Fungi 8:673. https://doi.org/10.3390/jof8070673
Keren S, Diaci J (2018) Comparing the quantity and structure of dead wood in selection managed and old-growth forests in south-east Europe. Forests 9:76. https://doi.org/10.3390/jof8070673
Kitson RE, Mellon MG (1944) Colorimetric determination of phosphorus as molybdivanadophosphoric acid. Industrial and Engineering Chemistry, Analytical Edition 16:379–383. https://doi.org/10.1021/i560130a017
Laiho R, Prescott CE (2004) Decay and nutrient dynamics of coarse woody debris in northern coniferous forests: a synthesis. Can J Res 34:763–777. https://doi.org/10.1139/x03-241
Lenth R (2022) emmeans: Estimated marginal means, aka least-squares means. R package version 1.7.4-1. https://CRAN.R-project.org/package=emmeans
Liers C, Arnstadt T, Ullrich R, Hofrichter M (2011) Patterns of lignin degradation and oxidative enzyme secretion by different wood- and litter-colonizing basidiomycetes and ascomycetes grown on beech-wood. FEMS Microbiol Ecol 78:91–102. https://doi.org/10.1111/j.1574-6941.2011.01144.x
Llamas O, Belinchón G, Ramos LA, Albadalejo PV (2011) El Rebollar. Tomero y Romillo Servicios Ambientales, León, Spain
Lombardi F, Cherubini P, Cocozza R, Tognetti C, Lasserre B, Marchetti M (2013) Investigating biochemical processes to assess dead wood decay of beech and silver fir in Mediterranean mountain forests. Ann for Sci 70:101–111. https://doi.org/10.1007/s13595-012-0230-3
Lonsdale D, Pautasso M, Holdenrieder O (2008) Wood-decaying fungi in the forest: conservation needs and management options. Eur J Res 127:1–22. https://doi.org/10.1007/s10342-007-0182-6
Lozano-García B, Muñoz-Rojas M, Parras-Alcántara L (2017) Climate and land use changes effects on soil organic carbon stocks in a Mediterranean semi-natural area. Sci Total Environ 579:1249–1259. https://doi.org/10.1016/j.scitotenv.2016.11.111
Martín JA, Solla A, Woodward S, Gil L (2005) Fourier transform-infrared spectroscopy as a new method for evaluating host resistance in the Dutch elm disease complex. Tree Physiol 25:1331–1338. https://doi.org/10.1093/treephys/25.10.1331
Micó E, Juárez M, Sánchez A, Galante E (2011) Action of the saproxylic scarab larva Cetonia Aurataeformis (Coleoptera: Scarabaeoidea: Cetoniidae) on woody substrates. J Nat Hist 45:2527–2542. https://doi.org/10.1080/00222933.2011.596953
Micó E, Marcos-García MA, Ramírez-Hernández A, Galante E (2021) El Bosque Adehesado como refugio de una entomofauna muy diversa. Publicacions de la Universitat d’Alacant, Alicante, Spain
Micó E, Martínez-Pérez S, Jordán-Núñez J, Galante E, Micó-Vicent B (2022) On how the abandonment of traditional forest management practices could reduce saproxylic diversity in the Mediterranean Region. Ecol Manag 520:120402. https://doi.org/10.1016/j.foreco.2022.120402
Mille-Lindblom C, von Wachenfeldt E, Tranvik LJ (2004) Ergosterol as a measure of living fungal biomass: persistence in environmental samples after fungal death. J Microbiol Methods 59:253–262. https://doi.org/10.1016/j.mimet.2004.07.010
Mononen K, Jääskeläinen A-S, Alvula L, Pakkanen TT, Vuorinen T (2005) Chemical changes in silver birch (Betula pendula Roth) wood caused by hydrogen peroxide bleaching and monitored by colour measurement (CIELab) and UV-Vis, FTIR and UVRR spectroscopy. Holxforschung 59:381–388. https://doi.org/10.1515/HF.2005.063
Montgomery HJ, Monreal CM, Young JC, Seifert KA (2000) Determination of soil fungal biomass from soil ergosterol analyses. Soil Biol Biochem 32:1207–1217. https://doi.org/10.1016/S0038-0717(00)00037-7
Morán-Ordóñez A, Ramsauer J, Coll L, Brotons L, Ameztegui A (2021) Ecosystem services provision by Mediterranean forests will be compromised above 2ºC warming. Glob Change Biol 27:4210–4222. https://doi.org/10.1111/gcb.15745
Moreno G, Pulido FJ (2009) The functioning, management and persistence of dehesas. In: Rigueiro-Rodríguez A, McAdam J, Mosquera-Losada MR (eds) Agroforestry in Europe. Advances in Agroforestry, vol 6. Springer, Dordrecht, pp 127–170
van der Wal A, Ottosson E, de Boer W (2015) Neglected role of fungal community composition in explaining variation in wood decay rates. Ecology 96:124–133. https://doi.org/10.1890/14-0242.1
Noll L, Leonhardt S, Arnstadt T, Hoppe B, Poll C, Matzner E, Hofrichter M, Kellner H (2016) Fungal biomass and extracellular enzyme activities in coarse woody debris of 13 tree species in the early phase of decomposition. Ecol Manag 378:181–192. https://doi.org/10.1016/j.foreco.2016.07.035
Öder V, Petritan AM, Schellenberg J, Bergmeier E, Walentowski H (2021) Patterns and drivers of dead wood quantity and variation in mid-latitude deciduous forests. Ecol Manag 487:118977. https://doi.org/10.1016/j.foreco.2021.118977
Økland B, Bakke A, Hågvar S, Kvamme T (1996) What factors influence the diversity of saproxylic beetles? A multiscaled study from a spruce forest in southern Norway. Biodivers Conserv 5:75–100. https://doi.org/10.1007/BF00056293
Oliver-Moscardó S, Luis-Calabuig E (1979) Factores termopluviométricos. In: Ballcells E (ed) Integrado Y Multidisciplinar De La Dehesa Salmantina. Centro Edafología Y Biología Aplicada De Salamanca Y Centro Pirenaico De Biología experimental. CSIC, Salamanca- Jaca, pp 101–155
Pandey KK, Pitman AJ (2003) FTIR studies of the change in wood chemistry following decay by brown-rot and white-rot fungi. Int Biodeterior Biodegr 52:151–160. https://doi.org/10.1016/S0964-8305(03)00052-0
Parisi F, Pioli S, Lombardi F, Fravolini G, Marchetti M, Tognetti R (2018) Linking deadwood traits with saproxylic invertebrates and fungi in European forests – a review. iForest 11:423–436. https://doi.org/10.3832/IFOR2670-011
Pascoal C, Cássio F, Nikolcheva L, Bärlocher F (2010) Realized fungal diversity increases functional stability of leaf litter decomposition under zinc stress. Microb Ecol 59:84–93. https://doi.org/10.1007/s00248-009-9567-z
Pastorelli R, Paletto A, Agnelli AE, Lagomarsino A, De Meo I (2021) Microbial diversity and ecosystem functioning in deadwood of black pine of a temperate forest. Forests 12:1418. https://doi.org/10.3390/f12101418
Pettersen RC (1984) The chemical composition of wood. In: Rowell R (ed) The chemistry of solid wood. American Chemical Society, Washington DC, pp 57–126
Peuravuori J, Passo N, Pihlaja K (1999) Kinetic study of the thermal degradation of lake aquatic humic matter by thermogravimetric analysis. Thermochimica Acta 325(2):181–193. https://doi.org/10.1016/S0040-6031(98)00582-6
Pietsch KA, Eichenberg D, Nadrowski K, Bauhus J, Buscot F, Purahong W, Wipfler B, Wubet T, Yu M, Wirth C (2019) Wood decomposition is more strongly controlled by temperature than by tree species and decomposer diversity in highly species rich subtropical forests. Oikos 128:701–715. https://doi.org/10.1111/oik.04879
Popescu CM, Popescu MC, Singurel G, Vasile C, Argyropoulos DS, Willfor S (2007) Spectral characterization of eucalyptus wood. Appl Spectrosc 61:1168–1177. https://doi.org/10.1366/000370207782597076
Popescu CM, Popescu MC, Vasile C (2010) Structural changes in biodegraded lime wood. Carbohydr Polym 79:362–372. https://doi.org/10.1016/j.carbpol.2009.08.015
Přívětivý T, Adam D, Vrška T (2018) Decay dynamics of Abies alba and Picea abies deadwood in relation to environmental conditions. Ecol Manag 427:250–259. https://doi.org/10.1016/j.foreco.2018.06.008
Purahong W, Tanuncha B, Muszynski S, Maurer F, Wahdan SFM, Malter J, Buscot F, Noll M (2022) Cross-kingdom interactions and functional patterns of active microbiota matter in governing dead wood decay. Proc R Soc B 289. https://doi.org/10.1098/rspb.2022.0130
R Core Team (2021) R: A language and environment for statistical computing. R foundation for statistical computing, Vienna. https://www.R-project.org
Rajala T, Tuomivirta T, Pennanen T, Mäkipää R (2015) Habitat models of wood-inhabiting fungi along a decay gradient of Norway spruce logs. Fungal Ecol 18:48–55. https://doi.org/10.1016/j.funeco.2015.08.007
Ruzicka S, Edgerton D, Norman M, Hill T (2000) The utility of ergosterol as a bioindicator of fungi in temperate soils. Soil Biol Biochem 32:989–1005. https://doi.org/10.1016/S0038-0717(00)00009-2
Sánchez A, Micó E, Galante E, Juárez M (2017) Chemical transformation of Quercus wood by Cetonia larvae (Coleoptera: Cetoniidae): an improvement of carbon and nitrogen available in saproxylic environments. Eur J Soil Biol 78:57–65. https://doi.org/10.1016/j.ejsobi.2016.12.003
Seibold S, Thorn S (2018) The importance of dead-wood amount for saproxylic insects and how it interacts with dead-wood diversity and other habitat factors. In: Ulyshen MD (ed) Saproxylic insects, zoological monographs 1. Springer, Switzerland, pp 607–637
Seibold S, Rammer W, Hothorn T, Seidl R, Ulyshen MD, Lorz J, Cadotte MW, Lindenmayer DB, Adhikari YP, Aragón R (2021) The contribution of insects to global forest deadwood decomposition. Nature 597:77–81. https://doi.org/10.1038/s41586-021-03740-8
Seibold S, Müller J, Allner S, Willner M, Baldrian P, Ulyshen MD, Brandl R, Bässler C, Hagge J, Mitesser O (2022) Quantifying wood decomposition by insects and fungi using computed tomography scanning and machine learning. Sci Rep 12:16150. https://doi.org/10.1038/s41598-022-20377-3
Seitz LM, Sauer DB, Burroughs R, Mohr HE, Hubbard JD (1979) Ergosterol as a measure of fungal growth. Phytopathol 69:1202–1203
Shannon VL, Vanguelova EI, Morison JIL, Shaw LJ, Clark JM (2022) The contribution of dead wood to soil carbon dynamics in contrasting temperate forest ecosystems. Eur J Res 141:241–252. https://doi.org/10.1007/s10342-021-01435-3
Siitonen J (2001) Forest management, coarse woody debris and saproxylic organisms: Fennoscandian boreal forests as an example. Ecol Bull 49:11–41. http://www.jstor.org/stable/20113262
Spaccini R, Piccolo A (2009) Molecular characteristics of humic acids extracted from compost at increasing maturity stages. Soil Biol Biochem 41:1164–1172. https://doi.org/10.1016/j.soilbio.2009.02.026
Stokland JN, Siitonen J, Jonsson BG (2012) Biodiversity in dead wood. Cambridge University Press, Cambridge
Strukelj M, Brais S, Quideau SA, Angers VA, Kebli H, Drapeau P, Oh SW (2013) Chemical transformations in downed logs and snags of mixed boreal species during decomposition. Can J Res 43:785–798. https://doi.org/10.1139/cjfr-2013-0086
Sverdrup-Thygeson A, Birkemoe T (2009) What window traps can tell us: effect of placement, forest openness and beetle reproduction in retention trees. J Insect Conserv 13:183–191. https://doi.org/10.1007/s10841-008-9141-x
Ulyshen MD (2016) Wood decomposition as influenced by invertebrates. Biol Rev Camb Philos Soc 91:70–85. https://doi.org/10.1111/brv.12158
Větrovský T, Voříšková J, Šnajdr J, Gabriel J, Baldrian P (2011) Ecology of coarse wood decomposition by the saprotrophic fungus Fomes Fomentarius. Biodegradation 22:709–718. https://doi.org/10.1007/s10532-010-9390-8
Walter J, Hein R, Beierkuhnlein C, Hammerl V, Jentsch A, Schädler M, Schuerings J, Kreyling J (2013) Combined effects of multifactor climate change and land-use on decomposition in temperate grassland. Soil Biol Biochem 60:10–18. https://doi.org/10.1016/j.soilbio.2013.01.018
Weedon JT, Cornwell WK, Cornelissen JH, Zanne AE, Wirth C, Coomes DA (2009) Global meta-analysis of wood decomposition rates: a role for trait variation among tree species? Ecol Lett 12:45–56. https://doi.org/10.1111/j.1461-0248.2008.01259.x
Woodall CW, Liknes GC (2008) Climatic regions as an indicator of forest coarse and fine woody debris carbon stocks in the United States. Carb Bal Manage 3:5. https://doi.org/10.1186/1750-0680-3-5
Woodall C, Monleon V, Fraver S, Russell MB, Hatfield MH, Campbell JL, Domke GM (2019) The downed and dead wood inventory of forests in the United States. Sci Data 6:180303. https://doi.org/10.1038/sdata.2018.303
Young JC (1995) Microwave-assisted extraction of the fungal metabolite ergosterol and total fatty acids. J Agric Food Chem 43:2904–2910. https://doi.org/10.1021/jf00059a025
Acknowledgements
We would like to thank the Junta de Castilla y León and its environmental agents for facilitating our fieldwork. To the anonymous reviewer whose suggestions have improved the quality of the paper.
Funding
Financial support was provided by the Spanish Ministerio de Economía y Competitividad (CGL2016-78181-R). JQ was supported by Spanish Ministry of Science and Innovation, project PID2020-115140RB-I00/AEI/https://doi.org/10.13039/501100011033.
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature.
Author information
Authors and Affiliations
Contributions
E.M., A.S. and M.J. conceived the idea and designed the method. E.M. and J.M. did the fieldwork. J.M and A.S. performed the chemical analysis. J.M., MJ, A.S. and E.M. participated in work conceptualization and the preliminary data analysis. M.A analysed the data and led the writing of the data analysis. E.M. and J.Q. led the writing of the manuscript. E.M. did the project administration and funding acquisition. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Communicated by Peter Biber.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Micó, E., Aguirrebengoa, M., Quinto, J. et al. Physical decomposition stage and ergosterol content predict the chemical composition of downed dead wood in Mediterranean dehesas. Eur J Forest Res (2024). https://doi.org/10.1007/s10342-024-01672-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10342-024-01672-2