Abstract
Crapemyrtle bark scale (CMBS), Acanthococcus lagerstroemiae (Kuwana) (Hemiptera: Eriococcidae), is a non-native scale insect that has spread throughout many urban areas of the Southeast and Middle Atlantic regions of the United States following its initial detection near Dallas, Texas in 2004, severely reducing the aesthetic value and health of the popular ornamental crapemyrtle tree (Lagerstroemia spp.). We infested crapemyrtles with known numbers of CMBS to determine the minimum number of individuals required for establishment after initial arrival on plants. We also investigated how netting—implemented to understand differences in establishment when scale dispersal and predation are inhibited—influenced population growth. We determined that one female CMBS egg sac can successfully establish a new population ~ 92% of the time and that netting had negligible effects on establishment. Our results underscore the importance of surveying and managing CMBS and scale insects with similar biology when attempting to prevent infestation of nursery stock, which is widely implicated as a vector for long-distance dispersal of scale insects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Key message
-
Crapemyrtle bark scale is a non-native pest that causes damage to high-value ornamental trees.
-
We determined that a single female crapemyrtle bark scale can lead to established infestations.
-
Our results show the importance of thorough surveying for crapemyrtle bark scale.
Introduction
Non-native scale insects, often arriving via imported live plants (Liebhold and Tobin 2008; Liebhold et al. 2012; Meineke et al. 2013; Meurisse et al. 2019), have become some of the most common and damaging pests of trees in North America. Invaders such as oystershell scale (Lepidosaphes ulmi L.) (Griswold 1925; Crouch et al. 2021), beech scale (Cryptococcus fagisuga Lind.) (Morin et al. 2007), Japanese maple scale (Lopholeucaspis japonica Cockerell) (Addesso et al. 2016), European elm scale (Gossyparia spuria Modeer) (Herbert 1924), and others cause damage to urban trees. Damage occurs directly by insects removing carbohydrates via feeding on phloem or parenchyma tissue and/or indirectly by facilitating growth of sooty mold and pathogenic fungi. Scale insects can also vector and increase susceptibility of trees to disease in urban and/or rural forests, such as in the insect–fungus complex beech bark disease (Morin et al. 2007), drippy blight of red oaks caused by a bacterium associated with kermes scale (Allokermes galliformis Riley) (Sitz et al. 2018), and grapevine leafroll virus, which is vectored by mealybugs and soft scales (Almeida et al. 2013; Hommay et al. 2021). While the mechanisms governing their invasions vary, ranging from number of arriving propagules at a site or plant to natural enemy pressure at the invasion site, certain invasive scale insect species, specifically phloem-feeding scales, exhibit high fecundity (producing hundreds of eggs per female), develop through a tiny crawler stage that is difficult to detect, and have multiple generations per year (Morin et al. 2007; Addesso et al. 2016; Crouch et al. 2021). These traits increase the number of potential propagules produced and, potentially, the ability of scale insects to establish and spread.
Propagule pressure, the number of individuals arriving per unit of space and time (Hill et al. 2016), is integral to establishment success (Lockwood et al. 2005; Brockerhoff et al. 2014); larger numbers of arriving conspecifics or multiple introduction events over time typically leads to a higher chance of establishment. Establishment success, especially for small founding populations, is also modulated by demographic (i.e., random variation in fecundity and survival) and environmental (i.e., variation in weather patterns and natural enemy prevalence) stochasticity (Leung et al. 2004; Lockwood et al. 2005; Drake and Lodge 2006; Chase et al. 2022). Many scale insect species might be robust to such stochasticity owing to their high fecundity and development through several overlapping generations per year, as well as the ability of many scale insect species to reproduce parthenogenetically (Liebhold et al. 2016). Moreover, scale insects produce a waxy or sclerotized covering that reduces penetration of many insecticides (Fondren and Mccullough 2005; Quesada et al. 2018) and may also reduce attack from natural enemies and/or buffer against adverse weather conditions, especially in armored scale species such as Japanese maple scale (Lopholeucaspis japonica Cockerell) (Addesso et al. 2016). Conversely, the establishment and growth of scale populations could be altered by females being sessile for most of their life cycle, which potentially increases mate-finding failure (especially in non-parthenogenetic species), intensifies intraspecific competition, and/or exacerbates predation (e.g., immobile prey may be more susceptible). The role of these multiple and potentially opposing factors in governing plant-level establishment of scale insects, which also influences spread across larger (e.g., between urban centers) spatial scales, is poorly understood. As scale insects are often transported on live plant material (Liebhold et al. 2012; Meurisse et al. 2019), understanding how scale insect populations establish on a plant is essential to informing phytosanitation practices for these small-bodied insects.
Crapemyrtle bark scale (CMBS), Acanthococcus lagerstroemiae (Kuwana) (Hemiptera: Eriococcidae), is a non-native, sap-feeding insect first detected near Dallas, TX in 2004 that has since invaded several urban centers in the eastern United States (US) (Wang et al. 2016). Since its initial detection, this scale insect has damaged the popular non-native ornamental tree crapemyrtle (Lagerstroemia spp. Linnaeus, Lythraceae) across urban landscapes of fifteen different states in the Southeast and Middle Atlantic regions of the US (Wang et al. 2016). Both female and male CMBS have a cryptic, mobile crawler stage immediately after hatching from eggs (Wang et al. 2019). While both males and females exhibit a sessile period in which crawlers insert their stylets into the tree and feed (hereafter termed “settled crawlers”), only males have a winged adult stage; females remain sessile as adults, laying eggs in a white, waxy coating covering their bodies called ovisacs (Wang et al. 2019). Parthenogenetic reproduction by CMBS has not been observed in lab settings (Xie et al. 2022). In North America, CMBS can produce up to three generations per year in the warm-summer humid continental climate of the Middle Atlantic region and up to four generations in the humid subtropical climate at more southern latitudes (Gu et al. 2014). There remain several uninvaded regions of the US that harbor crapemyrtles and other suitable hosts (Wang et al. 2019; Xie et al. 2021).
Knowledge of the role of founding population size in population establishment and growth, while being a fundamental aspect of an invasion, could also (1) reveal the importance of phytosanitation practices against CMBS, including in nurseries growing crapemyrtles that may be the source of many satellite CMBS infestations, and (2) potentially elucidate the number of propagules necessary to achieve between-tree movement. Our study objectives were to (1) determine the minimum founding population size for tree-level establishment of CMBS after initial arrival on a plant, and (2) quantify population growth following establishment. This work provides useful information for management practitioners, while also revealing key aspects of CMBS ecology in its non-native range.
Materials and methods
During a pilot study in early June 2021, potted crapemyrtles were infested with zero, one, two, three, or four settled CMBS crawlers to determine the minimum founding population size of CMBS. Two weeks following deployment of CMBS, multiple plants infested with one CMBS had establishment of new scales. Thus, to further evaluate establishment success of a single ovisac, 25 0.6-m-tall Lagerstroemia indica x fauriei 'Natchez’ (crapemyrtle) were infested with one female settled crawler in early July 2021. It was not known whether females had been mated at the time of infestation.
Plants were placed in 26.5-l pots using a soil mix containing pine bark fines and sphagnum peat (Pro-Line 44N, Jolly Gardener, Poland Spring, ME). Crapemyrtle bark scales were collected by clipping infested branches from crapemyrtles in Charlotte, NC. Infested twig fragments were zip-tied to the upper portion of our study trees on the same day that the twig fragments were prepared. Plants were then relocated using a truck to a large field with minimal shade at the Bartlett Tree Research Laboratory in Charlotte, NC, adjacent to the plants from the pilot study. Half of the plants had fine fabric mesh netting (0.5 mm × 0.6 mm) secured around the base of the pot using twine to exclude predators and inhibit dispersal loss of winged males and/or departure and arrival of wind-blown crawlers. The mesh netting occasionally blew open or was dislodged during thunderstorms and high wind events (e.g., potentially allowing alate males and natural enemies to enter or exit) but was always replaced within 48 h. We continued monitoring plants infested with zero CMBS in the pilot study to confirm that there was no colonization by CMBS between adjacent trees. There were mature crapemyrtles approximately 50 m away from our study trees, but these trees were monitored periodically throughout the study and CMBS was never found.
Per plant, we recorded establishment of CMBS (success/failure) and the number of male white coverings (male pupae), female settled crawlers, and female ovisacs two to three times per week from July through October 2021. Establishment was considered successful (1 = success, 0 = failure) if a plant had at least one settled scale at the conclusion of CMBS population density counts in October. Because CMBS is active in winter (Wright et al. 2023), we left all plants in the field until March and then destructively sampled plants to document persistence of populations or additional failed or successful establishments through winter. Each individual plant was checked visually for CMBS, the entirety of each plant being examined. Population density per plant per sample day was calculated as the sum of male pupae, female settled crawlers, and female ovisacs.
Logistic regression, analysis of variance (ANOVA), and analysis of deviance with type III sums of squares were used to compare establishment success and population density on the last sample day in October, respectively, as a function of netting. All statistical analyzes were performed using the R statistical programming environment v4.2.2 (R Core Team 2022) and the car package (Fox and Sanford 2019) was used for analysis of variance and deviance. We fit density (number of individual CMBS per plant) with a Poisson distribution but found that the model had overdispersion and thus re-fit the model with a negative binomial distribution with a log link function. Control plants were excluded from all analyzes, as none had any documented scale insects until the final counts were conducted in March 2022, when one control adjacent to a heavily infested plant was found to have 28 settled crawlers. Lastly, to evaluate population growth, we graphed the ln-transformed densities of scale insect populations as a function of time.
Results
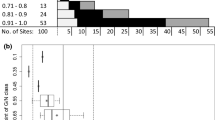
Of plants infested with a single CMBS female settled crawler, 92% had CMBS population establishment (Fig. 1). In the model comparing establishment success among netted and non-netted plants, no statistically clear effect was detected (\({X}_{1}^{2}\) = 2.78, p = 0.10; Fig. 1a). Despite that, CMBS populations established on 100% of netted plants compared with 85% on non-netted plants (Fig. 1a).
Average (a) establishment success (\({X}_{1}^{2}\) = 2.78, p = 0.10) of crapemyrtle bark scale (CMBS) on crapemyrtles infested with one CMBS and densities on the last sample day in October (b) of CMBS, ln-transformed (\({X}_{1}^{2}\) = 3.73, p = 0.05). Controls were excluded from average establishment success (a) and density (b) analyzes, as we only analyzed plants that were initially artificially infested and no controls ever became infested. When establishment success = 0, no scales were present. The vertical lines with horizontal bars in (a) are error bars, whereas in (b) the vertical lines represent the range of values and horizontal bars represent the median. The dots in both figures represent scale densities on individual plants
After being infested with one female settled crawler in July, CMBS populations grew to high densities by October, with an overall average density of 32 ± 4 (Fig. 1b). In the model comparing CMBS densities on the last sample day in October between netting treatments, there was no statistically clear effect of netting (\({X}_{1}^{2}\) = 3.73, p = 0.05; Fig. 1b) despite densities of CMBS averaging 39 ± 21 on netted plants compared with 11 ± 5 on non-netted plants (Fig. 1b).
Scale densities on individual plants reached high levels (mean per plant maximum density = 89 ± 48) despite being infested with just one settled crawler initially (Fig. 2). The highest single plant scale density recorded at one sample day was 1086 scales (Fig. 2). Densities of CMBS fluctuated on individual plants over time, for example, as one plant increased from 21 CMBS on August 16th to a peak of 157 on August 26th before declining to 97 on September 17th (Fig. 2).
Crapemyrtle bark scale (CMBS) density on individual crapemyrtle saplings infested with one CMBS with each plant represented by a gray line. Each line displays a moving average over time with the purple line indicating a moving average for CMBS populations for all plants over time. Controls were excluded from this analysis as we only analyzed population growth on plants that were initially artificially infested and no controls ever became infested
Discussion
The ability of a few propagules to establish can create management challenges for invading species (Leung et al. 2004; Lockwood et al. 2005; Drake and Lodge 2006; Chase et al. 2022), and our study demonstrates that CMBS can establish a new population on a plant following the introduction of a single, settled female. This finding highlights the difficulty of managing this invader, especially in nursery settings where large numbers of crapemyrtles may be cultivated directly adjacent to each other, potentially facilitating movement of scales from one plant to adjacent plants if branches are touching. Missing a single, mated female crawler during inspection and/or treatment is likely to result in population establishment on a plant (Fig. 1) and populations could reach densities greater than 500 scale insects in a matter of months (Fig. 2). Because we did not know the mating status of the settled females that we infested plants with, the likelihood of establishment and subsequent population growth could have been highly dependent upon the presence of male CMBS on the same or nearby trees. However, it is possible that we infested plants with a large proportion of mated females with mature ovisacs, leading to high establishment success. Moreover, detecting low density populations is inherently challenging (Venette et al. 2002). For example, 11 plants in our study harbored CMBS populations that our targeted surveys indicated had dropped to zero before rebounding, underscoring that CMBS first-instar crawlers are exceedingly difficult to detect. Because of their minute size, cryptic life history, and ability to establish from low densities, our findings highlight the importance of thorough inspections of nursery stock for CMBS before shipping and planting in the landscape to prevent the continued spread of this pest. Our results also show the potential for CMBS populations to rebound after treatment with insecticides if some scales are left unharmed. Even with thorough inspections, it is possible that large infestations could outbreak in both landscape and nursery settings if even a single mating pair—or one mated female—is not detected on a single tree.
Author contributions
EW collected data, conducted analyzes, and wrote initial draft of the manuscript; SW and KC helped conceive study and provided guidance on analysis, writing, and editing subsequent drafts of the manuscript; KC also helped collect data. All authors contributed critically to the drafts and gave final approval for publication.
Data availability
All data supporting results will be made available on Zenodo upon official acceptance of the manuscript.
References
Addesso KM, Blalock A, O’Neal PA (2016) Japanese Maple scale activity and management in field nursery production. J Environ Hortic 34:41–46. https://doi.org/10.24266/0738-2898-34.2.41
Almeida RPP, Daane KM, Bell VA, et al (2013) Ecology and management of grapevine leafroll disease. Front Microbiol 4. https://doi.org/10.3389/fmicb.2013.00094
Brockerhoff EG, Kimberley M, Liebhold AM et al (2014) Predicting how altering propagule pressure changes establishment rates of biological invaders across species pools. Ecol 95:594–601. https://doi.org/10.1890/13-0465.1
Chase KD, Kelly D, Liebhold AM, Brockerhoff EG (2022) The role of propagule pressure in experimental bark beetle invasions. J Appl Ecol 60:342–352. https://doi.org/10.1111/1365-2664.14326
Crouch CD, Grady AM, Wilhelmi NP et al (2021) Oystershell scale: an emerging invasive threat to aspen in the southwestern US. Biol Invasions 23:2893–2912. https://doi.org/10.1007/s10530-021-02545-0
Drake JM, Lodge DM (2006) Allee effects, propagule pressure and the probability of establishment: risk analysis for biological invasions. Biol Invasions 8:365–375. https://doi.org/10.1007/s10530-004-8122-6
Fondren KM, Mccullough DG (2005) Phenology, natural enemies, and efficacy of horticultural oil for control of Chionaspis heterophyllae (Homoptera: Diaspididae) on Christmas tree plantations. J Econ Entomol 98:1603–1613. https://doi.org/10.1093/jee/98.5.1603
Fox J, Sanford W (2019) An R companion to applied regression. Sage Publications, Thousand Oaks, CA, USA
Griswold GH (1925) A study of the oyster-shell scale, Lepidosaphes Ulmi (L.), and One of Its parasites, Aphelinus Mytilaspidis Le B. Cornell University, Ithaca, NY
Gu M, Merchant M, Robbins J, Hopkins J (2014) Crape myrtle bark scale: a new exotic pest. Texas A&M Agrilife Extension
Herbert F (1924) The European elm scale in the west. United States Department of Agriculture
Hill MP, Clusella-Trullas S, Terblanche JS, Richardson DM (2016) Drivers, impacts, mechanisms and adaptation in insect invasions. Biol Invasionskei 18:883–891. https://doi.org/10.1007/s10530-016-1088-3
Hommay G, Alliaume A, Reinbold C, Herrbach E (2021) Transmission of Grapevine leafroll-associated virus-1 (Ampelovirus) and Grapevine virus A (Vitivirus) by the Cottony Grape Scale, Pulvinaria vitis (Hemiptera: Coccidae). Viruses 13:2081. https://doi.org/10.3390/v13102081
Leung B, Drake JM, Lodge DM (2004) Predicting invasions: propagule pressure and the gravity of allee effects. Ecol 85:1651–1660. https://doi.org/10.1890/02-0571
Liebhold AM, Tobin PC (2008) Population ecology of insect invasions and their management. Annu Rev Entomol 53:387–408. https://doi.org/10.1146/annurev.ento.52.110405.091401
Liebhold AM, Brockerhoff EG, Garrett LJ et al (2012) Live plant imports: the major pathway for forest insect and pathogen invasions of the US. Front Ecol Environ 10:135–143. https://doi.org/10.1890/110198
Liebhold AM, Yamanaka T, Roques A et al (2016) Global compositional variation among native and non-native regional insect assemblages emphasizes the importance of pathways. Biol Invasions 18:893–905. https://doi.org/10.1007/s10530-016-1079-4
Lockwood JL, Cassey P, Blackburn T (2005) The role of propagule pressure in explaining species invasions. Trends Ecol Evol 20:223–228. https://doi.org/10.1016/j.tree.2005.02.004
Meineke EK, Dunn RR, Sexton JO, Frank SD (2013) Urban warming drives insect pest abundance on street trees. PLoS ONE 8:2–8. https://doi.org/10.1371/journal.pone.0059687
Meurisse N, Rassati D, Hurley BP et al (2019) Common pathways by which non-native forest insects move internationally and domestically. J Pest Sci 92:13–27. https://doi.org/10.1007/s10340-018-0990-0
Morin R, Liebhold A, Tobin P et al (2007) Spread of beech bark disease in the eastern United States and its relationship to regional forest composition. Can J For Res 37:726–736. https://doi.org/10.1139/x06-281
Quesada CR, Witte A, Sadof CS (2018) Factors influencing insecticide efficacy against armored and soft scales. Horttechnology 28:267–275. https://doi.org/10.21273/HORTTECH03993-18
R Core Team (2022) R-4.2.2 for Windows. R Foundation for Statistical Computing, Vienna, Austria. https://www.r-project.org/. Accessed 4 June 2023.
Sitz R, Zerillo M, Snelling J, et al (2018) Drippy blight, a disease of Red Oaks in Colorado, U.S., produced from the combined effect of the scale insect Allokermes galliformis and the Bacterium Lonsdalea quercina subsp. Quercina. AUF 44. https://doi.org/10.48044/jauf.2018.012
Venette RC, Moon RD, Hutchison WD (2002) Strategies and statistics of sampling for rare individuals. Annu Rev Entomol 47:143–174. https://doi.org/10.1146/annurev.ento.47.091201.145147
Wang Z, Chen Y, Gu M et al (2016) Crapemyrtle bark scale: a new threat for crapemyrtles, a popular landscape plant in the U.S. Insects 7:1–19. https://doi.org/10.3390/insects7040078
Wang Z, Chen Y, Diaz R (2019) Temperature-dependent development and host range of crapemyrtle bark scale, Acanthococcus lagerstroemiae (Kuwana) (Hemiptera: Eriococcidae). Fla Entomol 102:181. https://doi.org/10.1653/024.102.0129
Wright ER, Chase KD, Littlejohn C et al (2023) Winter activity for crapemyrtle bark scale, an urban landscape pest. Horts 58:1237–1241. https://doi.org/10.21273/HORTSCI17341-23
Xie R, Wu B, Gu M, Qin H (2022) Life table construction for crapemyrtle bark scale (Acanthococcus lagerstroemiae): the effect of different plant nutrient conditions on insect performance. Sci Rep 12:11472. https://doi.org/10.1038/s41598-022-15519-6
Xie R, Wu B, Gu M et al (2021) Confirmation of new crapemyrtle bark scale (Acanthococcus lagerstroemiae) Hosts (Spiraea and Callicarpa) through DNA barcoding. Horts 56:1549–1551. https://doi.org/10.21273/HORTSCI16151-21
Acknowledgements
We thank Bartlett Tree Experts for access to space and providing study materials. This publication is a contribution of the Mississippi Agricultural and Forestry Experiment Station and based upon work supported by the National Institute of Food and Agriculture, U.S. Department of Agriculture, Hatch project under accession number 1025843.
Funding
This publication is a contribution of the Mississippi Agricultural and Forestry Experiment Station and based upon work supported by the National Institute of Food and Agriculture, U.S. Department of Agriculture, Hatch project under Accession Number 1025843.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
The authors declare no conflicts of interest.
Additional information
Communicated by Jay Rosenheim.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wright, E.R., Chase, K.D. & Ward, S.F. Plant-level establishment can result from a single female Acanthococcus lagerstroemiae propagule. J Pest Sci 97, 1081–1086 (2024). https://doi.org/10.1007/s10340-024-01792-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10340-024-01792-z