Abstract
Soil rehabilitation involves restoring their capacity to provide goods and services; however, there is a lack of information regarding the survival and growth responses of individual species to local environmental variations. This study evaluated the adaptation capacity of three non-native multiple-use species in agroforestry design, in a degraded semiarid temperate climate zone located in the Tula watershed in Mexico, assessing their survival, growth of morphological variables, and death risk for use as soil restoration plants. According to the slope of the terrain, the species were planted in an agroforestry arrangement of lines and ridges. Plants were selected according to their multipurpose potential, including their taxonomy, form, function, and use, as well as their soil and climatic conditions. Survival rates for all three species were below acceptable levels \((< 49\%)\). Neither Senna multiglandulosa nor Sedum dendroideum adapted well to the soil and climate of the site, since their survival rates were below acceptable \((\sim 17<49\mathrm{\%})\). Aloe sp. adapted to the edaphoclimatic conditions of the site because its survival rate was excellent \((\sim 97 \ge 90\%)\). Climate conditions during the autumn and winter affected growth rates of Senna multiglandulosa and Sedum dendroideum. Aloe sp. growth rates were only affected by climatic conditions during the winter months. Senna multiglandulosa's final height reduced death risk by \(1.50\%\). Sedum dendroideum's final diameter, initial canopy cover and canopy growth rate reduced the risk of death by \(55.1\), \(5.1\) and \(30.7\%\), respectively. The height growth rate of aloe and initial canopy cover reduced the risk of death by 93.9 and 2.4%, respectively. Plant species evaluated showed varying levels of adaptation. Aloe sp. was the species that adapted best to the site's soil and climate.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Rehabilitating a soil is the process of restoring its capacity to provide goods and services again, but it is important to take into consideration that a rehabilitated soil is not the same as it was before degradation. Intervention strategies aim to restore the productivity of the area rather than to restore its original structure (Peri et al. 2021; Chazdon 2008). Seeding or planting of woody plants, soil retention and conservation tools, or a combination of the two, are the most common practices worldwide (Mata-Balderas et al. 2014). However, each ecosystem has its own dynamics of disturbance and subsequent recovery, depending on the type, intensity, and frequency of disturbance. Thus, there are no universally effective strategies, but rather complementary techniques that must be evaluated in order to determine which are the most effective methods to recover an ecosystem based on its particular properties (Ceccon 2013; Duarte et al. 2018; López-Barrera 2015). Rehabilitation activities are designed to ensure that plants achieve high levels of survival and growth during the field establishment step (Navarro et al. 2006).
Many factors influence the survival of species at an early stage and the success of plantations, such as structural characteristics, morphology, soil properties, nutrient availability, climate, soil preparation, planting dates, humidity, weed control, site-specific characteristics as well as many other factors (Aguilos et al. 2020; Davis and Jacobs 2005; Navarro et al. 2006; Bernaola-Paucar et al. 2015; Palacios-Romero et al. 2017). Identification of the limiting environmental factors in an area of interest prior to planting is important to determine what morphological and physiological characteristics the vegetative material should possess for fast rooting and adaptation to site conditions (Navarro et al. 2006; Landis et al. 2010). Despite the fact that diameter is recognized as an important attribute in nursery plants in order to ensure survival (Tsakaldimi et al. 2013), as well as tolerance of adverse climate conditions (Prieto et al. 2011), which is due to its relationship with root volume and taproots (Jacobs et al. 2009), morphological characteristics are not the only factors that explain the variation in responses after planting.
The Mexican ecosystem is highly heterogeneous, creating an environment that promotes great biological diversity and an important natural capital, and is among the five nations with the greatest degree of biological and cultural diversity in the world (Challenger et al. 2009; Domínguez et al. 2009; Sarukhán et al. 2015). However, natural capital has suffered alarming deterioration due to the conversion of habitats to other soil uses and their consequent fragmentation and degradation (Challenger et al. 2009; Sarukhán et al. 2015). The natural vegetation cover of the country was only 54% in 1993, and 38% in 2002, of which approximately 50% was degraded (Challenger et al. 2009). More than 45% of soil degradation in Mexico is associated to human activity (Cotler et al. 2007), with rates higher in semiarid regions with low annual precipitation, which has a ranged of 260 to 600 mm (Zamora et al. 2020). Tula watershed is located in the states of Hidalgo, Tlaxcala, and Mexico, and it has two main climate types: temperate subhumid and semiarid (Köppen classification modified by García, 1964). Some of the semiarid climate regions are experiencing population growth, an expedition of mining concessions for the exploitation of construction materials, and agricultural and urban increase, resulting in the degradation of soils because of increasing demands of natural resources (INEGI, 2017; Zamora et al. 2020).
Given these serious alterations to Mexico's ecosystems, it is important to develop conservation and rehabilitation strategies. Rehabilitating ecosystems can prevent and reverse the loss of biodiversity and restore ecosystem services (Ceccon 2013). For degraded ecosystems to be restored, it is necessary to reintroduce species that can thrive in the edaphoclimatic conditions of these regions. Incorporating shrubs and succulents as Senna multiglandulosa, Sedum dendroideum and Aloe sp. under agroforestry practices can lead to sustainable soil management, as well as the rehabilitation of degraded areas. Among the factors considered in selecting these species were their ability to grow in semiarid to temperate climates, their resistance to drought and low temperatures, their ability to form soil, their effectiveness in erosion control, and their use in Mexico as alternative crops (Gazca and Benavides 2018; Kumar et al. 2022; Gutiérrez 2009).
Survival analysis considers data on the number of deaths of each species to calculate the likelihood of survival (Crawley 2007), which indicates the species' ability to adapt to different habitat conditions (Sigala et al. 2015). Cox proportional hazards regression model (Bradburn et al. 2003; Cox 1972) predicts a species' death probability at a given time \(t\) based on certain morphological variables. Using survival analysis and Cox proportional hazards modeling, this study estimated the probability of survival for each species and determined their risk of death based on their morphological characteristics. During the early stages of any field trial, it is crucial to evaluate the progress of the trial, which is why forest plantations should receive more attention at this stage in the research process (Aguilos et al. 2020).
This study aimed to evaluate the adaptation capacity of three non-native multipurpose species (Senna multiglandulosa, Sedum dendroideun, and Aloe sp.) in a degraded area with a semiarid temperate climate, located in the Tula watershed in Mexico, for restoring degrades soils through agroforestry by assessing their survival, growth of morphological variables, and death risk.
Materials and methods
Geographical location of study area
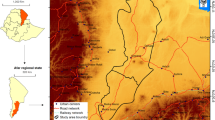
The study area was the Tula watershed located in the states of Mexico, Hidalgo, and Tlaxcala (Fig. 1), with elevations from 2333 to 3223 m and an area of 1037.66 km2. The average annual temperature is 14 °C while the temperature of the coldest month varies between − 3 °C and 18 °C, and the temperature of the hottest month is less than 22 °C. The predominant vegetation consists mainly of tascate forests, with species of the genus Juniperus spp., pine and oak, with species of the genus Pinus spp. and Quercus spp., and crasicaule scrub, with plants of the genus Opuntia spp. (Instituto Nacional de Estadística y Geografía [INEGI], 2017; García-Comisión Nacional para el Conocimiento y Uso de la Biodiversidad [CONABIO], 1998; Instituto Nacional de Investigaciones Forestales y Agrícolas y Pecuarias [INIFAP] and Comisión Nacional para el Conocimiento y Uso de la Biodiversidad [CONABIO], 1995; Zamora et al. 2020, Buendía-Espinoza et al. 2022). There are two main climates within the watershed: semiarid temperate (28% of the total area) and temperate sub-humid (72% of the total area). In the temperate semiarid climate, annual precipitation ranges from 500 to 600 mm per year. The predominant soils are haplic phaeozem and eutrophic cambisol, both of medium texture (loamy). In the sub-humid temperate climate, annual precipitation ranges from 600 to 1000 mm per year with rainfall in summer and the percentage of winter rainfall is 5 to 10.2% of the annual total. The precipitation in the driest month is less than 40 mm and the predominant soils are medium-textured haplic phaeozem and fine-textured pelic vertisol (INEGI 2017; García-CONABIO, 1998; INIFAP and CONABIO 1995; Zamora et al. 2020, Buendía-Espinoza et al. 2022).
Area of study and location of the experimental site within the Tula watershed, Mexico (Buendía-Espinoza et al., 2022)
Experimental site
Experimental site was established in Ejido San Felipe Teotitlán, municipality of San Felipe Teotitlán, located in the semiarid temperate zone within the Tula watershed at an elevation of 2 473 m and an area of 1 680 m2 (56 × 30 m). The average temperature is 14 °C, with thermal oscillations in the monthly averages of 6.6 °C, the coldest month (January) has an average temperature of 11.1 °C, and the warmest month (May) has an average temperature of 17.7 °C. Rainfall ranges from 500 to 600 mm annually, of which approximately 83% falls between June and October (INEGI 2009).
Experimental design
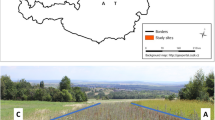
An agroforestry design based on contour planting was applied to the field experiment as a means of increasing organic matter production, reducing runoff, and contributing to the creation of a microclimate (Ceccon 2013). For this purpose, six 30 m-long trenches, 30 cm deep and wide, were dug perpendicular to the slope of the land in order to reduce runoff and sediment dragging, where 156 plants of Aloe sp. were planted each meter. Each trench was planted with one Senna multiglandulosa plant every ten m to provide nitrogen to the soil and to protect it. A total of 24 plants were planted in the six trenches. An internal ridge was created between the trenches, where 288 plants of Sedum dendroide species were transplanted in a real frame arrangement of 2 m in order to establish a microclimate and protect the soil. During the period July 30 to August 11, 2020, excavation and transplanting activities were conducted (Fig. 2).
Target species
Based on edaphoclimatic conditions at the experimental site as well as their multipurpose potential, taking into account taxonomy, form, function, and uses, multipurpose plants were selected.
Senna multiglandulosa, known as retama, grows at altitudes between 2100 and 4000 m from Central Mexico to Guatemala (INECOL 1997). This species is able to thrive in semiarid to temperate climates, is drought and cold temperature tolerant, prefers loamy to sandy soils, even if they contain high levels of stoniness, tolerates poor soils and tepetose soils, requires medium to high moisture levels, and is useful for erosion control (Gazca and Benavides 2018).
Aloe sp., also known as aloe, is a semiperennial succulent plant of the Liliaceae family and has succulent leaves arranged in the shape of roses. It is native to Africa, but has spread throughout Mexico, thriving in a wide variety of habitats, including forests and deserts. Aloe sp. grows in areas with rainfall between 200 and 600 mm, slowing down in areas with rainfall below this amount, but is resistant to drought and thrives at altitudes ranging from 0 to 2500 m. This species grows best in loamy soil, but can also thrive in sandy soils or soils with very little organic matter and a pH of alkaline-neutral. The thermal regime of this plant is from 18 to 27 °C, and at temperatures below 5 °C, it shows damage (Pedroza and Gómez 2006).
Sedum dendroideum, also known as Siempreviva, is a shrubby plant in the family Crassulaceae, which is native to Mexico, Guatemala, and Central America (Pérez-Calix 2008). The species is found in semi-warm, semiarid and cold climates, and in a wide range of habitats, including rocky areas, crags, slopes, forests, and scrub with altitudes between 1350 and 2750 m above sea level (Gutiérrez 2009). Plants can grow without difficulty at maximum temperatures, but during the winter they must be kept dry in order to prevent damage since they store water in their succulent leaves (López 2019). When temperatures are low during frosty conditions, this species is capable of surviving temperatures as low as 0 °C (Toogood 2007).
Vegetation sampling and measurement of variables
During a period of 12 months beginning in September 2020 and ending in August 2021, the survival and morphological variables of the species were evaluated monthly. In each evaluation, the survival of each seedling was assessed, assigning a 1 or 0 to dead or live plants, respectively, and measuring the height, diameter, and canopy cover of the seedlings. The height of each plant (cm) was measured from the base of the plant to the apex of its dominant vertical shoot using a flexometer. The stem diameter (cm) was measured at ground level with a vernier. The coverage (cm2) of the seedlings was calculated using the square method, which involves placing a 45.7 cm × 61 cm acrylic grid divided into 1 cm × 1 cm quadrants over the seedling and counting the number of squares occupied by the seedling. Squares with more than 50% covered were considered units.
Evaluation of species survival, vegetative growth, and mortality risk in the field
Based on the data collected on morphological variables and plant survival, the following indicators were calculated:
Survival estimation
The survival rate was calculated based on the Kaplan–Meier estimator, \({\widehat{S}}_{KM}\), also known as the limit product estimator, a nonparametric statistical method that takes censoring into account when studying the period during which an event must occur (Kaplan and Meier 1958). This estimator can be used when the individual times of the censored and uncensored individuals are known, so that survival rates can be calculated when an individual dies or reaches the end of their follow-up period. The Kaplan–Meier estimator is defined as:
where \(r\left({t}_{i}\right)\) indicates the live plants, \(d\left({t}_{i}\right)\) indicates the dead plants, and \({t}_{i}<t\) represents the time at which the measurement was made (Kaplan and Meier 1958; Rivas-Ruiz et al. 2014; Gallardo et al. 2016).
According to Kaplan and Meier (1958), survival function is defined as \(S\left(t\right)=P\left(t\ge t\right), t\ge 0\), where \(S(t)\) represents the probability of death occurring at time \(T\) equal to or greater than time \(t\). The status of each plant (alive or dead) at the end of the evaluation period and its duration (months) were considered. \(S(t) = 1 - F(t)\) indicates that \(S(t)\) is decreasing if \(F(t)\) is the distribution function of \(T\). \(S(t)\) decreases at a given rate at each instant, which represents the probability (risk) of an individual's death at that point (Kaplan and Meier 1958). SPSS v25 software was used for the analysis. Rodríguez-Echeverry and Leiton (2020) indicate that a survival rate exceeding 90% is excellent, between 70 and 89% is acceptable, between 50 and 69% is marginal, and less than 49% is inacceptable.
Estimation of death risks
Cox proportional hazards regression (Cox 1972) was used to examine the impact of planting site and production system on seedling morphology variables. The proportional hazards model used was as follows:
where \({h}_{i}(t)\) is the risk of death for individual \(i\) at time \(t\), derived by multiplying the unspecified baseline hazard function, \({h}_{o}\left(t\right)\), by an exponential function of \(k\) covariates (Allison 1995).
The model estimates a coefficient \(\beta\) for each factor or covariate and tests the null hypothesis that \({H}_{0}: \beta =0\) using the Chi-square test. When such a coefficient is negative, it indicates that the risk of death is reduced with increasing covariates, whereas a positive value indicates the opposite (Williams 2008). SPSS v25 software was used for the analysis.
Estimation of vegetative growth
The height growth rate (HGR, cm month−1), diameter growth rate (DGR, mm month−1), and canopy growth rate (CGR, cm2 month−1) were calculated using the following equations (Griscom et al. 2005):
where \(H, D, and C\) represent the diameter, height, or canopy, respectively, at \({t}_{1}\) at the beginning of the experiment and at \({t}_{2}\) at the end of the experiment on a monthly basis. Species response at the planting site was assessed over four periods of analysis (Spring, summer, autumn, and winter).
Results
Survival estimation
All species established in the experimental area survived 44.1% after a period of 12 months (Table 1). Considering the censored data, which consisted of plants that did not succumb to the death event during the analysis, the Aloe sp. had the highest survival estimate of 97.4%, followed by Senna multiglandulosa and Sedum dendroideum with survival rates of 17% each (Table 1).
Both Sedum dendroideum and Senna multiglandulosa species achieved median survival rates of 50% at 7 and 8 months following establishment, respectively. The survival rate of Aloe sp. plants during the evaluation period did not exceed the established median survival limits, since 97.4% of the plants survived (Fig. 3).
As shown in Fig. 4, each species has a different risk of death. Sedum dendroideum recorded a death risk of 10% from October to December 2020; however, this increased to 50% from March to August 2021. The species Aloe had an estimated death rate of approximately 2% in April (month 8), which remained unchanged throughout the assessment. Senna multiglandulosa had an increased mortality risk in April 2021.
Estimation of death risks
The Cox proportional hazards model was statistically significant for all three species (Senna multiglandulosa: Chi2 = 4.056, p = 0.044 < 0.05; Aloe sp. Chi2 = 65.091, p = 0.0000 < 0.05; and Sedum dendroideum: Chi2 = 200.780, p = 0.0000 < 0.05), thus rejecting the null hypothesis that \({H}_{0}: \beta =0\). This indicates that at least one of the covariates can explain survival time in the model.
Cox regression results indicate that the predictor variable final height (finhei) has a positive effect on survival in the species Senna multiglandulosa; that is, a 1 cm increase in height reduces the risk of death by 1.5% (1–0.985), provided all other variables remain constant (Table 2).
Height growth rate (HGR) and initial canopy cover (inican) decreased Aloe's risk of death by 93.9% (1-0.061) and 2.4% (1-0.976) based on Cox regression analysis (Table 3).
Cox regression analysis shows that final diameter (findia), initial canopy cover (inidos) and canopy cover growth rate (CGR) have a positive effect on survival in the species Sedum dendroideum. That is, a 1 cm increase in final diameter, 1 cm2 increase in initial canopy cover, and 1 cm2 increase in canopy cover growth rate reduce the risk of death, respectively, by 55.10% (1-0.449), 5.1% (1-0.994), and 30.7% (1-0.693) assuming all other variables remain unchanged (Table 4).
Estimation of vegetative growth
Tables 5, 6, 7, and 8 present the average growth rates of plant height, stem diameter, and canopy cover for each species during the evaluation period. During the autumn cycle, Sedum dendroideum and Aloe sp. both had negative growth rates in height and canopy cover, with − 0.29 cm month−1 and − 7.37 cm2 month−1 and − 0.53 cm month−1 and − 4.26 cm2 month−1, respectively. Senna multiglandulosa demonstrated a positive growth rate in height (0.87847 cm month−1) and a negative growth rate in canopy cover, the latter of which was the highest at − 22.80 cm2 month−1. Senna multiglandulosa and Sedum dendroideum showed positive growth rates at the stem diameter level (Table 5).
During the second period, which corresponds to the winter cycle, all three species showed negative growth rates for height, stem diameter, and negative canopy cover, with Senna multiglandulosa and Sedum dendroideum showing the greatest decreases in stem diameter and canopy cover, with − 0.005067 cm month−1 and − 10.44 cm2 month−1, respectively. Species Aloe sp. showed the greatest reduction in height, down − 1.15235 cm month−1 (Table 6).
In the springer cycle, which corresponds to the third period, all three species showed positive growth rates for height, stem diameter, and canopy cover, with the exception of Sedum dendroideum, which again displayed a decrease in height (− 0.44872 cm month−1). Senna multiglandulosa had the highest canopy cover growth rate with 59.5392 cm2 month−1 in this period (Table 7).
In the fourth period, which corresponds to the summer cycle, all three species had positive growth rates in height, stem diameter, and canopy cover, with the exception of Sedum dendroideum, which showed a decrease of − 0.02442 cm month−1 in stem growth rate. Aloe sp. and Senna multiglandulosa achieved the greatest growth rates in terms of height and canopy cover, measuring 0.94737 cm month−1 and 46.8333 cm2 month−1, respectively.
Discussion
Survival estimation
Plant survival is related to rainfall regime (del Campo 2002; Alloza 2003), so its scarcity during the early period following planting is the risk factor that generates the greatest risk for survival (Alloza and Vallejo 1999), particularly in those environments where vegetative activity can begin as early as the winter months. The average survival rate of the three species at the experimental site was below acceptable levels (\(< 49\%\), Rodríguez-Echeverry and Leiton 2020). According to Omary (2011) and Vallejo et al. (2012), plant survival can be affected by rainfall, slope, soil properties such as humidity, temperature, pH, electrical conductivity, nutrients, as well as planting or transplanting techniques (Ortega et al. 2006), water availability optimization processes used during plant establishment, and functional traits of plants developed in water-limited environments (Hernández et al. 2010). In this study, all three species were transplanted on the same soil substrate, so any differences in their survival rates may have been the result of the incidence of various factors which contribute to the establishment stage of plants, such as environmental conditions, plant management, morphology, physiology, and genetics (South 2000; Chen and Klinka 1998). The best example of adaptive power here is aloe, which is a Crassulacean Acid Metabolism (CAM) plant that maintains a series of regulatory mechanisms in its metabolism to adapt to seasonal changes (Honda et al 2000). Under stress conditions, aloe maintains this photosynthetic pathway of CAM plants and switches to the C3 metabolic pathway once conditions improve. Also, aloe has a thick cuticle that makes it more resistant to cold temperatures (Pedroza and Gómez 2006). Sedum dendroideum is also a CAM plant, but it has a thin cuticle which does not allow it to withstand cool season changes, whereas Senna multiglandulosa is a C3 plant, whose photosynthetic efficiency varies depending on the species, the phenological stage, and the aridity of the environment (Rundel et al. 1999).
Senna multiglandulosa and Sedum dendroideum showed survival rates below acceptable levels (\(\sim 17\%<49\%\), Rodríguez-Echeverry and Leiton 2020), while Aloe sp. displayed excellent survival rates (\(\sim 97 \ge\) 90%, Rodríguez-Echeverry and Leiton 2020), indicating adaptability to the site's edaphoclimatic conditions. Senna multiglandulosa and Sedum dendroideum showed the highest mortality during the months of March, April and May, likely due to excessive evapotranspiration and rainfall distribution in the study area, which averages 500 to 600 mm of rainfall per year, 83% of which falls between June and October (INEGI 2009). Margolis and Brand (1990) and Navarro et al. (2006) suggest that reforestation may be successful if plants are able to survive the establishment phase, which usually lasts less than two years, however the first year is crucial, as the plants begin to absorb water and nutrients from the soil once they come into contact with it. Therefore, factors that affect the plant's water status at the time of planting are critical to its survival (Burdett 1990; Heiskanen and Rikala 2000).
Estimation of death risks
Sigala et al. (2015) report that Cox proportional hazards regression can be used to estimate the proportion of species mortality risk based on morphological variables in conjunction with the site-specific edaphoclimatic conditions. Senna multiglandulosa's final height (finhei) is the most important predictor of survival, that is, it negatively affects mortality, which is consistent with Silva et al. (2009) and Stovall et al. (2019), who reported that juvenile trees have smaller canopy covers to allocate more resources to growth in height but are more likely to survive.
Sedum dendroideum's final diameter (findia) was the most significant factor in reducing its risk of death, followed by its canopy cover growth rate (CGR) and initial canopy cover (inidos). The final diameter (findia) reduced mortality by approximately 55%, which is consistent with Fontes (1999), who suggests that some species allocate more resources to developing their diameter as they develop, whereas early in their development, their diameter is greater than that of other species. The growth rate of canopy cover reduced (CGR) the risk of death by approximately 31%, which is in agreement with Moser et al. (2007), who assert that canopy leaf tissue, which catches sunlight and transpires water, plays an important role in establishing and growing new plants, as well as determining their geographic and temporal distribution. For aloe species, height growth rate (HGR) was the most important predictor variable in reducing the risk of death by 93.9%, followed by initial canopy cover (inican) by 2.4%. These findings are consistent with the findings of Valdecantos (2001), who states that height increases are typically positive under medium–high survival rates indicating good climatic conditions.
Estimation of vegetative growth
Navarro et al. (2006) point out that a plantation's success is determined by the plant's ability to grow and develop according to seasonal conditions and the species' capabilities, which are traditionally measured in terms of survival and growth. During the autumn cycle, when the rainy season ended and the cold season began, Senna multiglandulosa achieved a high survival rate and a positive growth rate in height (HGR: 0.87847 cm month−1) and diameter (DGR: 0.046528 cm month−1), however, its canopy cover area (CGR: -22.8056 cm2 month−1) declined. Seedlings of tree and shrub species generally develop narrow canopy covers to maximize their height and, to a lesser extent, diameter growth, and in response to light demands (Martínez-Sánchez 2008). Under subhumid conditions with summer rainfall in Chapultepec forest, Mexico City, Mexico, Gazca and Benavides (2018) also reported positive growth rates in height and diameter for Senna multiglandulosa. Moreover, Rico and Bachman (2006), as well as Terrones et al. (2004), recognize this species as fast-growing.
During this same period, Sedum dendroideum species showed a high survival rate and a positive diameter growth rate (DGR: 0.01888825 cm month−1), but negative height (HGR: − 0.292636 cm month−1) and canopy cover growth rates (CGR: − 7.377292 cm2 month−1). Negative canopy growth rates, which contribute to initial survival, but subsequent growth is positively related to lower canopy cover levels (Yang et al. 2011), may have affected plant diameter and height by decreasing photosynthesis (Moser et al. 2007; Olivas et al. 2013). During this time period, Aloe sp. had a high survival rate, but a negative growth rate in height (HGR: − 0.53082 cm month−1) and canopy area (CGR: − 4.26496 cm2 month−1). Although the planting season for Aloe sp. extends into August, its negative growth rates in height and canopy cover could be a result of early frosts, which are a major problem during this period due to the plant's slow rooting habit, low growth tendencies and susceptible leaves (Pedroza and Gomez 2006).
During the winter period, Senna multiglandulosa again maintained an excellent survival rate, while Sedum dendroideum obtained an acceptable survival rate, but their growth rates were negative for height (− 0.80694 and − 0.832245 cm month−1, respectively), diameter (− 0.00042 and − 0.005067 cm month−1), and canopy cover (− 10.4444 and − 4.357831 cm2 month−1). Gazca and Benavides (2018) also reported negative growth rates for Senna multiglandulosa during the autumn cycle in Chapultepec forest, Mexico City, Mexico, under subhumid conditions and summer rainfall. Negative growth rates in canopy cover could have led to negative growth rates in heights and diameters since plants' establishment and growth depend on leaf tissue that performs photosynthesis (Moser et al. 2007; Olivas et al. 2013). In plants, leaf area is affected by hydrological, biogeochemical, and biophysical processes (Peduzzi et al. 2012), as well as by solar radiation interception, photoassimilate conversion efficiency, use of water and nitrogen, and temperature regulation (de Freitas et al. 2007; Barrios and Cobo 2004). The canopy's negative growth rate may also be attributed to the high levels of stress caused by the distribution of rainfall at the study site, which resulted in, at best, regrowth, while in severe cases, a significant reduction in height and diameter (Burdett 1990; Heiskanen and Rikala 2000).
During the same period, Aloe sp. plants also survived well, but their growth rates were negative in height (− 1.15235 cm month−1) and canopy cover (− 4.88889 cm2 month−1). These results are in agreement with Pedroza and Gómez (2006) who explain that Aloe plants are restricted in their growth and development by frost, mainly at temperatures below 7 °C, causing severe damage at temperatures below 0 °C.
During the seasons of spring and summer, when the rainy season begins, Senna multiglandulosa y Sedum dendroideum obtained survival rates below acceptable levels (\(\sim 17\%\)) at the end of the spring season. Navarro et al. (2006) and Vallejo et al. (2012) note that high mortality of established plants in semiarid areas and degraded dry sites is primarily determined by rainfall amount and spatial distribution, evapotranspiration rates, the length of the growing season, and soil characteristics, including depth and moisture retention capacity. Nevertheless, Senna multiglandulosa showed positive growth rates in terms of height (HGR: 8.94444 cm month−1-spring, and HGR: 2.75 cm month−1-summer), diameter (DGR: 0.143611 cm month−1-spring, and DGR: 0.103333 cm month−1-summer), and canopy cover (CGR: 59.5392 cm2 month−1-spring, and CGR: 46.8333 cm2 month−1-summer). Growth rates for height, stem diameter, and canopy cover might have been positive because the rainy seasons have been linked to evapotranspiration (Navarro et al. 2006) and plant survival (Padilla and Pugnaire 2007), which facilitate a deeper root system (Grantz et al. 1998; Padilla and Pugnaire 2007).
During the spring period, Sedum dendroideum showed positive growth rates for diameter (DGR: 0.053577 cm month−1) and canopy cover (CGR: 0.76403 cm2 month−1), but negative growth rates for height (HGR: − 0.44872 cm month−1). However, during the summer, Sedum dendroideum exhibited positive growth rates in terms of height (HGR: 0.50239 cm month−1) and canopy cover (CGR: 2.82863 cm2 month−1) and slightly negative growth rates in terms of diameter (DGR: − 0.02442 cm month−1). Growth rates in diameter and height may have been positive due to canopy growth, as some species invest more resources in diameter growth during development compared to their beginnings, and as species progress through more advanced stages of development, they become taller (Fontes 1999). During these two seasons, Aloe sp. plants showed positive growth rates in height (HGR: 1.81499 cm month−1-spring, and HGR: 0.94737 cm month−1-summer), as well as in canopy cover (CGR: 3.705972 cm2 month−1-spring, and CGR: 6.730263 cm2 month−1-summer), corroborating what Pedroza and Gómez (2006) said that Aloe sp. plants resume their growth gradually after leaving the frost season with rainfall under 400 mm.
Conclusions
Based on this study, it's possible to determine whether a species can adapt to edaphoclimatic conditions where it's established, taking into account survival, vegetative growth, and death risk, so that agroforestry can be recommended as a land management method on degraded soils that benefits society as a whole as well as rural development. Among the three plant species evaluated, Aloe sp showed the greatest ability to adapt to the edaphoclimatic conditions of the study area. The seasonal conditions of the site affect the growth rates of morphological variables of the species evaluated in varying degrees. Climate conditions during autumn and winter affected the height, stem diameter, and canopy diameter of Senna multiglandulosa and Sedum dendroideum species. Plant height and canopy cover of Aloe sp. species were affected by the winter weather conditions. Each species' morphological variables determined its death risk. For Senna multiglandulosa species, the final height variable reduced the death risk while for Sedum dendroideum species, the final diameter, initial canopy cover, and growth rate in canopy cover reduced the death risk. Variable height growth rate and initial canopy cover in Aloe species reduced death risk.
Data availability
The analyzed datasets are available from the corresponding author on reasonable request.
Code availability
Not applicable.
References
Aguilos R, Marquez C, Adornado H, Aguilos M (2020) Domesticating commercially important native tree species in the Philippines: early growth performance level. Forests 11(8):885. https://doi.org/10.3390/f11080885
Allison PD (1995) Survival analysis using the SAS system: a practical guide. SAS Institute, Cary, NC, USA, p 292
Alloza JA (2003) Análisis de repoblaciones forestales en la Comunidad Valenciana. Tesis Doctoral, Universidad Politécnica de Valencia, Valencia, España, Desarrollo de criterios y procedimientos de evaluación, p 301
Alloza JA, Vallejo R (1999) Relación entre las características meteorológicas del año de plantación y los resultados de las repoblaciones. Ecología 13:173–187
Barrios E, Cobo JG (2004) Plant growth, biomass production and nutrient accumulation by slash/agroforestry systems in tropical hillsides of Colombia. Agroforestry System 40(3):255–265
Bernaola-Paucar RM, Pimienta-Barrios E, Gutiérrez-González P, Ordaz-Chaparro VM, Alejo-Santiago G, Salcedo-Pérez E (2015) Efecto del volumen del contenedor en la calidad y supervivencia de Pinus hartwegii Lindl. en sistema doble transplante. Revista Mexicana de Ciencias Forestales 6(28):174–187
Bradburn MJ, Clark TG, Love SB, Altman DG (2003) Survival analysis part II: multivariate data analysis—an introduction to concepts and methods. Br J Cancer 89:431–436. https://doi.org/10.1038/sj.bjc.6601119
Buendía-Espinoza JC, Martínez-Ochoa EdC, Díaz-Aguilar I, Cahuich-Damián JE, Zamora-Elizalde MC (2022) Identifying potential planting sites for three non-native plants to be used for soil rehabilitation in the tula watershed. Forests 13(2):270. https://doi.org/10.3390/f13020270
Burdett AN (1990) Physiological processes in plantation establishment and the development of specifications for forest planting stock. Can J For Res 20:415–427
Ceccon E (2013) Restauración en bosques tropicales: fundamentos ecológicos, prácticos y sociales, https://www.fisica.unam.mx/personales/mir/el/2013_libroRestauracion.pdf
Challenger A, Dirzo R, López JC, Mendoza E, Lira-Noriega A (2009) Factores de cambio y estado de la biodiversidad. In: Sarukhán J (eds) Capital natural de México. Vol. II: Estado de conservación y tendencias de cambio México D.F., Conabio, pp 37–73
Chazdon RL (2008) Beyond deforestation: restoring forests and ecosystem services on degraded lands. Science 320:1458–1460
Chen HYH, Klinka K (1998) Survival, growth, and allometry of planted Larix occidentalis seedlings in relation to light availability. For Ecol Manage 106:169–179
Cotler H, Sotelo E, Domínguez J, Zorrilla M, Cortina S, Quiñones L (2007) La conservación de suelos: Un asunto de interés público. Gaceta Ecológica 83:5–71
Cox DR (1972) Regression models and life-tables. J Roy Stat Soc 34:187–220
Crawley MJ (2007) The R book. Wiley, Hoboken
Davis AS, Jacobs DF (2005) Quantifying root system quality of nursery seedlings and relationship to outplanting performance. New Forest 30:295–311. https://doi.org/10.1007/s11056-005-7480-y
de Freitas CSR, de Moraes GJL, de Miranda MSL, Moreira MR, da Vinícius SE, Jean-Paul L (2007) Crescimento, nutrição et fixação biológica de nitroggenio em plantios mistos de eucalipto e leguminosas arbóreas. Pesq Agrop Brasileira 42(6):759–768
del Campo AD (2002) Régimen de cultivo, desarrollo en vivero, calidad de planta y respuesta al establecimiento en cuatro especies de frondosas mediterráneas. Tesis Doctoral, Universidad de Córdoba, España, p 310
Domínguez C, Bojórquez L, Boege K, Fornoni J, Gómez P, Valiente A, Orozco A (2009) Sinergias entre el cambio climático y las especies exóticas invasoras. Informe Final, Instituto de Ecología, UNAM-Semarnat, D.F., México
Duarte N, Cuesta F, Arcos I (2018) Selección y establecimiento de estrategias y prácticas de restauración. In: Proaño R, Duarte N, Cuesta F (eds) Guía para la restauración de bosques montanos tropicales. CONDESAN, Quito-Ecuador
Instituto de Ecología [INECOL] (1997) Flora del bajío y de regiones adyacentes. Resúmenes floba, Fascículo (51), pp 62–66, 85. http://www1.inecol.edu.mx/publicaciones/resumeness/FLOBA/Flora%2051.pdf
Fontes LMA (1999) Padroes alométricos em especies arbóreas pioneiras tropicais. Scientia Forestalis 1(55):79–87
Gallardo AE, Molina-Delgado M, Cordero CR (2016) Aplicación del análisis de sobrevivencia al estudio del tiempo requerido para graduarse en educación superior: El caso de la Universidad de Costa Rica. Páginas De Educación 9(1):61–87
García E-Comisión Nacional para el Conocimiento y Uso de la Biodiversidad [CONABIO] (1998) Climas, escala 1:1000000. http://geoportal.conabio.gob.mx/metadatos/doc/html/clima1mgw.html. Accessed 25 May 2021
García E (1964) Modificaciones al Sistema de Clasificación de Köppen (para Adaptarlo a las Condiciones de la República Mexicana). Instituto de Geografía. UNAM, Mexico City
Gazca GMO, Benavides MHM (2018) Ensayo de leguminosas para la reforestación de la 2a sección del bosque de Chapultepec. Revista Mexicana De Ciencias Forestales 3:39–54. https://doi.org/10.29298/rmcf.v3i14.473
Grantz D, Vaughn D, Farber RJ, Kim B, Vancurent T, Campbell R, Bainbridge D, Zink T (1998) Transplanting native plants to revegetate abandoned farmland in the western Mojave Desert. J Environ Qual 27:960–967. https://doi.org/10.2134/jeq1998.00472425002700040033x
Griscom HP, Ashton PMS, Berlyn GP (2005) Seedling survival and growth of native tree species in pastures: Implications for dry tropical forest rehabilitation in central Panama. For Ecol Manage 218:306–318. https://doi.org/10.1016/j.foreco.2005.08.026
Gutiérrez MA (2009) Siempreviva. http://jardindelasalud.blogspot.com/2009/02/siempreviva-sedum-praealtum-dc.html. Accessed 15 April 2021
Heiskanen J, Rikala R (2000) Effects of peat-based container media on establishment of scots pine, Norway spruce and silver birch seedlings after transplanting in contrasting water conditions. Scand J For Res 15:49–57
Hernández EI, Vilagrosa A, Bellot J, Pausas JG (2010) Morphological traits and water use strategies in seedlings of Mediterranean coexisting species. Plant Ecol 207:233–244
Honda H, Akagi H, Shimada H (2000) An isozyme of the NADP-malic enzyme of a CAM plant, Aloe arborescens, with variation on conservative amino acid residues. Genetics 243:85–92. https://doi.org/10.1016/S0378-1119(99)00556-9
Jacobs DF, Salifu KF, Davis AS (2009) Drought susceptibility and recovery of transplanted Quercus rubra seedlings in relation to root system morphology. Ann for Sci 66:1–12
Kaplan EL, Meier P (1958) Nonparametric estimation from incomplete observations. J Am Stat Assoc 53(282):457–481
Kumar A, Zechariah J, Ramchandra SAJ, Tripathi P (2022) Study on Aloe vera leaves farming in Rajasthan. Pharma Innov J 11(5S):289–291
Landis TD, Dumroese RK, Haase DL (2010) The container tree nursery manual, Volume seven: Seedling processing, storage and outplanting. Agricultura Handbook 674. USDA Forest Service, Washington, DC
López R (2019) Azoteas verdes sistemas que refrescan las grandes urbes. Gaceta UNAM, Issue 5086, pp 1–39. https://www.gaceta.unam.mx/azoteas-verdes-sistemas-que-refrescan-las-grandes-urbes/
López-Barrera F (2015) Restauración de bosques y selvas. Lección 2.1. Diplomado en línea: Restauración de ecosistemas y servicios ambientales. Edición 2015. Fundación Internacional para la Restauración de Ecosistemas, Madrid, España; Instituto de Ecología A.C., Veracruz, México y El Colegio de la Frontera Sur, Campeche, México.
Margolis HA, Brand DG (1990) An ecophysiological basis for understanding plantation establishment. Can J for Res 20:375–390
Martínez-Sánchez J (2008) Allometric variation of shade—tolerant tree species in a Mexican tropical rain forest. Revista De Biología Neotrop 5(1):41–51. https://doi.org/10.5216/rbn.v5i1.5626
Mata-Balderas JM, Treviño-Garza E, Jiménez-Pérez J, Aguirre-Calderón OA, Alanís-Rodríguez E, Foroughbakhch-Pournavab R (2014) Prácticas de rehabilitación en un ecosistema semiárido, afectado por el establecimiento de un banco de material, en el noreste de México. CienciaUAT 8:32–43
Moser G, Hertel D, Leuschner C (2007) Altitudinal change in LAI and stand leaf biomass in tropical Montane forests: a transect study in Ecuador and a pan-tropical meta-analysis. Ecosystems 10:924–993. https://doi.org/10.1007/s10021-007-9063-6
Instituto Nacional de Estadística y Geografía [INEGI] (2009) Prontuario de Información Geográfica Municipal de Los Estados Unidos Mexicanos. Nopaltepec, Mexico. Clave Geoestadística 15061. http://www3.inegi.org.mx/contenidos/app/mexicocifras/datos_geograficos/15/15061.pdf. Accessed 15 Dec 2021
Instituto Nacional de Estadística y Geografía [INEGI] (2017) Conjunto de Datos Vectoriales de Uso de Suelo y Vegetación. Escala 1:250000. Serie VI. http://www.conabio.gob.mx/informacion/gis/. Accessed 22 May 2020.
Instituto Nacional de Investigaciones Forestales y Agrícolas y Pecuarias [INIFAP], Comisión Nacional para el Conocimiento y Uso de la Biodiversidad [CONABIO] (1995) Edafología, Escalas 1:250000–1:1000000. http://geoportal.conabio.gob.mx/descargas/mapas/imagen/96/eda251mgw. Accessed 25 May 2020
Navarro RM, del Campo AD, Cortina J (2006) Factores que afectan al éxito de una repoblación y su relación con la calidad de la planta. In: Cortina J, Peñuelas J, Puertolas J, Savé R, Vilagrosa A (coords.) Calidad de planta forestal para la restauración en ambientes mediterráneos: estado actual de conocimientos. Organimo Autómo Parques Nacionales, Ministerio de Medio Ambiente, Madrid, España, pp 31–46
Olivas PC, Oberbauer SF, Clark DB, Clark DA, Ryan MG, O’Brien JJ, Ordoñez H (2013) Comparison of direct and indirect methods for assessing leaf area index across a tropical rain forest landscape. Agric for Meteorol 177:110–116
Omary AA (2011) Effects of aspect and slope position on growth and nutritional status of planted Aleppo pine (Pinus halepensis Mill.) in a degraded land semiarid area of Jordan. New for 42:285–300
Ortega U, Majada J, Mena PA, Sánchez ZJ, Rodríguez IN, Txarterina K, Azpitarte J, Duñabeitia M (2006) Field performance of Pinus radiata D. Don produced in nursery with different types of containers. New for 31:97–112
Padilla FM, Pugnaire FI (2007) Rooting depth and soil moisture control Mediterranean woody seedling survival during drought. Funct Ecol 21:489–495. https://doi.org/10.1111/j.1365-2435.2007.01267.x
Palacios-Romero A, Rodríguez-Laguna R, Razo-Zarate R, Meza-Rangel J, Prieto-García F, de la Hernández-Flores M, L (2017) Espuma fenólica de célula abierta hidratada como medio para mitigar estrés hídrico en plántulas de Pinus leiophylla. Madera y Bosques 23(2):43–52
Pedroza SA, Gómez LF (2006) La sábila (Aloe spp.) Propiedades, manejo agronómico, proceso agroindustrial y de mercado. Universidad Autónoma Chapingo, México
Peduzzi A, Wynne RH, Fox TR, Nelson RF, Thomas VA (2012) Estimating leaf area index in intensively managed pine plantations using airborne laser scanner data. For Ecol Manage 270:54–65
Pérez-Calix E (2008) Crassulaceae, Fasículo 156. Flora del bajío y de regiones adyacentes. Instituto de Ecología A.C., Centro Regional del Bajío Pátzcuaro, Michoacán, México, pp 1–143
Peri PL, Mártínez PG, Chauchard L, Schlichter T (2021) Uso sostenible del bosque: aportes desde la silvicultura Argentina. https://repositorio.inta.gob.ar/xmlui/bitstream/handle/20.500.12123/10384/INTA_CRPatagoniaSur_EEASantaCruz_PERI_PL_Capitulo_1_Uso_sostenible_del_bosque.pdf?sequence=1&isAllowed=y
Prieto RJA, García RJL, Sigala RJA, Mejía BJM, Rueda SA (2011) Indicadores de calidad de planta en viveros forestales del estado de Durango. Capítulo IV. Indicadores de calidad de planta en viveros forestales de la sierra Madre Occidental. Campo experimental Valle del Guadiana-INIFAP. Durango, Durango. México Libro Técnico Núm 3:57–86
Rico AML, Bachman S (2006) A taxonomic revision of Acaciella (Leguminosae, Mimosoideae). Anales Del Jardín Botánico De Madrid 63(2):189–244
Rivas-Ruiz R, Pérez-Rodríguez M, Palacios L, Talavera JO (2014) Investigación clínica XXI Del juicio clínico al análisis de supervivencia. Rev Med Inst Mex Seguro Soc 52(3):308–315
Rodríguez-Echeverry J, Leiton M (2020) Estrategias de restauración para el páramo de frailejones perturbado por incendios en el norte de Ecuador. Ecosistemas 29(3):2018. https://doi.org/10.7818/ECOS.2018
Rundel PW, Esler KJ, Cowling RM (1999) Ecological and phylogenetic patterns of carbon isotope discrimination in the winter-rainfall flora of the Richtersveld, South Africa. Plant Ecol 142:133–148. https://doi.org/10.1023/A:1009878429455
Sarukhán J, Urquiza-Haas T, Koleff P, Carabias J, Dirzo R, Ezcurra E, Cerdeira-Estrada S, Soberón J (2015) Strategic actions to value, conserve, and restore the natural capital of megadiversity countries: the case of Mexico. Bioscience 65:164–173
Sigala RJA, González TMA, Prieto RJA (2015) Survival of Pinus pseudostrobus Lindl. plantations in terms of theproduction system and pre-conditioning in the nursery. Revista Mexicana De Ciencias Forestales 6:20–31
Silva CS, Cerezini TM, Santos FC, Barbosa J, Mendoca HA (2009) Modelos alométricos como preditores dasestratégias de alocagao de recursos em árvores emergentes e de subdossel. Ecología da mata atlántica. Curso de Pós-Graduagao em Ecología-Universidade de Sao Paulo 1–3. http://ecologia.ib.usp.br/curso/2009/pdf/PO3/PO3_massaranduba.pdf
South DB (2000) Planting morphologically improvedpine seedlings to increase survival and growth. Forestry and Wildlife Research Series No. 1. Alabama Agricultural Experiment Station. Auburn University, Alabama
Stovall AEL, Shugart H, Yang X (2019) Tree height explains mortality risk during an intense drought. Nat Commun 10:4385. https://doi.org/10.1038/s41467-019-12380-6
Terrones RTR, González SC, Ríos RSA (2004) Arbustivas nativas de uso múltiple en Guanajuato. Libro Técnico No. 2. Campo Experimental Bajío, Centro de Investigación Regional del Centro, Instituto Nacional de Investigaciones Forestales, Agrícolas y Pecuarias, Celaya, Guanajuato, México
Toogood A (2007) Royal Horticultural Society Enciclopedia de la propagación de plantas. Primera edición en rústica ed. Barcelona: Blume, pp 231–251.
Tsakaldimi M, Ganastsas P, Jacobs DF (2013) Prediction of planted seedling survival of five Mediterranean species base on initial seedling morphology. New For 44:327–339
Valdecantos DA (2001) Aplicación de fertilizantes orgánicos en la repoblación de zonas forestales degradadas de la Comunidad Valenciana. Tesis Doctoral, Facultad de Ciencias, Departamento de Ecología, Universidad de Alicante, Alicante, España
Vallejo VR, Smanis A, Chirino E, Fuentes D, Valdecantos A, Vilagrosa A (2012) Perspectives in dryland restoration: approaches for climate change adaptation. New For 43:561–579. https://doi.org/10.1007/s11056-012-9325-9
Williams CS (2008) Surviving Survival Analysis. An Applied Introduction. In: Proceedings of the South East SAS Users Group. St Pete Beach, FL, USA. SESUG. http://analytics.ncsu.edu/sesug/2008/ST-147.pdf
Yang W, Liu F, Zhou L, Zhang S, An S (2011) Trade-offs between growth and survival of non-pioneer light-demanding tree seedlings in tropical forest of Hainan Island, China. J Trop Ecol 27:611–620
Zamora EMC, Buendía EJC, Martínez HPA, García NRM (2020) Diagnóstico del uso del suelo y vegetación en la microcuenca Tula, México. Revista Mexicana De Ciencias Agrícolas 11:57–68. https://doi.org/10.29312/remexca.v11i1.2213
Acknowledgements
Researchers would like to thank the community of San Felipe Teotitlán for their support during this study.
Funding
Funding for this research was provided by the Universidad Autónoma Chapingo and the Mexican National Council for Science and Technology (CONACyT).
Author information
Authors and Affiliations
Contributions
Conceptualization: OLFT, JCBE methodology: JCBE, MCZE investigation and resources: OLFT, JCBE, MCZE software: OLFT, JCBE formal analysis: OLFT, JCBE, MCZE validation: OLFT, JCBE, MCZE writing—original draft preparation: JCBE, data curation: OLFT, JCBE writing—review and editing: OLFT, JCBE, MCZE visualization, supervision and project administration: JCBE, MCZE.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
All the authors whose names appeared on the submission approved the version to be published and agreed to be accountable for all aspects of the work in ensuring that the questions related to the accuracy of integrity of any part of the work were appropriately investigated and resolved.
Additional information
Communicated by Agustin Merino.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Flores-Trejo, O.L., Buendía-Espinoza, J.C. & Zamora-Elizalde, M.C. Evaluation of the adaptive potential of three non-native multipurpose species for soil rehabilitation. Eur J Forest Res 142, 997–1010 (2023). https://doi.org/10.1007/s10342-023-01570-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10342-023-01570-z