Abstract
Due to the expected increases of number and intensity of summer droughts in Central Europe the identification of drought tolerant ecotypes becomes more important in future forestry. A common garden experiment with seedlings of Fagus sylvatica provenances from the center (Germany) and eastern margin (Poland) of the species’ distribution range was conducted. Responses of morphological, physiological, chemical and growth parameters to three drought treatments were analyzed. Relative growth rates of the marginal provenance were lower as compared to the central provenance. The marginal seedlings showed a tendency to higher total biomasses because of higher seed masses. In both provenances drought decreased biomass production and root/shoot ratio which was lower in the central provenance. A lower specific root area of the marginal provenance indicated a better adaptation to low xylem water potentials. Under moderate drought, lower leaf δ13C signatures may indicate lower stomatal limitation (or a reduced rate of CO2 assimilation) in the marginal provenance. We conclude that marginal beech provenances may exhibit a better drought adaptation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Climate warming is predicted to increase winter precipitation, but to decrease summer rainfall in parts of Central Europe (IPCC 2007; Schär et al. 2004). This may affect forestry in particular because of the long lifespan of trees. European beech (Fagus sylvatica L.) is a highly competitive species in Central Europe with a wide tolerance of contrasting soil chemical conditions (Ellenberg 1996; Leuschner 1998). However, beech is known to be sensitive to drought which is also reflected in its distribution area in a mostly sub-oceanic climate (Bohn 2004). Due to its great economic importance in European forestry, much research has recently focused on the drought response of juvenile and adult beech plants and the mechanisms of drought tolerance of this species (e.g., Grossoni et al. 1998; Peuke and Rennenberg 2004; Löf et al. 2005; Meier and Leuschner 2008a, b). In comparison to other Central European broad-leaved tree species, a sensitivity of beech upon drought was found particularly with respect to embolism in its conducting system (Cochard et al. 2005), stem increment reduction in dry summers, pre-senescent leaf shedding in drought periods (Granier et al. 2007), and a reduced fine root biomass in dry soil (Meier and Leuschner 2008c). In addition, beech seedlings were also found to be sensitive to dry air (Lendzion and Leuschner 2008). This has stimulated a vital debate on the future of Central European beech forests and the proper choice of tree species for forestry under the prospect of a drier and warmer climate (e.g., Rennenberg et al. 2004; Ammer et al. 2005).
Species that occupy large geographic ranges respond to contrasting environmental conditions by genotypic variation and phenotypic plasticity (Abrams et al. 1992; Peuke and Rennenberg 2004; Kriebitzsch et al. 2005). Several case studies have investigated the drought sensitivity of beech provenances along a gradient of decreasing precipitation from northern to southern Central Europe and to the Mediterranean region (García-Plazaola and Becerril 2000; Nielsen and Jørgensen 2003; Peuke et al. 2006), but fewer studies have been conducted on drought sensitivity of beech at the eastern margin of its distribution area. Czajkowski and Bolte (2006) conducted a drought experiment with beech seedlings from eastern provenances, but focused on aboveground drought responses only and gave no attention to root responses to drought which may be crucial in drought tolerance of beech (Meier and Leuschner 2008c).
Since the frequency of extreme drought and frost events is increasing in Central Europe with the growing continentality of the climate from west to east (Ellenberg 1996), eastern provenances might therefore be important sources for drought and frost resistant ecotypes (Wilmanns 1990; Czajkowski et al. 2006).
We conducted a growth experiment with beech seedlings from a provenance in the distribution center (Central Germany) and a provenance from South-eastern Poland close to the eastern range margin and observed the response of about 20 physiological, morphological and growth-related parameters in moderately or severely drought-stressed plants relative to the well-watered control. Based on earlier beech provenance trials conducted by Nielsen and Jørgensen (2003), Peuke et al. (2006) and Meier and Leuschner (2008a), we tested the following hypotheses: (1) beech provenances from the eastern range margin with a more continental climate are less sensitive to soil drought, and (2) the root system of beech seedlings is particularly sensitive to drought and thus represents a bottleneck in the strategy of young beech plants to cope with drought.
Methods
Plant material
Fagus sylvatica seeds from Central Germany (Neuhaus, Solling) and South-east Poland (Lutowiska, Podkarpackie) were chosen for study in order to compare autochthonous provenances from the center and the eastern margin of the species’ distribution range (Fig. 1). Because we intended to simulate the natural situation in the stands, we decided not to pre-select the seeds of the two provenances for equal seed weight or seed quality but conducted a random selection of seeds in the two locations. The seeds originated from several tree individuals of each one stand per region (forestry district Neuhaus, No. 81009; forestry district Lutowiska). We focused on a single stand per region because the genetic diversity of F. sylvatica is typically higher within a given stand than the diversity between different stands. For example, in a sample covering six beech forests in Central Germany (Hesse), Sander et al. (2000) found 99% of the diversity within the stands and only 1% of the diversity between the stands. Climatic data of the two regions are given in Table 1.
Location of the seed origin areas in the geographic range of beech after Bohn (2004), modified. Light gray planar–hilly, Dark gray hilly–mountainous, Black mountainous–high mountainous beech populations
Cultivation
For germination, the seeds were placed in regularly watered pots filled with loamy sand (Einheitserde B) in a climate chamber at 15/20°C (night/day), after weighing ten seeds per provenance for subsequent determination of the relative growth rate (RGR) (see below). On April 19, 2006, the seedlings were planted in the center of circular plastic containers (2 l) filled with a mixture of one part loamy sand, one part Perlite (Perligran G, Deutsche Perlite GmbH, Dortmund, Germany) and one part humus material (v:v:v). A commercial NPK-fertilizer (Triabon, COMPO GmbH & Co. KG, Münster, Germany; 16-8-12/N-P-K) was added.
The experiment took place in the Experimental Botanical Garden of the University of Göttingen between May 10, 2006, and September 21, 2006, under a mobile plexiglass roof equipped with a rain sensor, which automatically covered the plants when it rained. The roof was removed automatically a few minutes after the rain stopped. Thus, the beeches grew under local temperature and light conditions, but with complete control of soil water supply. To minimize potential influences of environmental gradients at the experimental site, the provenances and treatments were randomly positioned in alternating order and the positions were changed randomly four times during the experiment.
The pots were well-watered until the drought treatment (DT) was initiated after 14 weeks (July 25, 2006). In total, 36 plants per provenance were cultivated with each 12 plants treated with a different moisture regime, i.e., a control (40%), a moderate stress (20%), and a severe stress (10% soil water content) treatment. These soil moisture levels are roughly equivalent 5, 10 and 20 vol%. The limited volume of the pots made it necessary to add water every 2 days after water loss had been determined by weighing the pots.
Harvesting
At the end of the experiment (September 21, 2006), all leaves were removed from the stem, and the remaining shoot was cut off at the root collar after measuring shoot length and diameter of the stem and counting the number of leaves. All leaves were scanned with a flatbed graphics scanner, and the images were analyzed with the software WinFolia (WinFolia 2005b, Régent Instruments Inc., Québec, QC, Canada) to determine leaf area and calculate specific leaf area (SLA, in cm2 g−1 DM). The roots of the trees were harvested by carefully sifting the root-containing soil material of each pot through a sieve and washing the roots to clean them of soil residues. They were sorted by diameter (fine roots < 2 mm, coarse roots > 2 mm). The roots were spread out in water, scanned and the digitized images processed using the software WinRhizo (WinRhizo 2005c, Régent Instruments Inc., Québec, QC, Canada) which calculates the surface area of each root.
All plant organs were dried (70°C, approximately 80 h) and weighed. Specific root area (SRA, in cm2 g−1 DM), total fine root surface area, root dry weight and fine root/leaf area ratio were calculated from these data for each tree. The RGR (in g g−1 day−1) was calculated for the whole seedling by subtracting seed biomass from total harvested biomass and relating the difference to the duration of the experiment.
One day before the harvest, predawn water potential (Ψpre) of the leaves was measured at 4:00 a.m. using a Scholander pressure chamber (Scholander et al. 1965). The relative water content of the leaves (θ l) harvested around noon was determined by drying (fresh weight − dry weight/fresh weight).
Chemical analyses
The dried plant material of each organ of a plant was pooled and ground. The leaf δ13C signature and N concentration were determined by mass spectroscopy (Delta Plus, Finnigan MAT, Bremen, Germany) in the Stable Isotope Laboratory (KOSI) of the University of Göttingen. For analyzing plant cation concentration, 100 mg of plant powder were digested with 3 ml HNO3 at 185°C for 5 h and the concentrations of Ca and K measured by atomic absorption spectrometry (AAS Vario 6, Analytic Jena, Jena, Germany).
Statistical analyses
All statistical analyses were performed with SAS Version 8.02 (SAS Institute Inc., Cary, NC, USA) and JMP (JMPIN Version 4.0.4, SAS Institute 2001). Significance was determined at P < 0.05 throughout. Before statistical analyses, all data were tested for normal distribution (Shapiro–Wilk test) and homogeneity of variances (Bartlett test). To achieve normal distribution and homogeneity of variances, the data of fine root biomass and leaf calcium concentration were logarithmically transformed. Two-way analyses of variance with the sources treatment, provenance and their interaction were performed by the ANOVA procedure for balanced data of the variables maximum shoot length, number of leaves per plant, seed weight, total biomass, leaf biomass, root/shoot ratio, RGR and leaf N concentration. In the case of unbalanced data (fine root biomass, SLA, leaf calcium concentration, leaf potassium concentration), general linear models were calculated. Differences between two treatments were analyzed with a Scheffé test, except for root/shoot ratio and RGR which were analyzed with a post hoc Tukey test.
For non-normally distributed data, the influences of provenance and treatment were investigated with a Kruskal–Wallis test (leaf water content, predawn leaf water potential, root collar diameter, shoot biomass, SRA, FR/LA ratio, δ13C, leaf magnesium concentration). Differences between two treatments were analyzed with a U-test after Mann and Whitney. A summary of the results of the different tests comparing the plant morphological, physiological and chemical variables between different DTs and different provenances is given in Table 2.
Results
Plant water status
Neither predawn leaf water potential Ψpre nor leaf water content θ l measured at noon were influenced by the provenance in the three treatments (Table 3).
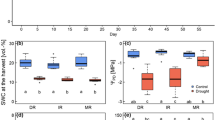
Leaf δ13C was not significantly different between the central and marginal provenances under ample soil moisture supply (control treatment), but was significantly higher (less negative) in the central beech population under moderate drought stress (Fig. 2). As expected Ψpre and θ l decreased with increasing drought but the response was not different between the provenances. A large and significant increase in δ13C occurred in both provenances between the control and the moderate stress treatment, but no further increase was visible toward the severely stressed plants.
Parameters of morphology and growth
The marginal provenance exhibited significantly higher seed weights than the central provenance (0.25 vs. 0.16 g, t-test < 0.001, F = 19.05). The RGR was significantly influenced by both provenance and treatment. Seedlings of the central provenance had a higher RGR in all treatments (difference significant only for the control and severe stress treatment, Fig. 3). The decrease in RGR with increasing drought was roughly similar in the two provenances. Total biomass produced at the day of harvest, however, was not different between the two provenances despite a higher RGR of the central provenance. This holds true under ample water supply and in the DTs and was a consequence of the considerably higher seed weight of the marginal provenance. The number of leaves produced per plant and the shoot length at harvest were affected neither by the DT nor by provenance. However, drought reduced the root collar diameter significantly (Table 3).
Total fine root biomass per plant was significantly lowered by drought. However, the marginal provenance showed only a small and non-significant difference in fine root biomass between the control and the severe stress treatment, whereas the central provenance exhibited a significant decrease (Fig. 4a). The root/shoot ratio was lower in the stressed than in the control plants, and lower in the central as compared to the marginal provenance (Fig. 4b).
Root/shoot ratio decreased with increasing drought stress in both provenances, but to a greater extent in that from the distribution center than in the marginal one. SRA of the central provenance significantly declined with increasing drought in contrast to nearly constant values in the marginal provenance (Fig. 5a). In other words, the proportion of finest roots decreased upon drought in the central but not in the marginal provenance. SLA was neither affected by drought nor provenance (Table 3).
The ratio of fine root to leaf area (FR/LA) was found to be significantly lower in stressed than in control plants. While the central provenance showed a significant decrease of FR/LA with increasing drought, the response of the marginal provenance was less pronounced (Fig. 5b). Thus, FR/LA of both provenances showed similar values in the severely stressed treatment, although FR/LA of the well-watered central seedlings was significantly lower than that of the marginal ones.
Leaf nutrient concentrations
The N concentration of the leaves was neither affected by soil moisture treatment nor provenance as was the leaf concentration of calcium (Table 3). The potassium concentrations of the leaves, however, were found to be influenced by both drought and provenance. The central provenance showed significantly lower means than the marginal one, which contained highest amounts of K in the moderate stress treatment (Fig. 6).
Discussion
The main differences between the two provenances to be compared in this study were detected with regard to seed weight, RGR of the seedlings and carbon allocation patterns. Although seeds of the central provenance showed considerably lower weights than those of the marginal one, which may reduce the fitness of the embryo, this initial disadvantage was compensated by higher subsequent growth rates of the seedlings from the range center. These differences may indicate different adaptations to the climate in Central Germany and South-East Poland. An experimental test of this hypothesis would require to grow plants of the two provenances under the same environmental conditions and to use the seeds of these plants for drought experiments. This would assure that different seed weights are inherited and do not reflect phenotypic plasticity. The results of Thomsen and Kjær (2002) with different beech genotypes indicate that seed weight seems to be at least partly under genetic control in this species.
A reduction of biomass is a frequently observed response to drought in beech seedlings (Madsen and Larsen 1997; Fotelli et al. 2001). However plants can prevent productivity losses to some extent by completing growth before the onset of drought stress (Schraml and Rennenberg 2002). Thus, how severely drought will affect the biomass production of juvenile beeches seems to depend on the timing of drought relative to the growth period. Nevertheless, Tognetti et al. (1995) found beech seedlings from drier regions always to produce less biomass than equally treated seedlings from regions with ample water supply, irrespective of the DT. García-Plazaola and Becerril (2000) showed similar results for the leaf biomass of beech seedlings. The overall lower RGRs of the marginal provenance in our study could therefore be interpreted as an adaptation to drier habitats.
In contrast to seedlings, mature trees can use water resources deeper in the soil because of their fully developed root system (Bolte and Roloff 1993). The allocation of carbon and nutrients between root and shoot may be crucial for the success of tree seedlings at sites with temporal water shortage. A higher seed mass may support the early development of a deep-reaching primary root which may protect the seedling from damage by summer droughts (Baker 1972; Leishman and Westoby 1994). A more frequent occurrence of dry spells in summer in the more continental climate of South-East Poland could have fostered a selection process toward beech plants with higher seed masses to increase the survivorship of the seedlings. That the strategy of providing larger acorns in a drier climate is successful is indicated by the fact of marginal seedlings having produced similar amounts of biomass after 22 weeks as compared to central ones despite lower RGRs.
A second important adaptive trait seems to be the root/shoot ratio which was higher in the marginal provenance than in the central one. Similarly, Tognetti et al. (1995) found higher root/shoot ratios in beech seedlings originating from drier regions. Adaptive change in the root/shoot ratio is an expression of the numerous trade-offs that exist between stress tolerance and growth processes. It may be an important adaptation securing access to soil water resources in a drier climate, since beech seedlings seem to be particularly sensitive to drought effects on the fine root system: various studies exposing beech seedlings to drought found a greater reduction of root than of shoot biomass, resulting in a decrease, and not an increase of root/shoot ratio upon drought (Davidson et al. 1992; Fotelli et al. 2001; Löf et al. 2005; Meier and Leuschner 2008a). In our experiment as well, shoot biomass was less affected by drought than root biomass. A similarly sensitive response of the fine root system was also reported for adult beech trees in a rainfall gradient studied by Meier and Leuschner (2008c). Thus, beech provenances with an inherently higher root/shoot ratio should have advantages in a drier environment, given the low belowground drought tolerance of beech. Moreover, there is evidence that beech provenances from drier environments have a less drought-sensitive root system than provenances from more humid climates. Hamp et al. (1999) found that the root/shoot ratio of beech seedlings was reduced to a greater extent in plants from moister habitats than in plants of a drier origin. Similarly, the relatively small reduction of the root/shoot and fine root/leaf area (FR/LA) ratio in the marginal provenance as compared to the central provenance in our study may be interpreted as a better adaptation to drought of the Polish plants. This is supported by a reduction in fine root biomass in the DTs that was significant only in the central, but not in the marginal provenance indicating a higher drought tolerance of the latter. An explanation of the greater reduction in fine root biomass upon drought of the central provenance could be the higher specific root surface area in these plants, indicating a greater proportion of very fine roots (i.e., roots < 0.5 mm in diameter) in total root mass and/or a lower average root tissue density. Both traits would support a faster root growth rate and a higher water and nutrient uptake per root mass, but should result in a greater sensitivity to drought (Ryser 1996).
The significant reduction of SRA of the central provenance under drought must be viewed as an acclimatization which reduces the drought exposure of the most sensitive elements of the root system. Different phenologies of growth may be another explanation of the greater drought-induced reduction of root biomass in the central provenance. Nielsen and Jørgensen (2003) found drought tolerant beech seedlings to complete growth 18 days earlier than equally treated drought-sensitive plants, thereby avoiding drought stress at least partly. We may speculate that root growth may have occurred mostly before the onset of drought in the marginal provenance as did leaf and shoot growth, whereas root growth of the central provenance occurred later under less favorable conditions. However, direct observation of root growth activity with minirhizotrons is required to test this hypothesis.
This study focused on above- and belowground growth, morphology and carbon partitioning patterns while only a few physiological variables were investigated. Remarkably, parameters of leaf water status (Ψpre and θ l) did not differ significantly between the central and the marginal provenances, neither under ample nor reduced water supply. However, the about 0.6‰ lower δ13C signature of the leaves of the marginal provenance under moderate drought stress may indicate that stomatal limitation probably has been higher in the plants of the central provenance (e.g., Ehleringer and Cooper 1988; Fotelli et al. 2003; Virgona and Farquhar 1996). If valid, the central provenance plants from a humid environment should have responded more sensitively to soil drought by partial stomatal closure. Unfortunately, the corresponding δ18O signatures are missing for these plants. However, a rather low sensitivity of photosynthetic efficiency and carbon gain over the summer was reported by Nahm et al. (2006) in beech plants growing at the relatively dry south-eastern range margin of the distribution area which supports our hypothesis. On the other hand, a negative effect of stomatal closure on CO2 assimilation is unlikely since RGR was higher, and not lower, in the seedlings of the central provenance as compared to the marginal one. According to Damesin and Lelarge (2003), the contrasting δ13C values could also be a consequence of different phenologies of growth: δ13C tends to increase with proceeding growth in beech with the consequence that slower growing plants (as in the marginal provenance) discriminate more against 13C than faster growing ones. In a genotype comparison of droughted Betula pendula seedlings, Aspelmeier and Leuschner (2004) also found generally lower δ13C signatures in plants from drier origins.
Calcium, which is mostly transported by the xylem sap and accumulates in the leaves, was remarkably constant across the provenances and DTs. A comparison of leaf K concentrations revealed particularly high values in the moderately stressed plants of the marginal provenance. A possible explanation is that these plants conducted a more active osmoregulation in the leaf tissue than those from the central provenance. This hypothesis needs testing by pressure–volume-curve analysis of the different provenances.
Conclusions
We conclude that the marginal beech provenance from a subcontinental to continental climate with a higher probability of summer drought reveals several traits characterizing this population as being better drought adapted than a central one from sub-oceanic Central Germany. Due to a considerably higher acorn mass, the seedlings of the marginal population may be more successful in surviving drought in the first year. Further, marginal plants were less sensitive to a drought-induced reduction in fine root biomass allowing them to maintain higher root/shoot and root surface area/leaf area ratios under drought than central plants. This is probably a consequence of a root morphology and/or root growth phenology better adapted to more frequent summer droughts at the range margin. The δ13C signature may point at a lower stomatal limitation of photosynthesis (or a reduced rate of CO2 assimilation) in the marginal provenance. We suggest that a combination of morphological, physiological and phenological adaptations enable beech seedlings of the marginal provenance to maintain an equal or even higher biomass production upon drought as compared to plants of the central provenance, even though their inherent RGR seems to be lower.
However, further results about the drought response and its underlying mechanisms among different beech provenances are needed to confirm the long term drought resistance of marginal provenances. In addition, experiments are required to show whether beech seed size is a genetically fixed trait or varies considerably with environmental conditions.
References
Abrams MD, Kloeppel BD, Kubiske ME (1992) Ecophysiological and morphological responses to shade and drought in two contrasting ecotypes of Prunus serotina. Tree Physiol 10:343–355
Ammer VC, Albrecht L, Borchert H, Brosinger F, Dittmar C, Elling W, Ewald J, Felbermeier B, Gilsa H, Huss J, Kenk G, Kolling C, Kohnle U, Meyer P, Mosandl R, Moosmayer HU, Palmer S, Reif A, Rehfuess KE, Stimm B (2005) Future suitability of beech (Fagus sylvatica L.) in Central Europe: critical remarks concerning a paper of Rennenberg et al. (2004). Allg Forst Jagdztg 176:60–67
Aspelmeier S, Leuschner C (2004) Genotypic variation in drought response of silver birch (Betula pendula): leaf water status and carbon gain. Tree Physiol 24:517–528
Baker HG (1972) Seed weight in relation to environmental conditions in California. Ecology 53:997–1010. doi:10.2307/1935413
Bohn U (2004) Karte der natürlichen Vegetation Europas. Ber Landesamtes Umweltschutz Sachsen-Anhalt Sonderheft, vol 2. Landesamt für Umweltschutz Sachsen-Anhalt, Halle, pp 4–13
Bolte A, Roloff A (1993) Einfluss von Buchenüberhältern auf Bodenvegetation und Naturverjüngung. Allg Forst Jagdztg 164:97–101
Cochard H, Coste S, Chanson B, Guehl JM, Nicolini E (2005) Hydraulic architecture correlates with bud organogenesis and primary shoot growth in beech (Fagus sylvatica). Tree Physiol 25:1545–1552
Czajkowski T, Bolte A (2006) Unterschiedliche Reaktion deutscher und polnischer Herkünfte der Buche (Fagus sylvatica L.) auf Trockenheit. Allg Forst Jagdztg 177:30–40
Czajkowski T, Kompa T, Bolte A (2006) Zur Verbreitungsgrenze der Buche (Fagus sylvatica L.) im nordöstlichen Mitteleuropa. Forstarchiv 77:203–216
Damesin C, Lelarge C (2003) Carbon isotope composition of current-year shoots from Fagus sylvatica in relation to growth, respiration and use of reserves. Plant Cell Environ 26:207–219. doi:10.1046/j.1365-3040.2003.00951.x
Davidson SR, Ashmore MR, Garretty C (1992) Effects of ozone and water deficit on the growth and physiology of Fagus sylvatica. For Ecol Manage 51:187–193
Ehleringer JR, Cooper TA (1988) Correlations between carbon isotope ratio and microhabitat in desert plants. Oecologia 76:562–566
Ellenberg H (1996) Vegetation Mitteleuropas mit den Alpen. Ulmer, Stuttgart
Fotelli MN, Geßler A, Peuke AD, Rennenberg H (2001) Drought affects the competitive interactions between Fagus sylvatica seedlings and an early successional species, Rubus fruticosus: responses of growth, water status and δ13C composition. New Phytol 151:427–435. doi:10.1046/j.1469-8137.2001.00186.x
Fotelli MN, Rennenberg H, Holst T, Mayer H, Geßler A (2003) Carbon isotope composition of various tissues of beech (Fagus sylvatica) regeneration is indicative of recent environmental conditions within the forest understorey. New Phytol 159:229–244. doi:10.1046/j.1469-8137.2003.00782.x
García-Plazaola JI, Becerril JM (2000) Effects of drought on photoprotective mechanisms in European beech (Fagus sylvatica L.) seedlings from different provenances. Trees (Berl) 14:485–490. doi:10.1007/s004680000068
Granier A, Reichstein M, Bréda N, Janssens IA, Falge E, Ciais P, Grunwald T, Aubinet M, Berbigier P, Bernhofer C, Buchmann N, Facini O, Grassi G, Heinesch B, Ilvesniemi H, Keronen P, Knohl A, Köstner B, Lagergren F, Lindroth A, Longdoz B, Loustau D, Mateus J, Montagnani L, Nys C, Moors E, Papale D, Peiffer M, Pilegaard K, Pita G, Pumpanen J, Rambal S, Rebmann C, Rodrigues A, Seufert G, Tenhunen J, Vesala I, Wang Q (2007) Evidence for soil water control on carbon and water dynamics in European forests during the extremely dry year: 2003. Agric Meteorol 143:123–145. doi:10.1016/j.agrformet.2006.12.004
Grossoni P, Bussott F, Tani C, Gravano E, Santarelli S, Bottacci A (1998) Morpho-anatomical alterations in leaves of Fagus sylvatica L. and Quercus ilex L. in different environmental stress condition. Chemosphere 36:919–924. doi:10.1016/S0045-6535(97)10148-5
Hamp R, Shi L, Guttenberger M, Nehls U (1999) Mykorrhizierung und Stresstoleranz von Ökotypen der Buche (Fagus sylvatica L.): “Conventwaldprojekt”. Forschungsbericht, FZKA-BWPLUS
IPCC (2007) Summary for policymakers. In: Salomon S, Qin D, Manning M et al (eds) Climate change 2007: the physical science basis Contribution of working group I to the fourth assessment report of the intergovernmental panel on climate change. University Press, Cambridge
Kriebitzsch W-U, Bick U, Degen B, Wühlisch v. G, Schweinle J, Schmitt U, Puls J, Welling J, Beck W (2005) Angepasstheit und Anpassungsfähigkeit von Buchen-Provenienzen an Klimabedingungen. BFH-Nachrichten, Issue 2, pp 2–3
Leishman MR, Westoby M (1994) The role of seed size in seedling establishment in dry soil-conditions—experimental-evidence from semiarid species. J Ecol 82:249–258. doi:10.2307/2261293
Lendzion J, Leuschner C (2008) Growth of European beech (Fagus sylvatica L.) saplings is limited by elevated atmospheric vapour pressure deficits. For Ecol Manage 256:648–655
Leuschner C (1998) Mechanismen der Konkurrenzüberlegenheit der Rotbuche. Ber Reinh-Tuxen-Ges 10:5–18
Löf M, Bolte A, Welander NT (2005) Interacting effects of irradiance and water stress on dry weight and biomass partitioning in Fagus sylvatica seedlings. Scand J For Res 20:322–328. doi:10.1080/02827580500201593
Lorenc H (2005) Atlas klimatu Polski. Instytut Meteorologii i Gospodarki Wodnej, Warszawa
Madsen P, Larsen JB (1997) Natural regeneration of beech (Fagus sylvatica L.) with respect to canopy density, soil moisture and soil carbon content. For Ecol Manage 97:95–105
Meier IC, Leuschner C (2008a) Genotypic variation and phenotypic plasticity in the drought response of fine roots of European beech. Tree Physiol 28:297–309
Meier IC, Leuschner C (2008b) Leaf size and leaf area index in Fagus sylvatica forests: competing effects of precipitation, temperature, and nitrogen availability. Ecosystems 11:655–669. doi:10.1007/s10021-008-9135-2
Meier IC, Leuschner C (2008c) Belowground drought response of European beech: fine root biomass and carbon partitioning in 14 mature stands across a precipitation gradient. Glob Change Biol 14:2081–2095. doi:10.1111/j.1365-2486.2008.01634.x
Nahm M, Radoglou K, Halyvopoulos G, Geßler A, Rennenberg H, Fotelli MN (2006) Physiological performance of beech (Fagus sylvatica L.) at its southeastern distribution limit in Europe: seasonal changes in nitrogen, carbon and water balance. Plant Biol 8:52–63. doi:10.1055/s-2005-872988
Nielsen CN, Jørgensen FV (2003) Phenology and diameter increment in seedlings of European beech (Fagus sylvatica L.) as affected by different soil water contents: variation between and within provenances. For Ecol Manage 174:233–249
Peuke AD, Rennenberg H (2004) Carbon, nitrogen, phosphorus, and sulphur concentration and partitioning in beech ecotypes (Fagus sylvatica L.): phosphorus most affected by drought. Trees (Berl) 18:639–648. doi:10.1007/s00468-004-0335-x
Peuke AD, Geßler A, Rennenberg H (2006) The effect of drought on C and N stable isotopes in different fractions of leaves, stems and roots of sensitive and tolerant beech ecotypes. Plant Cell Environ 29:823–835. doi:10.1111/j.1365-3040.2005.01452.x
Rennenberg H, Seiler W, Matyssek R, Geßler A, Kreuzwieser J (2004) Die Buche (Fagus sylvatica L.)—ein Waldbaum ohne Zukunft im südlichen Mitteleuropa? Allg Forst Jagdztg 175:210–224
Ryser P (1996) The importance of tissue density for growth and life span of leaves and roots: a comparison of five ecologically contrasting grasses. Funct Ecol 10:717–723. doi:10.2307/2390506
Sander T, König S, Rothe GM, Janßen A, Weisgerber H (2000) Genetic variation of European beech (Fagus sylvatica L.) along an altitudinal transect at mount Vogelsberg in Hesse, Germany. Mol Ecol 9:1349–1361. doi:10.1046/j.1365-294x.2000.01014.x
Schär C, Vidale PL, Luthi D, Frei C, Haberli C, Liniger MA, Appenzeller C (2004) The role of increasing temperature variability in European summer heatwaves. Nature 427:332–336. doi:10.1038/nature02300
Schipka F (2002) Blattwasserzustand und Wasserumsatz von vier Buchenwäldern entlang eines Niederschlagsgradienten in Mitteldeutschland. Ph.D. thesis, Georg-August-Universität Göttingen
Scholander PF, Bradstreet ED, Hemmingsen EA, Hammel HT (1965) Sap pressure in vascular plants. Science 148:339–346. doi:10.1126/science.148.3668.339
Schraml C, Rennenberg H (2002) Ökotypen der Rotbuche (Fagus sylvatica L.) zeigen unterschiedliche Reaktionen auf Trockenstreß. Forstw Cbl 121:59–72. doi:10.1046/j.1439-0337.2002.00059.x
Thomsen KA, Kjær ED (2002) Variation between single tree progenies of Fagus sylvatica in seed traits, and its implications for effective population numbers. Silvae Genet 51:183–190
Tognetti R, Johnson JD, Michelozzi M (1995) The response of European beech (Fagus sylvatica L.) seedlings from two Italian populations to drought and recovery. Trees (Berl) 9:348–354. doi:10.1007/BF00202499
Virgona J, Farquhar GD (1996) Genotypic variation in relative growth rate and carbon isotope discrimination in sunflower is related to photosynthetic capacity. Aust J Plant Physiol 23:227–236
Wilmanns O (1990) Pflanzen prägen Lebensräume Die Rotbuche, Fagus sylvatica L. Biol Unserer Zeit 20:60–62. doi:10.1002/biuz.19900200116
Acknowledgments
The authors wish to thank Boris Rewald for fruitful discussions regarding the field work. We are grateful to Jan Suszka, Institute of Dendrology, Polish Academy of Sciences, for providing the seed material of F. sylvatica. This study was financially supported by the Helmholtz-Gemeinschaft (HGF) and was conducted in the framework of the Virtual Institute of Macroecology. We thank two anonymous reviewers for helpful comments on an earlier version of this paper.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by R. Matyssek.
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Rose, L., Leuschner, C., Köckemann, B. et al. Are marginal beech (Fagus sylvatica L.) provenances a source for drought tolerant ecotypes?. Eur J Forest Res 128, 335–343 (2009). https://doi.org/10.1007/s10342-009-0268-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10342-009-0268-4