Abstract
Ungulate herbivory can alter functional plant communities of early-successional forest ecosystems. The consequences of such vegetation changes on soil carbon cycling are still not fully understood. Here, we used an ungulate exclusion experiment to investigate how different levels of herbivory and associated changes in vegetation succession modulate soil CO2 efflux and its heterotrophic and autotrophic sources following windthrow in temperate mountain forests. Our results indicate that only high levels of ungulate herbivory and associated vegetation shifts from tree to rather grass dominated plant communities affect soil CO2 fluxes. We did not find evidence that a moderate herbivory level and accompanied smaller shifts in the functional plant community affect soil CO2 fluxes. A greater soil CO2 efflux under the influence of high herbivory pressure was primarily attributed to accelerated heterotrophic respiration, likely due to warmer soil conditions. Moreover, autotrophic respiration from grass roots and associated microbial communities is suggested to contribute to higher soil CO2 fluxes. We conclude that intense herbivory and accompanied successional changes in the functional plant community enhance soil carbon losses following forest windthrow. This might have negative consequences for the soil carbon stocks and for the climate system.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Temperate forests store large amounts of carbon (C) and act thereby as important sink for atmospheric CO2 (Goodale et al. 2002; Luyssaert et al. 2010). Natural disturbances, such as from windthrows or bark beetle attacks, can turn forest net CO2 sinks into distinct net CO2 sources due to reduced photosynthetic uptake and continuing respiratory losses (Amiro et al. 2010; Matthews et al. 2017; Yamanoi et al. 2015). The magnitude of net CO2 losses after disturbance depends largely on vegetation regrowth. With tree regeneration, it takes roughly one to two decades until disturbed forests become CO2 neutral again (Amiro et al. 2010; Matthews et al. 2017; Yamanoi et al. 2015). Across many European forests, tree regeneration is, however, delayed or completely inhibited following disturbance, often as a result of ungulate herbivory (Ammer 1996; Pröll et al. 2014; Ramirez et al. 2019; Thrippleton et al. 2018). In Austria, for example, about 60% of the regenerating forests are heavily affected by browsing from deer and chamois (Schodterer 2016). Moreover, herbaceous species such as Calamagrostis sp. can represent strong competitors for seedlings and saplings and once a dense ground vegetation layer has established, sites can remain in herbaceous dominated, non-forest states for several decades post-disturbance (Kupferschmid and Bugmann 2005; Pröll et al. 2014; Rebele and Lehmann 2001; Thrippleton et al. 2018). This raises the question of whether ungulate herbivory and accompanied shifts in the functional plant community affect C cycle dynamics of early-successional forests, with potential consequences for the ecosystem C balance and the CO2 exchange with the climate system.
Soil CO2 efflux (Fs; = soil respiration) represents the largest respiratory flux across forest ecosystems (~ 60% of ecosystem respiration), and its relative contribution to the forest C balance could be shown to increase following disturbance (Janssens et al. 2001; Paul-Limoges et al. 2015). Soil CO2 efflux is strongly mediated by vegetation type and plant community characteristics (Bond-Lamberty et al. 2004b; Metcalfe et al. 2011; Raich and Tufekciogul 2000). Plants drive Fs principally via effects on belowground allocation of photosynthetically fixed C, litter quality and quantity, and microclimatic conditions (e.g. temperature and moisture) (Metcalfe et al. 2011). Moreover, plant groups can be associated with distinct microbial communities (e.g. mycorrhizal symbionts) (Brundrett 2009; Van Der Heijden et al. 2015), thereby affecting Fs differently. Successional shifts in the functional plant community composition due to herbivory are therefore assumed to affect both, the autotrophic (i.e. CO2 from roots and associated micro-organisms) and heterotrophic (i.e. CO2 from decomposition of soil organic matter) source of Fs. Particularly, the response of heterotrophic respiration to herbivory-affected succession might be a key factor influencing both, the net ecosystem–atmosphere CO2 exchange and the amount of C stored in forest soils.
Several studies have investigated forest disturbance effects on Fs and its sources (Kobler et al. 2015; Kulmala et al. 2014; Mayer et al. 2014, 2017b; Zehetgruber et al. 2017) and herbivory effects on a variety of soil characteristics and processes, including soil C storage, litter decomposition, and Fs (Andriuzzi and Wall 2017; Bardgett and Wardle 2003; Ellis and Leroux 2017; Prietzel and Ammer 2008; Tanentzap and Coomes 2012). Yet, it remains largely unknown how ungulate herbivory and accompanied shifts in successional plant community composition modulate in situ soil CO2 fluxes after disturbance in temperate forests. Here, we evaluate how different levels of herbivory by deer and chamois affect the heterotrophic and autotrophic sources of Fs at forest windthrow sites in the Austrian Alps using ungulate exclusion (fence) and root exclusion (trenching) treatments. We hypothesized that Fs would increase with an increasing influence of herbivores and an accompanied increase in grass cover (Fig. 1a). An increase in Fs was thereby primarily attributed to accelerated heterotrophic respiration rates due to more favourable microclimatic conditions (e.g. warmer) under herbs/grasses (Mayer et al. 2014, 2017a). Additionally, higher autotrophic respiration rates from a dense herbaceous vegetation layer (Mayer et al. 2014, 2017b; Zehetgruber et al. 2017) were assumed to contribute to higher Fs under the influence of herbivores (Fig. 1a).
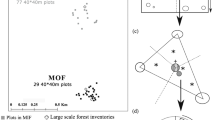
Hypothesized effect of ungulate herbivory and succession on soil CO2 efflux and its heterotrophic and autotrophic respiratory sources (a). Control and fence treatments at windthrow sites in Höllengebirge and Reutte, respectively; a stronger herbivory pressure and accompanied stronger vegetation changes are apparent at Reutte (b)
Materials and methods
Study sites
The study took place in the Höllengebirge mountain range (henceforth ‘Höllengebirge’; 47° 47′ 19″ N, 13° 38° 21″ E) and in the Tannheimer mountain area near the town of Reutte (henceforth ‘Reutte’; 47° 27′ 27″ N, 10° 40′ 12″ E), located in the Austrian Calcareous Alps. Höllengebirge is a south-west exposed site at an altitude of 1000 m a.s.l, and Reutte is a south-east exposed site at an altitude of 950 m a.s.l. Average air temperature and precipitation in 2016 were 7.1 °C and 1903 mm at the Höllengebirge site and 8.6 °C and 1351 mm at the Reutte site (data provided by Zentralanstalt für Meteorologie und Geodynamik—ZAMG). The Höllengebirge and Reutte sites were similar to one another regarding bedrock which was mainly limestone in paragenesis with dolomite. Chromic Cambisols, Rendzic Leptosols, and Folic Histosols (IUSS Working Group WRB 2006) were the dominant soil types and Moder and Tangel (Zanella et al. 2019) the main humus forms. Mean soil properties of the sites are given in Table 1.
The natural woodland community at both sites is dominated by Picea abies, Abies alba, and Fagus sylvatica (Kilian et al. 1994). Both sites were affected by a storm event during which several hectares of the forest stand were either blown over or destroyed by wind snap. The Höllengebirge and Reutte site was disturbed in 2007 and 2003, respectively. At both sites, the timber (mainly the stem fraction) was removed after the disturbance event. The Höllengebirge windthrow site (henceforth ‘Höllengebirge control’) was dominated by herbaceous ground vegetation (Calamagrostis sp., Carex alba, and Adenostyles glabra); a mixture of naturally regenerating and planted trees (Picea abies and Larix decidua) covered roughly a quarter to a third of the site. The Reutte windthrow site (henceforth ‘Reutte control’) was dominated by a dense layer of Calamagrostis sp. and other grass species. Based on the tree regeneration density/cover at the control plots, the herbivory effects were classified as ‘moderate herbivory level’ at the Höllengebirge site and ‘high herbivory level’ at the Reutte site (Fig. 1b).
A fence (height 2 m) covering an area of ~ 0.7 ha was installed at each site. Year of installation was 2008 and 2010 at Reutte and Höllengebirge, respectively. This ungulate exclusion treatment allowed for studying the effect of herbivory (particularly browsing) on tree regeneration and successional plant communities after windthrow. In 2016, vegetation inside the fence was dominated by a mixture of naturally regenerating and planted trees (Picea abies, Larix decidua, Abies alba, Acer pseudoplatanus, Sorbus aucuparia, Salix sp., Fagus sylvatica, and Pinus sylvestris) which covered most of the surface (Fig. 1b). Fence treatments at Höllengebirge and Reutte are henceforth denoted as ‘Höllengebirge fence’ and ‘Reutte fence’, respectively.
Normalized difference vegetation index (NDVI) values were 0.15 ± 0.07, 0.29 ± 0.05, 0.19 ± 0.05, and 0.32 ± 0.04 at Höllengebirge control, Höllengebirge fence, Reutte control, and Reutte fence, respectively.
Field measurements
Prior to Fs measurements, plastic collars (4 cm height, 10 cm diameter, 3 cm inserted into soil) were installed across Höllengebirge control (n = 23), Höllengebirge fence (n = 16), Reutte control (n = 16), and Reutte fence (n = 16) treatments; locations were distributed randomly. Root-exclusion plots (Subke et al. 2006) were established at each site by digging trenches down to either bedrock or a maximum depth of 80 cm, each encompassing an area of 1 × 1 m. All roots within the trenches were cut, and a plastic sheet was installed to inhibit root and mycorrhizal in-growth. For Fs measurements, one plastic collar (see above) was installed in the centre of each root-exclusion plot. In total, 14 and 10 root-exclusion plots were installed across the Höllengebirge and Reutte site, respectively (distributed equally between control and fence treatments). Ground vegetation within root-exclusion plots and collars was removed prior to measurements, and regrowth was cut regularly (Bahn et al. 2008).
During the vegetation period of 2016, Fs was measured in monthly intervals by means of the closed chamber technique, using a portable infrared gas analyser (EGM-4; PP Systems International Inc., Amesbury, MA, USA). Measurements were taken by connecting a respiration chamber (SRC-1; PP Systems International Inc.) to the plastic collars. Soil temperature at 5 cm depth (handheld thermometer) and soil moisture between 0 and 7 cm depth (Field Scout TDR Soil Moisture Meter; Spectrum Technologies Inc., Aurora, IL, USA) were measured next to the collars at the same time. To avoid a temporal sampling bias, sequential arrangement of treatments and plots was alternated between measurements. At each site, soil temperature and moisture were also monitored continuously (1 h interval) by means of five GS3 sensors (installation depth 5 cm) and EM50 data loggers (Decagon Devices, USA). To get spatially representative hourly temperature and moisture data for each site and treatment, continuous measurements were corrected by linear regression to the manually gathered soil temperature and moisture measurements during the corresponding time (Wangdi et al. 2017).
At Reutte, tree and grass cover was estimated for each plot using a 1 × 1 m frame; collars represented the centre.
Statistical analysis
The effect of herbivory exclusion on Fs, soil temperature, and soil moisture were tested by means of analysis of variance with a mixed-effects model structure at each site (Pinheiro et al. 2014). To account for the repeated measurement structure within the data, the plots were assigned random effects and the treatments were assigned fixed effects in each model. Linear mixed-effects models were used to predict Fs (µmol CO2 m− 2 sec− 1) as a function of soil temperature (°C) and moisture (vol%) (Reichstein and Beer 2008):
Due to an exponential relationship between Fs and soil temperature, data were log-transformed prior to analysis. Fixed effects model coefficients β0 − β2 were estimated from the data and are given in Table 2. The terms Fsij, Tij, Mij are the measured soil CO2 efflux, soil temperature, and soil moisture, respectively (for observation j at plot i). Plots were assigned random effects where terms ai and bi depict random intercepts and slope coefficients for the response to soil temperature, respectively. The term εij is the residual error. Including a random slope to describe a plot-wise response of Fs to soil moisture and an interaction of temperature and moisture did not improve the model fit. Marginal and conditional R2 served as indicators for model goodness of fit (Nakagawa and Schielzeth 2013). Additionally, the relationship between Fs and temperature only was tested (Table S1).
The linear mixed-effects model (Table 2) and the average continuous soil climate data of the control and fence treatments were used to predict log(Fs) for each plot (also for root-exclusion plots) and each day between 7 June until 7 November. Soil CO2 efflux from root-exclusion plots was assumed to be from heterotrophic respiration only. Autotrophic respiration rates were calculated by subtracting the predicted daily mean heterotrophic respiration rates from log(Fs) predictions of non-root exclusion plots. The log(Fs) predictions were transformed to Fs by taking the exponent.
Structural equation modelling (Beaujean 2014; Grace 2006) was used to explore the effects of grass and tree cover on the sums of Fs. This analysis was conducted for Reutte only, as no vegetation data were available for Höllengebirge plots. An initial a priori model included pathways between Fs, tree and grass cover and a correlation between tree and grass cover. Two alternative final models were obtained by removing pathways in a stepwise procedure. Only significant pathways were kept in the final models. Model selection was based on Akaike’s information criterion (AIC), chi-squared test results, and comparative fit index (CFI) (Beaujean 2014; Grace 2006).
Statistical analyses and plotting were done in R (R Core Team 2014) using packages ‘nlme’ (Pinheiro et al. 2014), ‘MuMIn’ (Barton 2018), and ‘lavaan’ (Rosseel 2012). The level of significance for the statistical analyses was a p value < 0.05.
Results and discussion
In this study, we investigated how ungulate herbivory and associated differences in successional plant communities affect soil CO2 efflux (Fs) and its heterotrophic and autotrophic sources at two windthrow sites in the Austrian Alps. Herbivory effects were assessed by means of fence treatments, and the sources of Fs were separated by means of root exclusion treatments. Fenced treatments were characterized by a dense tree regeneration. Herbivory promoted the growth of herbal and grass species. A moderate herbivory level at Höllengebirge control plots resulted in a mixture of regenerating trees and herbaceous plants while a high herbivory level at Reutte control plots resulted in a dense layer of Calamagrostis grasses and other graminoids, respectively (Fig. 1b). This successional pattern is in line with Tremblay et al. (2006), who showed that the abundance of browse-tolerant species, such as grasses, was positively related to deer density in harvested forest stands.
Soil CO2 efflux was significantly higher at herbivory-affected control plots at Reutte when compared to fence plots, while no fence effect was present at Höllengebirge (Fig. 2a, b). Average sums of Fs of 457 ± 29, 449 ± 36, 860 ± 67, and 594 ± 40 g C m−2 (mean ± SE for a 5 months’ period) were calculated for Höllengebirge control, Höllengebirge fence, Reutte control, and Reutte fence, respectively (Fig. 3). The control plots at Reutte respired on average 45% more CO2 than the fence plots. Our hypothesis, Fs would increase with increasing herbivory pressure, was therefore only supported in the case of a high herbivory level and accompanied large plant community differences inside and outside the fence (i.e. tree versus grass dominated plots). Conversely, it appears that a moderate herbivory level and smaller plant community differences inside and outside the fence (i.e. tree versus tree/grass/herb dominated plots) did not affect Fs rates. Generally, these findings are partly in line with the results of a recent meta-analysis by Andriuzzi and Wall (2017), who reported an overall positive response of Fs to herbivory across temperate ecosystems. However, their meta-analysis was biased towards temperate grasslands and herbivory by sheep and included also laboratory measurements of heterotrophic respiration. Support that Fs would increase with decreasing tree and increasing grass abundance is also given by structural equation modelling. The best model reveals a negative relation between Fs and tree cover (Fig. 4a). A clear negative correlation between grass and tree cover masked a positive relation between Fs and grass cover. This relationship was significant after removing the path between tree cover and Fs from the model (alternative model, Fig. 4b).
Soil CO2 efflux, heterotrophic respiration (= soil CO2 efflux from root exclusion plots), soil temperature, and soil moisture at windthrow sites in Höllengebirge and Reutte (mean ± SE). Measurements were conducted in control and fence treatments. Given are p-values of ANOVA with mixed effects model structure
Modelled, cumulative soil CO2 efflux sums (7 June until 7 November 2016) from control and fence treatments at Höllengebirge and Reutte. Sums are separated into heterotrophic and autotrophic respiration. Autotrophic respiration was calculated as the difference between soil CO2 efflux and heterotrophic respiration. Error bars represent standard error of the mean
Structural equation models describing the influence of tree (a) and grass (b) cover (%) on soil CO2 efflux. Single- and double-headed arrows represent significant relationships and correlations between variables, respectively. Values represent model coefficients and their standard errors (in brackets). Comparative fit index (CFI) is a measure for the goodness of the model fit
Heterotrophic respiration represented the dominant CO2 flux at the studied sites, with an average contribution to Fs ranging between 56 and 77% (Fig. 3). These values are in good agreement with previous findings from a fire-chronosequence study in boreal forests (Bond-Lamberty et al. 2004a) and from studies on clear-cut sites in temperate forests (Williams et al. 2014; Zehetgruber et al. 2017). Similar to Fs, heterotrophic respiration was only affected by high herbivory pressure at Reutte (Fig. 2c, d). There, herbivory resulted in 33% higher CO2 fluxes from heterotrophic respiration as suggested by our modelling results (Fig. 3). We therefore argue that greater Fs rates under high herbivory levels can be primarily explained by enhanced heterotrophic respiration from the decomposition of soil organic matter.
Soil CO2 efflux and heterotrophic respiration showed distinct seasonal variations in all treatments, strongly following the patterns in soil climate (Fig. 2). Soil temperature and moisture explained 34% and 69% of the overall and plot specific variation in Fs (Table 2). However, soil temperature only explained 32% of the overall and 64% of the plot specific variation in Fs (Table S1). As temperature was the major abiotic driver of Fs, the greater heterotrophic respiration rates at Reutte control plots can, to a large degree, be explained by warmer soil conditions (Kulmala et al. 2014; Mayer et al. 2014, 2017a; Zehetgruber et al. 2017); soil temperature was on average 1.9 ± 0.5 °C higher at the grass-dominated control plots when compared to the fence plots (Fig. 2f). In contrast, no differences in soil temperature could be seen at the Höllengebirge site where regenerating trees were present at both, fence and control plots (Fig. 2e). Thus, higher temperatures at Reutte control plots are mainly caused by a lack in crown shading from regenerating trees. These results correspond well to Mayer et al. (2017a), where the absence of regenerating trees increased soil temperature and decomposition processes following forest gap disturbance, while their presence kept temperature and decomposition even close to control stand levels. Additionally, higher heterotrophic respiration rates at Reutte control plots might be related to changes in the litter provided to the microbial community (Bardgett and Wardle 2003). As shown for clear-cut sites, particularly grasses can produce leaf biomass stocks (and thus litter) which exceed those of regenerating trees by far (Tremblay et al. 2006). Herbivores may have also directly affected microbial activity by the deposition of faeces or urine (Bardgett and Wardle 2003).
The higher Fs under the influence of intense herbivory is also likely due to a greater contribution of autotrophic respiration from roots and associated micro-organisms. Estimates for autotrophic respiration suggest ~ 30% higher plant-associated soil CO2 fluxes at Reutte (Fig. 3). Particularly, Calamagrostis species develop large root biomass pools which can be in a similar size of the fine root pools of mature forest stands (Brunner et al. 2013; Rebele and Lehmann 2001). The supply of fresh C from roots to the microbial community might have additionally enhanced the decomposition of older soil organic matter, a process which is known as rhizosphere priming (Gavazov et al. 2018; Kuzyakov 2010). Herbaceous species are, moreover, associated with different root symbionts when compared to trees. While most tree species form symbioses with ectomycorrhizal fungi, grasses and herbs form symbioses primarily with arbuscular mycorrhizal fungi (Brundrett 2009; Van Der Heijden et al. 2015). Respiration from mycorrhizal fungi can make a great contribution to Fs (Heinemeyer et al. 2007; Subke et al. 2011). Differences in mycorrhizal symbionts are therefore likely to additionally modulate autotrophic soil CO2 fluxes under an herbivory associated shift in the plant community composition.
It should be stressed that our results are based on pseudo-replicated measurements (Hurlbert 1984) from only one site per herbivory stage. Thus, we cannot claim for an ecological generalization. The results are representative for an intermediate stage after forest disturbance only (i.e. ~ one decade after windthrow). Moreover, the pre-fence conditions are unknown and the sites differed with regard to time since disturbance and soil properties (e.g. organic layer thickness). A thicker organic layer, for example, may be a cause for the generally higher Fs rates at Reutte (Fig. 3). It is also likely that the Reutte site is in a different phase of soil organic matter decomposition due to a later successional stage after windthrow. A conclusion regarding temporal changes of post-disturbance development of Fs is therefore not possible. A conclusion regarding herbivory levels, related plant-community changes, and how this affects soil CO2 fluxes is, nonetheless, possible.
Collectively, our results indicate that only high levels of ungulate herbivory and accompanied shifts from tree to rather grass dominated plant communities affect Fs and its heterotrophic and autotrophic sources at the studied windthrow sites. We did not find evidence that a moderate herbivory level and accompanied smaller shifts in the functional plant community affect soil CO2 fluxes. Higher Fs rates under the influence of intense herbivory were primarily attributed to accelerated heterotrophic respiration, likely due to warmer soil conditions. Moreover, autotrophic respiration from grass roots and associated microbial communities might have additionally promoted higher Fs rates. Disturbances, such as windthrows, can cause significant C losses from the forest soil and can turn former forest CO2 sinks into distinct CO2 sources to the atmosphere (Amiro et al. 2010; Thom and Seidl 2015). Greater heterotrophic respiration rates due to intense ungulate herbivory may further enhance net soil C losses following disturbance, with consequences for both the soil C storage and the climate system. On the other hand, higher autotrophic respiration rates from grasses might indicate higher photosynthetic fixation rates and thus greater input rates of fresh C to soil (Litton et al. 2007). This could mitigate post-disturbance net C losses at least to some degree (Zehetgruber et al. 2017). Additionally, grass roots have a higher root turnover than tree roots suggesting higher belowground litter input rates when compared to trees (Solly et al. 2014). Further studies on how ungulate herbivory and associated changes in vegetation affect soil C cycling are, therefore, urgently required to better understand plant–soil–atmosphere interactions following forest disturbance.
References
Amiro BD et al (2010) Ecosystem carbon dioxide fluxes after disturbance in forests of North America. J Geophys Res 115:1–13. https://doi.org/10.1029/2010jg001390
Ammer C (1996) Impact of ungulates on structure and dynamics of natural regeneration of mixed mountain forests in the Bavarian Alps. For Ecol Manage 88:43–53. https://doi.org/10.1016/S0378-1127(96)03808-X
Andriuzzi WS, Wall DH (2017) Responses of belowground communities to large aboveground herbivores: meta-analysis reveals biome-dependent patterns and critical research gaps. Glob Change Biol 23:3857–3868
Bahn M et al (2008) Soil respiration in European grasslands in relation to climate and assimilate supply. Ecosystems 11:1352–1367. https://doi.org/10.1007/s10021-008-9198-0
Bardgett RD, Wardle DA (2003) Herbivore-mediated linkages between aboveground and belowground communities. Ecology 84:2258–2268. https://doi.org/10.1890/02-0274
Barton K (2018) MuMin: multi-model inference
Beaujean AA (2014) Latent variable modeling using R. Routledge, New York
Bond-Lamberty B, Wang C, Gower ST (2004a) Contribution of root respiration to soil surface CO2 flux in a boreal black spruce chronosequence. Tree Physiol 24:1387–1395. https://doi.org/10.1093/treephys/24.12.1387
Bond-Lamberty B, Wang C, Gower ST (2004b) A global relationship between the heterotrophic and autotrophic components of soil respiration? Glob Change Biol 10:1756–1766. https://doi.org/10.1111/j.1365-2486.2004.00816.x
Brundrett MC (2009) Mycorrhizal associations and other means of nutrition of vascular plants: understanding the global diversity of host plants by resolving conflicting information and developing reliable means of diagnosis. Plant Soil 320:37–77. https://doi.org/10.1007/s11104-008-9877-9
Brunner I et al (2013) Fine-root turnover rates of European forests revisited: an analysis of data from sequential coring and ingrowth cores. Plant Soil 362:357–372. https://doi.org/10.1007/s11104-012-1313-5
Darabant A, Dorji S, Gratzer G, Katzensteiner K (2009) Pilotstudie: Resilienz von Schutzwäldern in den Nördlichen Kalkalpen. Forschungsbericht
Ellis NM, Leroux SJ (2017) Moose directly slow plant regeneration but have limited indirect effects on soil stoichiometry and litter decomposition rates in disturbed maritime boreal forests. Funct Ecol 31:790–801
Gavazov K et al (2018) Vascular plant-mediated controls on atmospheric carbon assimilation and peat carbon decomposition under climate change. Glob Change Biol 24:3911–3921. https://doi.org/10.1111/gcb.14140
Goodale CL et al (2002) Forest Carbon Sinks in the Northern Hemisphere. Ecol Appl 12:891–899. https://doi.org/10.1890/1051-0761(2002)012%5b0891:fcsitn%5d2.0.co
Grace J (2006) Structural equation modeling and natural systems. Cambridge University Press, Cambridge
Heinemeyer A, Hartley IP, Evans SP, Carreira De La Fuente JA, Ineson P (2007) Forest soil CO2 flux: uncovering the contribution and environmental responses of ectomycorrhizas. Glob Change Biol 13:1786–1797. https://doi.org/10.1111/j.1365-2486.2007.01383.x
Hurlbert SH (1984) Pseudoreplication and the design of ecological field experiments. Ecol Monogr 54:187–211. https://doi.org/10.2307/1942661
IUSS Working Group WRB (2006) World reference base for soil resources 2006 vol 2nd edition. World Soil Resources Reports No. 103. FAO Rome
Janssens IA et al (2001) Productivity overshadows temperature in determining soil and ecosystem respiration across European forests. Glob Change Biol 7:269–278. https://doi.org/10.1046/j.1365-2486.2001.00412.x
Kilian W, Müller F, Starlinger F (1994) Die forstlichen Wuchsgebiete Österreichs—Eine Naturraumgliederung nach waldökologischen Gesichtspunkten. Forstliche Bundesversuchsanstalt Waldforschungszentrum Seckendorff-Gudent-Weg 8 A-1131 Wien, Vienna
Kobler J, Jandl R, Dirnböck T, Mirtl M, Schindlbacher A (2015) Effects of stand patchiness due to windthrow and bark beetle abatement measures on soil CO2 efflux and net ecosystem productivity of a managed temperate mountain forest. Eur J Forest Res 134:683–692. https://doi.org/10.1007/s10342-015-0882-2
Kulmala L et al (2014) Changes in biogeochemistry and carbon fluxes in a boreal forest after the clear-cutting and partial burning of slash. Agric For Meteorol 188:33–44. https://doi.org/10.1016/j.agrformet.2013.12.003
Kupferschmid AD, Bugmann H (2005) Effect of microsites, logs and ungulate browsing on Picea abies regeneration in a mountain forest. For Ecol Manage 205:251–265. https://doi.org/10.1016/j.foreco.2004.10.008
Kuzyakov Y (2010) Priming effects: interactions between living and dead organic matter. Soil Biol Biochem 42:1363–1371
Litton CM, Raich JW, Ryan MG (2007) Carbon allocation in forest ecosystems. Glob Change Biol 13:2089–2109. https://doi.org/10.1111/j.1365-2486.2007.01420.x
Luyssaert S et al (2010) The European carbon balance Part 3: forests. Glob Change Biol 16:1429–1450. https://doi.org/10.1111/j.1365-2486.2009.02056.x
Matthews B, Mayer M, Katzensteiner K, Godbold DL, Schume H (2017) Turbulent energy and carbon dioxide exchange along an early-successional windthrow chronosequence in the European Alps. Agric For Meteorol 232:576–594. https://doi.org/10.1016/j.agrformet.2016.10.011
Mayer M, Matthews B, Schindlbacher A, Katzensteiner K (2014) Soil CO2 efflux from mountainous windthrow areas: dynamics over 12 years post-disturbance. Biogeosciences 11:6081–6093. https://doi.org/10.5194/bg-11-6081-2014
Mayer M, Matthews B, Rosinger C, Sandén H, Godbold DL, Katzensteiner K (2017a) Tree regeneration retards decomposition in a temperate mountain soil after forest gap disturbance. Soil Biol Biochem 115:490–498. https://doi.org/10.1016/j.soilbio.2017.09.010
Mayer M, Sandén H, Rewald B, Godbold DL, Katzensteiner K (2017b) Increase in heterotrophic soil respiration by temperature drives decline in soil organic carbon stocks after forest windthrow in a mountainous ecosystem. Funct Ecol 31:1163–1172. https://doi.org/10.1111/1365-2435.12805
Metcalfe DB, Fisher RA, Wardle DA (2011) Plant communities as drivers of soil respiration: pathways, mechanisms, and significance for global change. Biogeosciences 8:2047–2061. https://doi.org/10.5194/bg-8-2047-2011
Nakagawa S, Schielzeth H (2013) A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol Evol 4:133–142
Paul-Limoges E, Black TA, Christen A, Nesic Z, Jassal RS (2015) Effect of clearcut harvesting on the carbon balance of a Douglas-fir forest. Agric For Meteorol 203:30–42. https://doi.org/10.1016/j.agrformet.2014.12.010
Pinheiro JC, Bates DM, DebRoy S, Sarkar D, R Core Team (2014) nlme: Linear and nonlinear mixed effects models. http://CRAN.R-project.org/package=nlme
Prietzel J, Ammer C (2008) Montane Bermischwälder der Bayerischen Kalkalpen: reduktion der Schalenwilddichte steigert nicht nur den Verjüngungserfolg, sondern auch die Bodenfruchtbarkeit. Allgemeine Forst- und Jagdzeitung 179:104–112
Pröll G, Darabant A, Gratzer G, Katzensteiner K (2014) Unfavourable microsites, competing vegetation and browsing restrict post-disturbance tree regeneration on extreme sites in the Northern Calcareous Alps. Eur J Forest Res 134:293–308. https://doi.org/10.1007/s10342-014-0851-1
R Core Team (2014) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Raich JW, Tufekciogul A (2000) Vegetation and soil respiration: correlations and controls. Biogeochemistry 48:71–90. https://doi.org/10.1023/a:1006112000616
Ramirez JI, Jansen PA, den Ouden J, Goudzwaard L, Poorter L (2019) Long-term effects of wild ungulates on the structure, composition and succession of temperate forests. For Ecol Manage 432:478–488. https://doi.org/10.1016/j.foreco.2018.09.049
Rebele F, Lehmann C (2001) Biological Flora of Central Europe: Calamagrostis epigejos (L.) Roth. Flora 196:325–344. https://doi.org/10.1016/S0367-2530(17)30069-5
Reichstein M, Beer C (2008) Soil respiration across scales: the importance of a model–data integration framework for data interpretation. J Plant Nutr Soil Sci 171:344–354. https://doi.org/10.1002/jpln.200700075
Rosseel Y (2012) lavaan: an r package for structural equation modeling. J Stat Softw 48:1–36
Schodterer H (2016) Bundesweites Wildeinflussmonitoring 2004–2015 Periode 1–4 BFW. Praxisinformation 42:1–36
Solly EF et al (2014) Factors controlling decomposition rates of fine root litter in temperate forests and grasslands. Plant Soil 382:203–218. https://doi.org/10.1007/s11104-014-2151-4
Subke J-A, Inglima I, Cotrufo FM (2006) Trends and methodological impacts in soil CO2 efflux partitioning: a metaanalytical review. Glob Change Biol 12:921–943. https://doi.org/10.1111/j.1365-2486.2006.01117.x
Subke J-A, Voke NR, Leronni V, Garnett MH, Ineson P (2011) Dynamics and pathways of autotrophic and heterotrophic soil CO2 efflux revealed by forest girdling. J Ecol 99:186–193. https://doi.org/10.1111/j.1365-2745.2010.01740.x
Tanentzap AJ, Coomes DA (2012) Carbon storage in terrestrial ecosystems: do browsing and grazing herbivores matter? Biol Rev 87:72–94. https://doi.org/10.1111/j.1469-185X.2011.00185.x
Thom D, Seidl R (2015) Natural disturbance impacts on ecosystem services and biodiversity in temperate and boreal forests. Biol Rev 91:760–781. https://doi.org/10.1111/brv.12193
Thrippleton T, Bugmann H, Snell RS (2018) Herbaceous competition and browsing may induce arrested succession in central European forests. J Ecol 106:1120–1132
Tremblay J-P, Huot J, Potvin F (2006) Divergent nonlinear responses of the boreal forest field layer along an experimental gradient of deer densities. Oecologia 150:78–88
Van Der Heijden MG, Martin FM, Selosse MA, Sanders IR (2015) Mycorrhizal ecology and evolution: the past, the present, and the future. New Phytol 205:1406–1423
Wangdi N et al (2017) Soil CO2 efflux from two mountain forests in the eastern Himalayas, Bhutan: components and controls. Biogeosciences 14:99
Williams CA, Vanderhoof MK, Khomik M, Ghimire B (2014) Post-clearcut dynamics of carbon, water and energy exchanges in a midlatitude temperate, deciduous broadleaf forest environment. Glob Change Biol 20:992–1007. https://doi.org/10.1111/gcb.12388
Yamanoi K, Mizoguchi Y, Utsugi H (2015) Effects of a windthrow disturbance on the carbon balance of a broadleaf deciduous forest in Hokkaido. Jpn Biogeosci 12:6837–6851. https://doi.org/10.5194/bg-12-6837-2015
Zanella A et al (2019) TerrHum: an iOS application for classifying terrestrial humipedons and some considerations about. Soil Class Soil Sci Soc Am J. https://doi.org/10.2136/sssaj2018.07.0279
Zehetgruber B, Kobler J, Dirnböck T, Jandl R, Seidl R, Schindlbacher A (2017) Intensive ground vegetation growth mitigates the carbon loss after forest disturbance. Plant Soil 420:239–252. https://doi.org/10.1007/s11104-017-3384-9
Acknowledgements
Open access funding provided by University of Natural Resources and Life Sciences Vienna (BOKU). This study was funded by the projects ‘C-Alp’ and ‘C-Alp II’ (funded by the Austrian Academy of Sciences, ÖAW - Research initiative ‘Earth System Sciences (ESS)’). The initial establishment of the experimental sites was funded by the Office of the Tyrolean government, the Austrian Government, the Austrian Federal Forests (ÖBf AG), and the European Regional Development Fund of the European Union. We would like to thank Bradley Matthews for help in the field and for providing the soil climate data. We also thank Rosi and Franz Ebner for hosting the field team. The authors furthermore acknowledge important contributions of the ÖBf AG and the Tyrolean government, who, respectively, provided the experimental sites and funded the fence treatments.
Author information
Authors and Affiliations
Contributions
KK and MM conceived and designed the experiments. DK performed the measurements. MM and DK analysed the data. MM wrote the manuscript with the help of DK and KK.
Corresponding author
Additional information
Communicated by Christian Ammer.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Mayer, M., Keßler, D. & Katzensteiner, K. Herbivory modulates soil CO2 fluxes after windthrow: a case study in temperate mountain forests. Eur J Forest Res 139, 383–391 (2020). https://doi.org/10.1007/s10342-019-01244-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10342-019-01244-9