Abstract
The plant immune system has evolved to sense and respond to potential threats. When an insect attacks a plant, endogenous molecules called damage-associated molecular patterns (DAMPs) are released into the apoplast, triggering a cascade of intracellular signals. Extracellular DNA (eDNA) is a DAMP signal which activates the plant’s immune responses. However, our understanding of whether the detection of eDNA can lessen the damage caused by herbivores is still restricted. Here, we demonstrate that eDNA treatment in Arabidopsis leaves induced plant resistance against the herbivorous insect Frankliniella occidentalis without compromising the plant’s growth. The number of leaves, rosette diameter, fresh weight, and other growth-related parameters in eDNA-treated plants was comparable to water-treated plants. Besides, eDNA treatment reduced the feeding symptoms of F. occidentalis on Arabidopsis leaves. We further found that enhanced resistance in eDNA-treated plants was accompanied by callose accumulation in the affected area, and using the callose-deficient mutant pmr4-1, we demonstrated the positive role of callose in eDNA-induced resistance (eDNA-IR). Additionally, the induction in the jasmonic acid (JA)-signaling marker genes LOX2 and AOS, and the higher accumulation of Jasmonyl-isoleucine (JA-Ile) and JA revealed the role of jasmonates in eDNA-IR. Finally, we demonstrated that the JA signaling mediates callose deposition in eDNA-treated plants by using the JA response mutant jar1-1. These results advance our knowledge of the ability of eDNA to trigger plant resistance and the underlying mechanisms involved in eDNA-IR.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The plants’ innate immune system relies on rapid recognition of danger signals of different origins to respond to environmental stresses. Endogenous danger signals, also known as damage-associated molecular patterns (DAMPs), are elicitor molecules originated from the attacked host plant due to cell disruption. On the other hand, herbivore-associated molecular patterns (HAMPs) and pathogen-associated molecular patterns (PAMPs) are exogenous danger signals from the attacker (Gust et al. 2017). After the interaction between the plant and the attacker, these elicitor molecules are released to the apoplast and perceived by pattern recognition receptors (PRRs) that trigger defense responses through an array of intracellular signaling activations (Heil et al. 2012; Li et al. 2020). For example, the perception of HAMPs signals like the insect-derived elicitor inceptin (In11), present in the oral secretion (OS) of caterpillars, or the salivary peptide tetranins, derived from spider mites (Tetranychus urticae), enhanced defense responses against herbivory in the host plant (Iida et al. 2019; Steinbrenner et al. 2020). Recently, the importance of damage-self-recognition has been highlighted. However, unlike in mammals, not many DAMPs have been thoroughly studied in plants, and to date, only a few specific receptors have been identified for them (Quintana-Rodriguez et al. 2018). Therefore, more research is still needed to understand how self-danger signals are perceived and how plants react to them.
DAMP signals can be passively released to the apoplast due to cell leakage, known as primary DAMPs, or can be synthesized as secondary DAMPs, also known as phytocytokines. Examples of primary DAMPs are extracellular ATP (eATP), extracellular DNA (eDNA), and cell wall fragments like oligogalacturonides, cellobiose, and β‐glucans, and for secondary DAMPs, Pep1, PIP1, PSK, and other inducible peptides (Gust et al. 2017; Pastor et al. 2022). Upon recognition of DAMPs, plants activate a series of immune responses, including the burst of Ca2+ and reactive oxygen species (ROS), transcriptional changes, activation of mitogen-activated protein kinases (MAPKs), and phytohormone induction (Li et al. 2020). Studies showed that the exogenous application of DAMPs such as oligogalacturonides (OGs) or plant elicitor peptides like systemin, HypSys, Pep1, and its homologues Pep2 to Pep7 promotes resistance to a wide variety of necrotrophic and biotrophic pathogens (Ferrari et al. 2013; Huffaker et al. 2006; Pastor-Fernandez et al. 2020). Moreover, the application of extracellular nucleotides such as extracellular nicotinamide adenine dinucleotide (NAD), NAD phosphate (NADP), eATP, eDNA, eRNA, and single-stranded DNA oligodeoxynucleotides (ssODNs) triggers intracellular signaling, enhancing plant resistance against pathogens (Duran-Flores and Heil 2018; Kim et al. 2022; Mou 2017; Tanaka et al. 2014; Toum et al. 2020; Tripathi et al. 2018; Vega-Munoz et al. 2018). However, the potential fitness cost associated with induced resistance is another issue that should be considered in these studies. In nature, plants have acquired the ability to establish a balance between normal growth conditions and immunity. Hence, when defenses are induced, the metabolic resources are reallocated to defense-related metabolism, which can be accompanied by entailing plant growth costs (Hulten et al. 2006).
eDNA as a danger signal has recently gained much attention. Treating plants with self-eDNA fragments generates a series of general defense responses including Ca2+ signaling, ROS burst, phosphorylation of MAP kinases, and plasma membrane depolarization (Barbero et al. 2016, 2021; Duran-Flores and Heil 2018). Some studies reported that plants responses after self-eDNA treatments are stronger than after non-self-DNA exposure (Barbero et al. 2016; Carbajal-Valenzuela et al. 2022). For example, the levels of several phenylpropanoids and the ROS burst activation in tomato plants after self-eDNA treatments were up to tenfold higher than after non-self-eDNA (Carbajal-Valenzuela et al. 2022). On the other hand, other studies indicated that the perception of both molecules triggers similar responses, such as the induction of the expression level of defense-related genes and the accumulation of flavonoids and phenolic compounds (Rassizadeh et al. 2021; Vega-Munoz et al. 2018; Yakushiji et al. 2009). However, the mechanisms by which plants can discern between self-DNA and non-self-DNA remain elusive. Recently, a study on Arabidopsis thaliana exposed to extracellular self- and non-self-DNA showed that cells are capable of discriminating between both molecules by observing that non-self-DNA enters root tissues and within cells down to nuclei, while self-DNA remains outside (Chiusano et al. 2021). However, despite the existence of some hypotheses, no specific receptor for eDNA has been identified in plants (Bhat and Ryu 2016; Heil and Vega-Muñoz 2019). Although the initial stages of eDNA recognition are not yet fully understood, several studies have observed its capacity to elicit defense responses. For instance, we recently reported that the application of self-eDNA activates defense signaling and induces broad-range resistance against pathogens with different lifestyles such as Hyaloperonospora arabidopsidis, Botrytis cinerea, and Pseudomonas syringae and a phloem-feeding insect Myzus persicae (Rassizadeh et al. 2021). However, resistance generated by eDNA against cell-content feeder insects has not been investigated yet.
Plant defense responses against herbivory mainly depend on defense-related phytohormones such as jasmonic acid (JA), salicylic acid (SA), ethylene (ET), and abscisic acid (ABA) (Verhage et al. 2010), although it is usually correlated with the insect feeding style and the amount of damage (Howe and Jander 2008). For instance, plant resistance against cell-content feeder arthropods such as spider mites and thrips relies on the role of JA (Abe et al. 2008; Li et al. 2002), and almost 70% of transcriptomic changes in Arabidopsis plants upon thrips infestation are related to JA-responsive genes (De Vos et al. 2005). Additionally, the tomato JA-signaling mutant, Defenceless1 (def1), displayed high susceptibility to thrips feeding (Escobar-Bravo et al. 2017), and the exogenous application of JA or methyl jasmonate (MeJA) increased resistance against thrips in cotton and soybean plants (Abe et al. 2009; Selig et al. 2016).
Among the early defenses in plants, callose has been widely studied as a common trait following pathogen infection, albeit studies in plant–insect interactions are rather scarce (Wang et al. 2021). Callose deposition is one of the main plant resistance mechanisms acting as a physical barrier against the penetration of the attacker in plants (Luna et al. 2011; Wang et al. 2021). For example, the induction of callose deposition in Arabidopsis is necessary to stop pathogen growth upon chemical priming with I3CA and β-aminobutyric acid (BABA) (Gamir et al. 2018; Nishimura et al. 2003; Ton and Mauch-Mani 2004). Moreover, a recent report demonstrated a clear link between the induction of callose synthase activity (CalS) and a reduced expression of genes encoding hydrolytic enzymes. Very few studies have considered the interplay between JA levels and callose deposition. The induction of specific CalS genes and the JA marker gene PR-2 seems to be correlated with thrips herbivory (Qian et al. 2019). Furthermore, JA is necessary for callose deposition in response to the necrotrophic pathogens B. cinerea (Vicedo et al. 2009). However, to the best of our knowledge, the role of callose deposition in eDNA-IR has not been studied yet.
The western flower thrips, Frankliniella occidentalis (Thysanoptera: Thripidae), is one of the most severe pests in greenhouses and fields, with a global impact on a variety of vegetables and ornamental crops (Abe et al. 2008; Reitz et al. 2020). Thrips cause significant agricultural economic losses both directly through feeding and oviposition and indirectly by transmitting plant pathogenic tospovirus species (Rotenberg et al. 2015; Reitz et al. 2020). Thrips management is challenging due to specific characteristics, including their small size, short life cycle, high reproductive rate, and rapid adaptation to insecticides (Rotenberg et al. 2015; Steenbergen et al. 2018). Hence, the development of innovative and environmentally friendly methods for controlling thrips damage is crucial.
The extensive potential of eDNA in increasing plant resistance as a less toxic bioactive compound and an eco-friendly alternative for current chemical pesticides (Ferrusquía-Jiménez et al. 2020) encouraged us to use eDNA for controlling western flower thrips without environmental damage. In this study, we initially evaluated the possible negative impact of eDNA treatment on plant growth conditions. Furthermore, we investigated the eDNA effect as a DAMP signal on plant resistance to thrips attack. Our findings shed some light on the mechanism of eDNA-IR to insect pest F. occidentalis.
Materials and methods
Plant materials and insect infestation
Seeds of wild-type Arabidopsis thaliana (Col-0) and mutants in the same background including callose-deficient powdery mildew resistant-4 (pmr4-1) (Nishimura et al. 2003), Arabidopsis non-expressor of PR genes1 mutant (npr1-1) (Cao et al. 1997), double mutants pmr4-npr1 (Nishimura et al. 2003), and the JA-signaling defective mutant (jar1-1) (Matthes et al. 2010) were cultivated under short-day conditions (photoperiod of 8 h light at 22 °C and 16 h dark at 19 °C, with 70% relative humidity). Five-week-old plants were used for experiments.
The western flower thrips colony was maintained and reared in octagonal plastic bottles with fresh bean pods. Fifteen larvae were placed in the central leaflets, which were pre-treated with 150 ng µL–1 of eDNA and distilled water as control, 24 h before infestation. Infested plants were kept in the chamber under 16 h light/8 h dark at 25 °C for 4 days. Subsequently, the area of feeding scars (silver damage) was measured using GIMP software (2.10.32). Three separate experiments were performed using 10–12 replicates per treatment.
DNA extraction and treatment
Plant leaves from A. thaliana (Col-0) were harvested for genomic DNA extraction according to Duran-Flores and Heil (2018) with some changes as we described in our previous study (Rassizadeh et al. 2021). Briefly, 1 g of grinned plant leaves was kept in 4 ml of extraction buffer (200 mM Tris–HCl pH 7.5, 250 mM NaCl, 25 mM EDTA-Na2, and 0.5% SDS) at room temperature for 45 min. Samples were centrifuged at 10 °C and 14000 rpm for 10 min, and isopropanol was added. Samples were kept at −20 °C for 1 h following by another centrifugation, and thereafter, the pellet was rinsed twice with 70% ethanol, dried and dissolved in 50 µl of nuclease-free water. DNA extract purity was assessed at 260 nm on a NanoDrop (Thermo Scientific, Wilmington, DE, USA). Fifteen minutes of sonication at a maximum amplitude of 40–50 kHz was carried out to obtain DNA fragments using an ultrasonic bath (NAHITA 610/6 ultrasonic). Fragments of eDNA (150 ng µL–1) were sprayed on five-week-old plants’ leaves. (The final applied eDNA volume was approximately 200 µL per plant.)
RNA extraction and RT-qPCR analysis
Thirty minutes after infestation, leaves were harvested and grinned in liquid nitrogen. Plants’ total RNA was extracted according to the instructions of Kiefer et al. (2000) with some modifications. Briefly, 1 ml of Trizol was added to 500 mg of grinned plant material. Samples were centrifuged at 4 °C and 13000 rpm for 5 min, the supernatant was collected in a new tube, and 220 µl of CHCl3 was added. Another centrifugation was performed, the supernatant was collected in a new tube, and 350 µl aliquot of isopropanol and 350 µl of 0.8 M monosodium citrate anhydrous/1.2 mM NaCl were added and mixed. After centrifugation, the supernatant was removed, and the pellet was rinsed twice with 70% ethanol, dried and dissolved in 50 µl of nuclease-free water. Complementary DNA (cDNA) was synthesized with the high-capacity cDNA reverse transcription kit (Applied Biosystems). qRT-PCR was performed with the SYBR® Premix Ex Taq™ (TaKaRa, Kyoto, Japan) using a StepOne instrument (Applied Biosystems). Three biological replicates, each a pool of three individual plants, and two technical replicates were used for analysis. Arabidopsis UBIQUITIN21 (At5g25760) and PP2A (At1g13320) were used as housekeeping genes to normalize the expression values. The relative expression level was calculated via the 2–ΔΔCT method. Specific qRT-PCR primers that were used in this study are listed in supplementary Table S1.
Callose staining and quantification
Aniline blue staining was used to determine callose deposition level as described by Ton and Mauch-Mani (2004). Leaves were kept overnight in 95% ethanol to remove the chlorophyll, stained in 0.05% aniline blue (in 0.1 M Na2HPO4 phosphate buffer, pH 8), and kept overnight before being mounted on microscope slides. Pictures were taken using a fluorescence microscope (Olympus-IX71, Olympus CO., Japan). Accumulation of callose was quantified using GIMP software.
Targeted HPLC–MS for hormonal analysis
For hormonal analyses, 250 mg of fresh material sample was powdered in liquid nitrogen 30 min after thrips infestation and transferred to a 2-ml microcentrifuge tube. Next, a pool of internal standards including salicylic acid-d5 (SA-d5), dehydrojasmonic acid (dhJA), and jasmonate-isoleucine-d6 (JA-Ile-d6) was added to the samples, along with 990 µL of extraction buffer (H2O: MeOH + 0.01% HCOOH (90:10)). Crystal balls were added to the tubes, which were then placed in a shaker for 30 s. The samples were incubated on ice for 30 min, followed by centrifugation at 15000 rpm for 10 min at 4 °C. The supernatant was collected in a new tube and kept at −20 °C for 24 h before being filtrated using a 0.2-µm regenerated cellulose filter (Phenomenex). Five biological replicates, each a pool of two individual plants, were used for analysis. A quantification was performed by using external calibration curves with each pure chemical in an ACQUITY ultra-performance liquid chromatography system (UPLC; Waters, Mildford, MA, USA) connected to a triple quadrupole mass spectrometer (TQD, Waters, Manchester, UK). The HPLC Kinetex C18 analytical column with a 5 μm particle size of 2.1 100 mm (Phenomenex) was used for the LC separation. The chromatographic conditions and process were performed according to Sánchez-Bel et al. (2018).
Statistical analyses
Before turning to further analysis, Shapiro–Wilk’s test was carried out to verify the normality of the collected data. Thereafter, for those which their normality was confirmed, one-way analysis of variance (ANOVA) followed by Fisher’s least significant difference (LSD) test was performed. Concerning the collected data for which normality was not affirmed, the Kruskal–Wallis test followed by pairwise Mann–Whitney for multiple comparisons was performed. All such tests were carried out using the R programming language (version 4.2.2). The R package ggplot2 (version 3.4.0) (Wickham 2016) was employed for visualization purposes. P-value of < 0.05 was considered as statistically significant.
Results
eDNA treatment does not limit plant growth
We first investigated the potential fitness cost caused by eDNA to contrast the observations with other elicitors (Duran-Flores and Heil 2018; Hulten et al. 2006). For this purpose, we treated one-week-old Col-0 seedlings with 150 ng µl−1 of eDNA extracted from Arabidopsis Col-0 plants. Then, weekly treatments continued for one group with the same concentration of eDNA and the other with water. Control plants were treated with water from the first week. After 3 months, the growth-related parameters including the number of leaves, the size of the rosette, plant height, the number of siliques, plant fresh weight, and seeds weight as well as the leaves’ chlorophyll content were measured. We did not observe growth-limiting effects in plants under eDNA treatment. In most of the measured parameters, no significant differences were detected between groups. However, the seed weight and plant fresh weight in eDNA-treated plants were higher compared to the water-treated plants (Fig. 1a–g).
Box plot diagrams showing the effect of eDNA (conc. 150 ng μl−1) on the growth-related parameter after 3 months of weekly treatment, a number of leaves (ANOVA, LSD test; n = approx. 20), b size of the rosette (Kruskal–Wallis, Mann–Whitney test; n = approx. 26), c plant fresh weight (ANOVA, LSD test; n = approx. 12), d plant height (ANOVA, LSD test; n = approx. 16), e chlorophyll content (Kruskal–Wallis, Mann–Whitney test; n = approx. 16), f the number of siliques (Kruskal–Wallis, Mann–Whitney test; n = approx. 16), and g the seed weight per plant (Kruskal–Wallis, Mann–Whitney test; n = approx. 5). Kruskal–Wallis test, pairwise Mann–Whitney for the nonparametric data, and ANOVA, Fisher’s test (LSD) for normal data were performed, respectively. P-value of < 0.05 was considered as statistically significant. Different letters indicate significant differences among treatments. The results are derived from two independent experiments
eDNA induces resistance against thrips
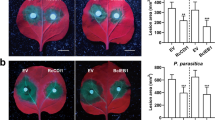
To investigate whether eDNA application can induce resistance against F. occidentalis, we inoculated 15 thrips larvae on each eDNA-treated and water-treated plant 24 h after treatments and kept them during 4 days for herbivory. On day four after the infestation, the area of the silver damage caused by thrips feeding was measured. Although no difference in the number of scars per leaf between eDNA-treated and control plants was observed (Fig. 2a), eDNA-treated plants displayed a statistically significant reduction in the silver damage area compared to the water-treated plants (Fig. 2b), showing a reduction of the symptoms.
Box plot diagrams showing a the number of silver damage per leaf in control (water-treated) and eDNA (eDNA-treated) plants 4 days post-infestation (Kruskal–Wallis test; n = 35), b the percentage of thrips feeding area (Silver damage) per leaf (Kruskal–Wallis test; n = 77); P-value of < 0.05 was considered as statistically significant, “ns” indicates not significant and asterisk indicates significant difference, and c image of silver damage on the Col-0 leaf. The experiment was repeated three times yielding to the similar results
Callose plays a role in eDNA-IR
Elevated CO2 levels trigger callose deposition in Phaseoulus vulgaris against F. occidentalis (Qian et al. 2019). To determine the role of cell wall reinforcement in eDNA-IR, we measured the callose accumulation in eDNA-treated and control plants. At 96-h post-thrips infestation (hpi), eDNA-treated infested plants showed significantly enhanced callose deposition than control infested plants, being not significant at earlier timepoints (Fig. 3a). Thereafter, we used the callose-deficient mutant (pmr4-1) to elucidate the importance of callose deposition in eDNA-IR against thrips. Since the PR-1 gene is constitutively expressed in this mutant (Nishimura et al. 2003), we added the Arabidopsis non-expressor of PR genes1 (npr1) and the double mutant pmr4-npr1 to our study. Upon eDNA treatment, none of the mutants showed a reduction in the feeding area compared to water-treated plants, whereas wild-type plants displayed a significant reduction (Fig. 3b). Additionally, the absence of callose in the callose-deficient mutants (Fig. 3c) supports our hypothesis, indicating the role of callose in eDNA-IR against thrips. Furthermore, higher susceptibility observed in pmr4-npr1, in both control and eDNA-treated plants, might suggest a potential role of SA in basal defense resistance against thrips.
a Percentage of callose accumulation in control (water-treated) and eDNA (eDNA-treated) Arabidopsis Col-0 plants after thrips infestation for 96 h (t-test; n = approx. 25), b percentage of thrips feeding area, 4 days post infestation (dpi) in Arabidopsis Col-0, callose-deficient mutant pmr4-1, SA impaired mutant npr1-1, and double mutant pmr4-npr1 upon eDNA treatment, and c image of callose deposition in control and eDNA-treated Col-0 and pmr4-1 mutant leaves 4 dpi. Bars represent mean ± standard error. Kruskal–Wallis, Mann–Whitney test; n = approx. 30 P-value of < 0.05 was considered as statistically significant, and asterisks and different letters indicate significant differences. The experiment was repeated three times separately
Defense-related pathways involved in eDNA-IR
To gain a better understanding of the potential mechanisms involved in eDNA-IR, we conducted analyses of the gene expression and hormone levels in the main defense pathways. Additionally, to evaluate the plant’s response over time, we analyzed the expression levels of JA-related genes at different timepoints following infestation (30 min, 1, 7, and 24 hpi). Accordingly, eDNA-treated plants showed an induction in the expression levels of marker genes at the earlier timepoints (30 min and 1hpi), which then decreased at later timepoints (7 and 24 hpi). On the contrary, in control plants, the upward trend of gene expression was initiated after 7 h of infestation (Supplementary Fig. S1), suggesting that eDNA treatment can accelerate the plant’s response to the infestation. Hence, 30-min post-thrips infestation, the expression levels of two JA-related marker genes, allene oxide synthase (AOS) and lipoxygenase2 (LOX2), ABA biosynthesis marker gene, nine-cis-epoxycarotenoid dioxygenase 3 (NCED3), and pathogenesis-related gene-1 (PR-1) as a marker for SA pathway with and without thrips infestation were quantified. The AOS, LOX2, and NCED3 transcript levels showed a significant upregulation in eDNA-treated plants infested with herbivore thrips, while the relative expression level of PR-1 remained unaltered. Furthermore, eDNA-treated plants in the absence of pests and the infested-control plants displayed a reduction of NCED3 expression (Fig. 4a–d). Subsequently, as determined by the hormonal profile, upon encountering the pest challenge, eDNA-treated plants showed an increase in the level of JA-Ile, JA, and ABA content. In contrast, the level of SA in all treatments remained unaltered (Fig. 4e–h).
a, b, c, and d Quantitative reverse transcription-polymerase chain reaction analysis of JA biosynthesis marker genes LOX2 and AOS, ABA biosynthesis marker gene NCED3, and SA marker gene PR-1 in control (water-treated), eDNA (eDNA-treated), control infested (water-treated infested with thrips) and eDNA infested (eDNA-treated infested with thrips), 30 min post-infestation (ANOVA, LSD test; n = 3). e, f, g, and h The hormone quantitative levels (ng g−1 fresh weight) of JA-Ile, JA, ABA, and SA without and with herbivore (thrips), 30 min post-infestation measured by targeted HPLC–MS analysis (ANOVA, LSD test; n = 5). Bars represent mean ± standard error. P-value of < 0.05 was considered as statistically significant, and different letters above bars indicate significant differences among treatments
JA mediates callose accumulation in eDNA-IR
We, therefore, hypothesized that JA could mediate callose accumulation in eDNA-IR against thrips. Consistent with this hypothesis, we first checked the resistance phenotype in the JA-insensitive mutant jar1-1. This gene encodes an amino synthetase protein that catalyzes the formation of JA-Ile, and the exogenous application of JA-Ile complements the jar1-1 mutation (Staswick and Tiryaki 2004). On day 4 after infestation, jar1-1 plants showed a larger lesion area than wild-type plants. As expected, wild-type eDNA-treated plants displayed a reduction of the symptoms compared to control plants, but the thrips feeding area in the jar1-1 mutant was similar in both eDNA- and water-treated plants. Hence, jar1-1 was insensitive to eDNA-IR against thrips (Fig. 5a). Finally, we tested whether callose deposition is compromised in jar1-1 mutant plants. On day 4 after infestation, Col-0 plants displayed high callose accumulation in eDNA-treated plants compared to the control. However, in the JA-deficient mutant, neither the control nor eDNA-treated plants showed any callose accumulation (Fig. 5b).
Box plot diagrams showing a the percentage of thrips feeding area in Arabidopsis Col-0 and JA-deficient mutant jar1-1 in eDNA (eDNA-treated) and control (water-treated) plants 4 dpi (Kruskal–Wallis, Mann–Whitney; P-value < 0.05, n = approx. 90), b quantification of the callose area in Col-0 and jar1-1 in eDNA-treated and control plants 4 dpi (Kruskal–Wallis, Mann–Whitney; n = approx. 40). P-value of < 0.05 was considered as statistically significant, and different letters above bars indicate significant differences among treatments. The results are derived from two independent experiments.
Exogenous application of JA-Ile restores eDNA-IR in jar1 mutant
To corroborate the importance of JA signaling in eDNA-IR, we exogenously applied JA-Ile to jar1-1 plants to complement the lower JA-Ile levels (Staswick and Tiryaki 2004). Hence, we treated adult jar1-1 and wild-type Arabidopsis plants with eDNA or water and infested them with thrips larvae 24 h after treatment. Then, we daily treated half of the control and eDNA-treated plants with JA-Ile 100 μM for 4 days. The percentage of the damaged area in Col-0 showed that plants with individual or combined eDNA and JA-Ile treatments have the same level of resistance compared to control plants (Fig. 6a). For jar1-1, eDNA and JA-Ile individual applications failed to protect the mutant, but the combination of both treatments restored the eDNA-IR (Fig. 6b). Finally, we quantified the callose accumulation in wild-type and jar1-1 plants to confirm the role of callose deposition in eDNA-IR. Wild-type plants displayed higher levels of callose in eDNA-treated plants, irrespective of JA-Ile treatments (Fig. 6c), and consistent with our hypothesis, the simultaneous application of eDNA and JA-Ile in jar1-1 mutant plants triggered significantly higher levels of callose than control or individual eDNA- or JA-Ile treatments (Fig. 6d), indicating that JA signaling is necessary for callose accumulation in eDNA-IR.
Box plot diagrams showing a, b the percentage of thrips feeding area upon water (as control), eDNA, JA-Ile and combination of eDNA/JA-Ile treatment in Arabidopsis Col-0, and JA deficient mutant jar1-1, 4 dpi (Kruskal–Wallis, Mann–Whitney; n = approx. 90) and c, d quantification of the callose area in Arabidopsis Col-0 and JA-deficient mutant jar1-1, 4 dpi (Kruskal–Wallis, Mann–Whitney; n = approx. 50). P-value of < 0.05 was considered as statistically significant, and different letters above bars indicate significant differences among treatments. The results are derived from two independent experiments
Discussion
This study aims to investigate the mechanisms behind eDNA as a DAMP signal in the induction of plant defense response. Our findings indicate that eDNA treatment does not negatively impact the plants’ normal growth. Besides, we also show that eDNA induces Arabidopsis plant resistance against herbivorous thrips (F. occidentalis). The perception of DAMPs as danger signals is a crucial strategy of the plant immune system, activating defense mechanisms and inducing resistance to further stress (Gust et al. 2017; Quintana-Rodriguez et al. 2018). While the study of eDNA as a DAMP signal in plant immunity is expanding, we still know little about how it is perceived and processed inside cells (Barbero et al. 2016, 2021; Carbajal-Valenzuela et al. 2022; Duran-Flores and Heil 2018; Vega-Munoz et al. 2018). Future experiments are needed to enhance our understanding of the mechanisms behind eDNA-IR.
We showed that plant growth was not negatively affected by eDNA treatment. More specifically, we demonstrated that the level of growth-related parameters in Col-0 Arabidopsis treated with eDNA was comparable to water-treated plants. Although previous studies have reported that eDNA induces root inhibition in organisms from different phyla, not many have demonstrated inhibition on the aerial part (Duran-Flores and Heil 2015; Mazzoleni et al. 2015a, b). For example, self-DNA treatments induced lettuce defenses and inhibited seed germination and root growth (Vega-Munoz et al. 2018). Also, a recent study showed that single-stranded DNA oligodeoxynucleotides inhibited A. thaliana root growth by around 50% in a concentration-dependent manner (Toum et al. 2020). However, our findings suggest that the perception of eDNA in aerial parts, 1 week after germination, does not suppress growth but induces seed production. Consistent with our results, eDNA treatment in Arabidopsis cells promoted plant growth and root development when used as phosphorus source (Paungfoo-Lonhienne et al. 2010). Additionally, pepper plants treated with self- and non-self-RNAs showed no difference in fruit yield at the end of the growing season (Kim et al. 2022). Our results, for the first time, show the Arabidopsis response to long-term eDNA treatments, in different morphological and physiological parameters. Nevertheless, more research is needed to examine the potential impact of eDNA source and concentration on plant growth and whether this effect is related to its role in plant immunity.
Our results demonstrate that eDNA treatment induces plant resistance against thrips (F. occidentalis). Here, we showed a reduction in the size of the thrips feeding symptoms on eDNA-treated plants, while the number of scars remained unchanged in both treatments. Thrips damage plant tissues by piercing and sucking the epidermal/mesophyll plant cells, resulting in silvery symptoms in the affected area and acting as a vector of tospoviruses, causing extensive damage to plants (Reitz 2009; Reitz et al. 2020). Therefore, developing green methods to control thrips damage is needed. The capacity of eDNA as a danger signal to induce resistance was confirmed in previous studies, where eDNA treatments induced resistance against a broad range of pathogens and a phloem-sucking insect Myzus persicae (Duran-Flores and Heil 2018; Hawes et al. 2011; Rassizadeh et al. 2021; Wen et al. 2009). However, the ability of eDNA to induce resistance against insects with different feeding styles and the mechanisms behind needs to be studied more.
In the present study, we provide evidence that eDNA enhances plant resistance through callose deposition. Our results indicated that high resistance in eDNA-treated plants against thrips is always accompanied by a high accumulation of callose in the damaged area. Callose deposition is one of the main resistance mechanisms acting as a physical barrier against the penetration of attackers (Hao et al. 2008; Luna et al. 2011; Wang et al. 2021). Here, we showed a significant induction in the callose accumulation 96 h after thrips infestation in eDNA-treated plants. We hypothesized that callose deposition decreases the symptoms by preventing the insect from continuously feeding on eDNA-treated plants, explaining why we could not find differences in the number of scars but a significant reduction in the size of these lesions. Consistent with this hypothesis, another study reported the importance of callose in Phaseolus vulgaris resistance against F. occidentalis, enhancing the expression levels of two callose synthases genes and the callose deposition (Qian et al. 2019). We further confirm the role of callose when eDNA treatment in the callose-deficient mutant pmr4-1 failed to induce resistance against thrips. Surprisingly, pmr4-1 plants displayed comparable susceptibility against thrips to wild-type plants, which might be correlated with the constitutive expression of SA-inducible marker gene PR-1 in this mutant (Nishimura et al. 2003). Moreover, high susceptibility to thrips in mutant pmr4-npr1 could indicate the possible role of SA in basal resistance against thrips. The defense mechanisms against thrips may involve different basal and induced defense responses, and further research is needed to determine their contributions.
We further found that thrips infestation elevates JA and ABA levels in eDNA-treated plants. Plants activate different signaling pathways depending on the feeding behavior of insects. JA signaling plays a prominent role in resistance against chewing and pierce-sucking herbivores (Abe et al. 2009; Howe et al. 2018). Our findings showed a significant upregulation of JA-biosynthesis genes AOS, LOX2, and hormonal content in eDNA-treated plants, indicating a crucial role of the JA pathway in the eDNA-IR against thrips. Previous studies on Arabidopsis genome arrays reported the relationship between thrips feeding, upregulation of JA-related genes, and the JA content (Abe et al. 2008; De Vos et al. 2005). For instance, JA levels are elevated in Arabidopsis plants after thrips infestation, and the insensitive JA mutant coi1-1 showed higher susceptibility and increased oviposition than wild-type (WT) plants (Abe et al. 2008; Abe et al. 2009). While our study mainly focuses on the role of JA in eDNA-IR, we also observed changes in ABA transcripts and hormonal levels, suggesting a possible involvement of ABA in eDNA-IR. ABA signaling is an important component of plant immunity, and it is involved in tomato plant resistance against thrips (Escobar-Bravo et al. 2018). Additionally, ABA positively regulates JA-dependent defense responses against herbivores in systemic tissues (Nguyen et al. 2016). A recent study found that self-eRNA induces both ABA- and JA-signaling marker genes CaDEF and CaCHI2, activating pepper plant immunity against bacterial and viral pathogens (Kim et al. 2022). Here, in eDNA-treated plants, we showed an increase in the ABA key biosynthesis marker gene NCED3 upon thrips infestation. However, further research using mutants deficient in ABA and JA could help expand our understanding of this possible synergistic interaction induces plant resistance to herbivores at the molecular level.
Finally, we demonstrated that JA mediates callose deposition, enhancing resistance against thrips. The JA-insensitive mutant jar1-1 displayed high susceptibility to thrips attack either in control or eDNA-treated plants. jar1-1 plants, due to a mutation in the JAR1 gene, contain reduced JA-Ile levels, playing an essential role in jasmonate signaling (Staswick and Tiryaki 2004). Here, we showed that the simultaneous application of eDNA and JA-Ile in jar1-1 plants could effectively restore the induced-resistance phenotype to thrips and correlated with the higher callose accumulation in both Col-0 and jar1-1 plants. Therefore, we hypothesized that a functional JA pathway is required for callose accumulation in eDNA-IR against thrips. Interestingly, intact JA signaling is essential for proper callose deposition in response to the necrotrophic pathogen Botrytis cinerea in tomato plants (Vicedo et al. 2009). However, additional signaling pathways might be involved in callose biosynthesis. For example, a study showed the role of ABA-signaling pathway in callose formation against the piercing-sucking pest brown planthopper in rice (Hao et al. 2008), and the link between ABA and JA in pathogen-induced callose deposition was demonstrated with the Arabidopsis ocp3 mutant (García-Andrade et al. 2011). Hence, the process from the eDNA perception to callose accumulation in plant cells might be the result of the cooperation of multiple pathways, which should be considered in future experiments.
Overall, this study considers the involved signaling pathways and defensive mechanisms in eDNA-IR against rasping-sucking pest thrips. Our findings, which are the first indication of the mechanisms of eDNA-IR against insects, contribute to a deeper understanding of the potential of eDNA as a tool for inducing plant resistance. However, the plant defensive response triggered by insect damage is surprisingly complex. Future experiments will help to clarify the possible interaction between eDNA-IR-related signaling pathways.
Author contributions
All authors contributed to the study conception and design. Experiments designed by VF, JG, and LR. LR, RC, and JG performed the experiments. Data collection and analysis were performed by LR. The manuscript was written by LR, VF, and JG.
Data availability
The datasets generated during the current study are available from the corresponding author on reasonable request.
References
Abe H, Ohnishi J, Narusaka M, Seo S, Narusaka Y, Tsuda S, Kobayashi M (2008) Function of jasmonate in response and tolerance of Arabidopsis to thrip feeding. Plant Cell Physiol 49:68–80. https://doi.org/10.1093/pcp/pcm168
Abe H et al (2009) Jasmonate-dependent plant defense restricts thrips performance and preference. BMC Plant Biol 9:97. https://doi.org/10.1186/1471-2229-9-97
Barbero F, Guglielmotto M, Capuzzo A, Maffei ME (2016) Extracellular self-DNA (esDNA), but not Heterologous plant or insect DNA (etDNA), induces plasma membrane depolarization and calcium signaling in lima bean (Phaseolus lunatus) and maize (Zea mays). Int J Mol Sci. https://doi.org/10.3390/ijms17101659
Barbero F, Guglielmotto M, Islam M, Maffei ME (2021) Extracellular fragmented self-DNA is involved in plant responses to biotic stress. Front Plant Sci 12:686121. https://doi.org/10.3389/fpls.2021.686121
Bhat A, Ryu C-M (2016) Plant perceptions of extracellular DNA and RNA. Mol Plant 9:956–958. https://doi.org/10.1016/j.molp.2016.05.014
Cao H, Glazebrook J, Clarke JD, Volko S, Dong X (1997) The Arabidopsis NPR1 gene that controls systemic acquired resistance encodes a novel protein containing ankyrin repeats. Cell 88:57–63. https://doi.org/10.1016/s0092-8674(00)81858-9
Carbajal-Valenzuela IA et al (2022) Response of plant immunity markers to early and late application of extracellular dna from different sources in tomato (Solanum lycopersicum). Agriculture 12:1587. https://doi.org/10.3390/agriculture12101587
Chiusano ML et al (2021) Arabidopsis thaliana response to extracellular DNA: self versus nonself exposure. Plants 10:1744. https://doi.org/10.3390/plants10081744
De Vos M et al (2005) Signal signature and transcriptome changes of Arabidopsis during pathogen and insect attack. Mol Plant Microbe Interact 18:923–937. https://doi.org/10.1094/mpmi-18-0923
Duran-Flores D, Heil M (2015) Growth inhibition by self-DNA: a phenomenon and its multiple explanations. New Phytol 207:482–485. https://doi.org/10.1111/nph.13542
Duran-Flores D, Heil M (2018) Extracellular self-DNA as a damage-associated molecular pattern (DAMP) that triggers self-specific immunity induction in plants. Brain Behav Immun 72:78–88. https://doi.org/10.1016/j.bbi.2017.10.010
Escobar-Bravo R, Klinkhamer PGL, Leiss KA (2017) Induction of jasmonic acid-associated defenses by thrips alters host suitability for conspecifics and correlates with increased trichome densities in tomato. Plant Cell Physiol 58:622–634. https://doi.org/10.1093/pcp/pcx014
Escobar-Bravo R, Ruijgrok J, Kim HK, Grosser K, Van Dam NM, Klinkhamer PGL, Leiss KA (2018) Light intensity-mediated induction of trichome-associated allelochemicals increases resistance against thrips in tomato. Plant Cell Physiol 59:2462–2475. https://doi.org/10.1093/pcp/pcy166
Ferrari S, Savatin DV, Sicilia F, Gramegna G, Cervone F, Lorenzo GD (2013) Oligogalacturonides: plant damage-associated molecular patterns and regulators of growth and development. Front Plant Sci 4:49. https://doi.org/10.3389/fpls.2013.00049
Ferrusquía-Jiménez NI, Chandrakasan G, Torres-Pacheco I, Rico-Garcia E, Feregrino-Perez AA, Guevara-González RG (2020) Extracellular DNA: a relevant plant damage-associated molecular pattern (DAMP) for crop protection against pests—a review. J Plant Growth Regul 40:451–463. https://doi.org/10.1007/s00344-020-10129-w
Gamir J, Pastor V, Sanchez-Bel P, Agut B, Mateu D, Garcia-Andrade J, Flors V (2018) Starch degradation, abscisic acid and vesicular trafficking are important elements in callose priming by indole-3-carboxylic acid in response to Plectosphaerella cucumerina infection. Plant J 96:518–531. https://doi.org/10.1111/tpj.14045
García-Andrade J, Ramírez V, Flors V, Vera P (2011) Arabidopsis ocp3 mutant reveals a mechanism linking ABA and JA to pathogen-induced callose deposition. Plant J 67:783–794. https://doi.org/10.1111/j.1365-313X.2011.04633.x
Gust AA, Pruitt R, Nurnberger T (2017) Sensing danger: key to activating plant immunity. Trends Plant Sci 22:779–791. https://doi.org/10.1016/j.tplants.2017.07.005
Hao P et al (2008) Herbivore-induced callose deposition on the sieve plates of rice: an important mechanism for host resistance. Plant Physiol 146:1810–1820. https://doi.org/10.1104/pp.107.111484
Hawes MC, Curlango-Rivera G, Wen F, White GJ, VanEtten HD, Xiong Z (2011) Extracellular DNA: the tip of root defenses? Plant Sci 180:741–745. https://doi.org/10.1016/j.plantsci.2011.02.007
Heil M, Vega-Muñoz I (2019) Nucleic acid sensing in mammals and plants: facts and caveats. Int Rev Cell Mol Biol 345:225–285. https://doi.org/10.1016/bs.ircmb.2018.10.003
Heil M, Ibarra-Laclette E, Adame-Alvarez RM, Martinez O, Ramirez-Chavez E, Molina-Torres J, Herrera-Estrella L (2012) How plants sense wounds: damaged-self recognition is based on plant-derived elicitors and induces octadecanoid signaling. PLoS ONE 7:e30537. https://doi.org/10.1371/journal.pone.0030537
Howe GA, Jander G (2008) Plant immunity to insect herbivores. Annu Rev Plant Biol 59:41–66. https://doi.org/10.1146/annurev.arplant.59.032607.092825
Howe GA, Major IT, Koo AJ (2018) Modularity in jasmonate signaling for multistress resilience. Annu Rev Plant Biol 69:387–415. https://doi.org/10.1146/annurev-arplant-042817-040047
Huffaker A, Pearce G, Ryan CA (2006) An endogenous peptide signal in Arabidopsis activates components of the innate immune response. Proc Natl Acad Sci USA 103:10098–10103. https://doi.org/10.1073/pnas.0603727103
Iida J et al (2019) Tetranins: new putative spider mite elicitors of host plant defense. New Phytol 224:875–885. https://doi.org/10.1111/nph.15813
Kiefer E, Heller W, Ernst D (2000) A simple and efficient protocol for isolation of functional RNA from plant tissues rich in secondary metabolites. Plant Mol Biol Rep18:33–39. https://doi.org/10.1007/BF02825291
Kim D, Riu M, Oh SK, Ryu CM (2022) Extracellular self-RNA: a danger elicitor in pepper induces immunity against bacterial and viral pathogens in the field. Front Plant Sci 13:864086. https://doi.org/10.3389/fpls.2022.864086
Li C, Williams MM, Loh YT, Lee GI, Howe GA (2002) Resistance of cultivated tomato to cell content-feeding herbivores is regulated by the octadecanoid-signaling pathway. Plant Physiol 130:494–503. https://doi.org/10.1104/pp.005314
Li Q, Wang C, Mou Z (2020) Perception of damaged self in plants. Plant Physiol 182:1545–1565. https://doi.org/10.1104/pp.19.01242
Luna E, Pastor V, Robert J, Flors V, Mauch-Mani B, Ton J (2011) Callose deposition: a multifaceted plant defense response. Mol Plant Microbe Interact 24:183–193. https://doi.org/10.1094/mpmi-07-10-0149
Matthes MC, Bruce TJA, Ton J, Verrier PJ, Pickett JA, Napier JA (2010) The transcriptome of cis-jasmone-induced resistance in Arabidopsis thaliana and its role in indirect defence. Planta 232:1163–1180. https://doi.org/10.1007/s00425-010-1244-4
Mazzoleni S et al (2015a) Inhibitory and toxic effects of extracellular self-DNA in litter: a mechanism for negative plant–soil feedbacks? New Phytol 205:1195–1210. https://doi.org/10.1111/nph.13121
Mazzoleni S et al (2015b) Inhibitory effects of extracellular self-DNA: a general biological process? New Phytol 206:127–132. https://doi.org/10.1111/nph.13306
Mou Z (2017) Extracellular pyridine nucleotides as immune elicitors in Arabidopsis. Plant Signal Behav 12:e1388977. https://doi.org/10.1080/15592324.2017.1388977
Nguyen D, Rieu I, Mariani C, van Dam NM (2016) How plants handle multiple stresses: hormonal interactions underlying responses to abiotic stress and insect herbivory. Plant Mol Biol 91:727–740. https://doi.org/10.1007/s11103-016-0481-8
Nishimura MT, Stein M, Hou BH, Vogel JP, Edwards H, Somerville SC (2003) Loss of a callose synthase results in salicylic acid-dependent disease resistance. Science 301:969–972. https://doi.org/10.1126/science.1086716
Pastor V, Cervero R, Gamir J (2022) The simultaneous perception of self- and non-self-danger signals potentiates plant innate immunity responses. Planta 256:10. https://doi.org/10.1007/s00425-022-03918-y
Pastor-Fernandez J, Gamir J, Pastor V, Sanchez-Bel P, Sanmartin N, Cerezo M, Flors V (2020) Arabidopsis plants sense non-self peptides to promote resistance against Plectosphaerella cucumerina. Front Plant Sci 11:529. https://doi.org/10.3389/fpls.2020.00529
Paungfoo-Lonhienne C, Lonhienne TGA, Mudge SR, Schenk PM, Christie M, Carroll BJ, Schmidt S (2010) DNA is taken up by root hairs and pollen, and stimulates root and pollen tube growth. Plant Physiol 153:799–805. https://doi.org/10.1104/pp.110.154963
Qian L et al (2019) Elevated CO2 enhances the host resistance against the western flower thrips, Frankliniella occidentalis, through increased callose deposition. J Pest Sci 94:55–68. https://doi.org/10.1007/s10340-019-01123-7
Quintana-Rodriguez E, Duran-Flores D, Heil M, Camacho-Coronel X (2018) Damage-associated molecular patterns (DAMPs) as future plant vaccines that protect crops from pests. Sci Hortic 237:207–220. https://doi.org/10.1016/j.scienta.2018.03.026
Rassizadeh L, Cervero R, Flors V, Gamir J (2021) Extracellular DNA as an elicitor of broad-spectrum resistance in Arabidopsis thaliana. Plant Sci 312:111036. https://doi.org/10.1016/j.plantsci.2021.111036
Reitz SR (2009) Biology and ecology of the western flower thrips (Thysanoptera: Thripidae): the making of a pest. Fla Entomol 92:7–13. https://doi.org/10.1653/024.092.0102
Reitz SR, Gao Y, Kirk WDJ, Hoddle MS, Leiss KA, Funderburk JE (2020) Invasion biology, ecology, and management of western flower thrips. Annu Rev Entomol 65(1):17–37. https://doi.org/10.1146/annurev-ento-011019-024947
Rotenberg D, Jacobson AL, Schneweis DJ, Whitfield AE (2015) Thrips transmission of tospoviruses. Curr Opin Virol 15:80–89. https://doi.org/10.1016/j.coviro.2015.07.009
Sánchez-Bel P et al (2018) Mycorrhizal tomato plants fine tunes the growth-defence balance upon N depleted root environments. Plant Cell Environ 41:406–420. https://doi.org/10.1111/pce.13105
Selig P, Keough S, Nalam VJ, Nachappa P (2016) Jasmonate-dependent plant defenses mediate soybean thrips and soybean aphid performance on soybean. Arthropod-Plant Interact 10:273–282. https://doi.org/10.1007/s11829-016-9437-9
Staswick PE, Tiryaki I (2004) The oxylipin signal jasmonic acid is activated by an enzyme that conjugates it to isoleucine in Arabidopsis. Plant Cell 16:2117–2127. https://doi.org/10.1105/tpc.104.023549
Steenbergen et al (2018) Thrips advisor: exploiting thrips-induced defences to combat pests on crops. J Exp Bot 69(8):1837–1848. https://doi.org/10.1093/jxb/ery060
Steinbrenner AD et al (2020) A receptor-like protein mediates plant immune responses to herbivore-associated molecular patterns. Proc Natl Acad Sci USA 117:31510–31518. https://doi.org/10.1073/pnas.2018415117
Tanaka K, Choi J, Cao Y, Stacey G (2014) Extracellular ATP acts as a damage-associated molecular pattern (DAMP) signal in plants. Front Plant Sci 5:446. https://doi.org/10.3389/fpls.2014.00446
Ton J, Mauch-Mani B (2004) Beta-amino-butyric acid-induced resistance against necrotrophic pathogens is based on ABA-dependent priming for callose. Plant J 38:119–130. https://doi.org/10.1111/j.1365-313X.2004.02028.x
Toum L et al (2020) Single-stranded oligodeoxynucleotides induce plant defence in Arabidopsis thaliana. Ann Bot 126:413–422. https://doi.org/10.1093/aob/mcaa061
Tripathi D, Zhang T, Koo AJ, Stacey G, Tanaka K (2018) Extracellular ATP acts on jasmonate signaling to reinforce plant defense. Plant Physiol 176:511–523. https://doi.org/10.1104/pp.17.01477
van Hulten M, Pelser M, van Loon LC, Pieterse CM, Ton J (2006) Costs and benefits of priming for defense in Arabidopsis. Proc Natl Acad Sci USA 103:5602–5607. https://doi.org/10.1073/pnas.0510213103
Vega-Munoz I, Feregrino-Perez AA, Torres-Pacheco I, Guevara-Gonzalez RG (2018) Exogenous fragmented DNA acts as a damage-associated molecular pattern (DAMP) inducing changes in CpG DNA methylation and defence-related responses in Lactuca sativa. Funct Plant Biol 45:1065–1072. https://doi.org/10.1071/FP18011
Verhage A, van Wees SC, Pieterse CM (2010) Plant immunity: it’s the hormones talking, but what do they say? Plant Physiol 154:536–540. https://doi.org/10.1104/pp.110.161570
Vicedo B et al (2009) Hexanoic acid-induced resistance against Botrytis cinerea in tomato plants. Mol Plant-Microbe Interact 22:1455–1465. https://doi.org/10.1094/mpmi-22-11-1455
Wang Y, Li X, Fan B, Zhu C, Chen Z (2021) Regulation and function of defense-related callose deposition in plants. Int J Mol Sci. https://doi.org/10.3390/ijms22052393
Wen F, White GJ, VanEtten HD, Xiong Z, Hawes MC (2009) Extracellular DNA is required for root tip resistance to fungal infection. Plant Physiol 151:820–829. https://doi.org/10.1104/pp.109.142067
Wickham H (2016) ggplot2: elegant graphics for data analysis. Springer, New York
Yakushiji S, Ishiga Y, Inagaki Y, Toyoda K, Shiraishi T, Ichinose Y (2009) Bacterial DNA activates immunity in Arabidopsis thaliana. J Gen Plant Pathol 75:227–234. https://doi.org/10.1007/s10327-009-0162-4
Acknowledgements
We express our gratitude to the Scientific Instrumentation Service (SCIC) at Universitat Jaume I for their technical assistance. This research was financially supported by the Spanish Government through the PID2020-118787RA-I00 Grant from the Ministerio de Ciencia e Innovación, and by Universitat Jaume I through the Plan de Promoción de la investigación Grants UJI-A2022-16, as well as the CDEIGENT/2018/015 fellowship from the Generalitat Valenciana.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. This research was financially supported by the Spanish Government through the PID2020-118787RA-I00 Grant from the Ministerio de Ciencia e Innovación, and by Universitat Jaume I through the Plan de Promoción de la investigación Grants UJI-A2022-16, as well as the CDEIGENT/2018/015 fellowship from the Generalitat Valenciana.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Yulin Gao.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rassizadeh, L., Cañadas, E., Cervero, R. et al. Extracellular DNA induces resistance against Frankliniella occidentalis through callose accumulation. J Pest Sci (2024). https://doi.org/10.1007/s10340-023-01733-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10340-023-01733-2