Abstract
New approaches to managing the cabbage root fly (Delia radicum L.) are needed because pesticide regulations continue to limit the availability of effective control products. Soil amendment with black soldier fly (Hermetia illucens L.) frass has recently been shown to reduce D. radicum survival. In a greenhouse experiment, soil from a field on which brassicaceous plant species had repeatedly been grown (brassica field soil) was mixed with frass at ratios of 1, 2 or 5 g/kg. In a second greenhouse experiment, 5 g/kg were added to (a) brassica field soil, (b) soil from a different field on which non-brassicaceous species had been rotated (crop rotation field soil) or (c) blocks of potting soil that were later transplanted to unamended field soil. Brussels sprouts (Brassica oleracea L.) plants were grown in amended soil and were infested with D. radicum larvae after 4 weeks. While amendment with 1 or 2 g/kg did not affect D. radicum performance compared with unamended soil, 5 g/kg reduced D. radicum survival and pupal biomass in brassica field soil. In crop rotation field soil, amendment with 5 g/kg reduced pupal biomass but did not reduce D. radicum survival. Amendment with 5 g/kg had no effect on D. radicum performance in potting soil. In general, D. radicum survival was lower in brassica field soil than in either other soil, irrespective of soil amendment. The effects of black soldier fly frass on D. radicum appear to depend on soil type.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
One of the major threats to the production of cruciferous crops in temperate climates is the cabbage root fly or cabbage maggot, Delia radicum L. (Diptera: Anthomyiidae). Infestations can cause crop losses of up to 100%, notably if young plants are affected (Ferry et al. 2009). Female flies lay their eggs around the plant stem and hatched larvae burrow into the soil to feed on the roots. Feeding damage and the facilitated entry of pathogens into injured roots often result in slow growth, yellowing, stunting and eventually plant death (Santolamazza-Carbone et al. 2017).
As the availability of effective insecticides to control D. radicum has been limited by stricter legislation in many European countries, growers find themselves forced to use alternative management approaches. However, methods such as the deployment of physical barriers, predators or microbial agents are often considered impractical or provide insufficient or inconsistent control (Collier et al. 2020). Moreover, there is a lack of brassica crops with effective resistance to D. radicum (Santolamazza-Carbone et al. 2017; Shuhang et al. 2016). It is therefore necessary to combine different control methods that are not sufficiently effective when applied separately. In addition, new tools for D. radicum control that can be used in an integrated pest management approach are needed.
Soil amendment with the by-products of insect farming has been suggested to stimulate naturally occurring beneficial microbes that could contribute to the control of plant pests (Barragán-Fonseca et al. 2022). Insect production for food and feed is a rapidly growing industry that generates residual streams consisting of insect feces, exuviae (molted exoskeletons) and unconsumed substrate. This mixture is commonly referred to as insect ‘frass,’ and potential applications in agriculture have been the subject of extensive research in recent years (Poveda 2021; Schmitt and de Vries 2020; Torgerson et al. 2021). One of the most important species for the commercial production of insects is the black soldier fly, Hermetia illucens L., as its larvae are particularly versatile and can be reared on various organic residual streams (van Huis 2021). Interestingly, unlike the residual streams of other insect species, exuviae and frass of black soldier fly larvae were recently found to negatively affect the performance of D. radicum when added to the soil (Wantulla et al. 2023b).
Insect exuviae, which are present in frass in variable proportions, have been shown to stimulate a variety of bacteria with biocontrol potential in the cabbage rhizosphere. In particular, the exuviae of black soldier fly larvae appeared to stimulate the abundance of bacterial species in the genus Pseudomonas and different members of the Burkholderiaceae more effectively than the exuviae of other insect species (Wantulla et al. 2023a). Certain species in the genera Pseudomonas and Burkholderia have been shown to exhibit insecticidal activity (Cordova-Kreylos et al. 2013; Kupferschmied et al. 2013). Furthermore, soil amendment with black soldier fly frass was reported to promote the fungal genus Mortierella in the rhizosphere of red clover and Italian ryegrass (Fuhrmann et al. 2022). Insecticidal activity of some Mortierella species has been demonstrated, e.g., by soil inoculation (Edgington et al. 2014). Bacteria and fungi belonging to these different groups might thus be responsible for negative effects of black soldier fly residual streams on D. radicum.
Amending soil with residues from black soldier fly rearing has been proposed as a new method for D. radicum management. While soil amendment with 5 g/kg of exuviae of black soldier fly larvae was previously found to reduce D. radicum biomass, amendment with frass at a ratio of 10 g/kg resulted in lower larval survival (Wantulla et al. 2023b). Since the actual by-product of insect rearing is frass rather than separated exuviae, the present study aimed to further investigate the potential of black soldier fly frass for controlling D. radicum. To reduce the amount of frass that was used in a previous study, a lower effective soil amendment ratio was determined. As the stimulation of native soil microbes is thought to be vital to the effectiveness of black soldier fly frass, effects of the amendment on D. radicum were subsequently compared between different types of soil.
Materials and methods
Plants and growth conditions
Brassica oleracea L. var. gemmifera cv. Cyrus (Brussels sprouts) plants were kept in a greenhouse compartment at 20 ± 3 °C, 60–80% relative humidity and 16 h light/8 h dark photoperiod for Experiment 1. In Experiment 2, temperature in the greenhouse fluctuated between a minimum of 13 °C (at night) and maximum of 32 °C (during the day) before plant infestation with D. radicum and between 12 and 30 °C after plants were infested under ambient humidity and light conditions.
Insect rearing and plant infestation
The D. radicum laboratory strain used originated from a population that had been collected in Zeewolde (Flevoland, the Netherlands) in 2013. All life stages were kept in a climate cabinet at 20 ± 1 °C and 16 h light/8 h dark photoperiod. Larvae were kept on B. napus L. subsp. rapifera (swede) roots of 10-week-old plants until pupation. Eclosed adult root flies were kept in gauze cages and were fed with a 1:1:1 mixture of sugar, milk powder and yeast. In addition, a solution of honey in tap water was offered in a Petri dish and tap water was offered in a Petri dish with moist filter paper on top of wet cotton wool. Oviposition was stimulated by providing slices of swede in Petri dishes to the flies in the cages. Eggs were collected and placed on a new swede prior to larval hatching.
To obtain larvae for plant infestation, eggs were incubated in Petri dishes with moist filter paper. Larvae hatched from the eggs after 4 days and plants were infested by carefully placing five neonate larvae on plastic plant labels that were inserted into the soil surface close to the stem. Labels were checked after 30 min and remaining larvae were replaced. This was repeated until five larvae had moved into the soil in every pot.
Insect residual stream
Frass of larvae of black soldier flies, H. illucens, used in this study was provided by Bestico (Berkel en Rodenrijs, the Netherlands). The material was inspected for the presence of insects or insect fragments, which were removed. Although current EU regulations for insect production residues require heating at 70 °C for 1 h (European Union 2021), such guidelines did not exist when the experiments reported here were conducted. For the present study, frass was oven-dried at 60 °C for 24 h to allow for dosing the soil amendment on a dry matter basis. The dried material was subsequently ground to a powder with an SM 100 cutting mill (Retsch, Haan, Germany).
Soil
Potting soil was provided by Unifarm (Wageningen, the Netherlands). Agricultural soil was collected from the topsoil layers of two organically managed fields in Wageningen and Lelystad, the Netherlands, in 2020. The field in Wageningen had been used to grow various brassicaceous plant species since 2011 and black mustard (Brassica nigra L.) had recently been grown at the location selected for soil collection. In the field in Lelystad, sugar beet (Beta vulgaris L.), onion (Allium cepa L.), parsley (Petroselinum crispum Mill.), wheat (Triticum aestivum L.) and potato (Solanum tuberosum L.) had been grown consecutively from 2016 to 2020. The composition of each field soil as assessed by Eurofins Agro (Wageningen, the Netherlands) is shown in Table 1. Both field soils were homogenized by sieving (particle size < 5 mm). Soil was stored at ambient temperature for 10 months before being used in Experiment 1 and for a maximum of 1 month before being used in Experiment 2.
Experiment 1: effects of soil amendment with black soldier fly frass at different ratios on D. radicum performance
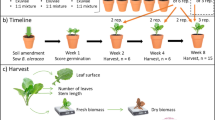
Brassica field soil was mixed with dried black soldier fly frass at a ratio of 1, 2 or 5 g/kg of dry soil or left unamended. In a completely randomized design, 20 plants per treatment were grown in 1 L plastic pots, which were individually placed in saucers. Three seeds were sown per pot and gently pressed down. If more than one seed germinated, one or two seedlings were randomly removed from each pot after 1 week. Excess seedlings were transplanted to pots of the same treatment in which no seeds had germinated or were discarded together with ungerminated seeds. Plants were watered three times per week by filling saucers and emptying them after 2 h. Starting 1 week after sowing, plants were fertilized for 3 weeks with an optimized fertilizer solution (Table S1). Each plant received 160 ml of fertilizer per week. Fertilizer amounts per 1 L of field soil were based on a nitrogen fertilization advice for cabbage provided by Eurofins Agro (Wageningen, the Netherlands). Plants were grown for 4 weeks before being infested with D. radicum larvae and were uprooted 3 weeks after infestation. Roots were rinsed to remove adhering soil and were checked for remaining larvae before washing all soil through a 1 mm aperture sieve to collect larvae and pupae. Living larvae and pupae were counted and pupae were weighed on a CP2P-F microbalance (Sartorius, Göttingen, Germany). Plants were oven-dried at 105 °C for 24 h before measuring shoot and root dry biomass.
Experiment 2: effects of soil amendment with black soldier fly frass on D. radicum performance in different soils
In a full factorial design with soil type and soil amendment as factors, potting soil, brassica field soil and crop rotation field soil were each mixed with black soldier fly frass at a ratio of 5 g/kg or left unamended. Amended and unamended potting soil was pressed into 5 cm3 blocks using a soil block machine (Visser NM71, Visser Tuinbouwtechniek, ‘s Gravendeel, the Netherlands). Eighty blocks of each treatment were placed in separate plastic trays. One B. oleracea seed was sown per soil block and gently pressed down. Field soils were filled into 1 L plastic pots, which were individually placed in saucers. Brassica field soil was used to prepare 20 pots with amended soil and 60 pots with unamended soil. Two seeds were sown per pot in 20 pots of each treatment, while 40 pots containing unamended soil were left without seeds in order to plant seedlings growing in blocks of potting soil later on. Crop rotation field soil was used to prepare 20 pots with amended and unamended soil, respectively, and two seeds were sown per pot. All seeds were gently pressed down. If both seeds in a pot germinated, one seedling was randomly removed after 1 week. Excess seedlings from pots were transplanted to pots of the same treatment in which seeds had not germinated or were discarded together with ungerminated seeds. Due to the low germination rate in amended brassica field soil, two pots had to be planted with seedlings from unamended brassica field soil.
All plants were watered three times per week by filling saucers or flooding trays and emptying them after 2 h. Starting 1 week after sowing, field soils were fertilized for 3 weeks with an optimized fertilizer solution (Table S1). Pots containing brassica field soil received 160 ml of fertilizer per week, while pots containing crop rotation field soil received 60 ml of fertilizer per week. Fertilizer amounts per 1 L of field soil were determined according to a nitrogen fertilization advice for cabbage provided by Eurofins Agro (Wageningen, the Netherlands) which was based on soil nutrient analyses. After 3 weeks of plant growth, 20 soil blocks with seedlings were randomly selected from amended and unamended blocks of potting soil, respectively, and planted into pots containing unamended brassica field soil. Plants were grown for a total of 4 weeks before being infested with D. radicum larvae and were uprooted 3 weeks after infestation. Roots were rinsed to remove adhering soil and were checked for remaining larvae before washing all soil through a 1 mm aperture sieve to collect larvae and pupae. Living larvae and pupae were counted and pupae were weighed on a CP2P-F microbalance (Sartorius, Göttingen, Germany). Plants were oven-dried at 105 °C for 24 h before measuring root dry biomass. Shoot biomass was not included in the evaluation, as a natural infestation with Pieris rapae L. caterpillars had led to feeding damage on most plants during the last week of the experiment. The incidence of P. rapae infestation was approximately equal across treatments.
Statistical analysis
All statistical analyses were performed using R (Version 4.1.0; R Core Team 2021) and the packages car (Fox and Weisberg 2019), dunn.test (Dinno 2017), emmeans (Lenth 2021), rcompanion (Mangiafico 2022) and stats (R Core Team 2021). Statistical models for Experiment 1 included only the factor soil amendment, whereas models for Experiment 2 included the factors soil type, soil amendment and their interaction. Generalized linear models (GLM) with binomial distributions were used to analyze seed germination and D. radicum survival, while a linear model (LM) was used to analyze root biomass. Pairwise comparisons were performed using estimated marginal means (EMM). Models were validated by plotting residuals and, if necessary, homoscedasticity and normality were confirmed using Levene’s test and the Shapiro–Wilk test, respectively. If model assumptions were violated, nonparametric tests were conducted instead. The Kruskal–Wallis test was used to analyze shoot biomass and average pupal biomass per plant in Experiment 1. For Experiment 2, the Scheirer–Ray–Hare (SRH) test was used to analyze seed germination, root biomass and average pupal biomass per plant. Dunn’s test was used for pairwise comparisons.
Results
Experiment 1: effects of soil amendment with black soldier fly frass at different ratios on D. radicum performance
D. radicum performance
Amendment of brassica soil with black soldier fly frass significantly affected D. radicum survival (GLM: χ2 = 26.97, df = 3, P < 0.001). When soil was amended with 5 g/kg, survival was significantly reduced compared with the control (EMM: P = 0.002; Fig. 1a) and compared with amendment with 1 g/kg (EMM: P = 0.002; Fig. 1a) or 2 g/kg (EMM: P < 0.001; Fig. 1a). Soil amendment had a significant effect on pupal fresh biomass (Kruskal–Wallis test: H = 13.9428, df = 3, P < 0.001) and amendment with 5 g/kg significantly reduced pupal biomass compared with the control (Dunn’s test: P = 0.002; Fig. 1b).
Survival (a) and pupal fresh biomass (b) of Delia radicum after feeding on Brassica oleracea plants growing in brassica soil amended with black soldier fly (BSF) frass at different ratios. Treatments denoted with the same letter are not significantly different (EMM/Dunn's test, P > 0.05). Error bars (a) represent standard errors. Box plot whiskers (b) represent largest values within 75% quantiles + 1.5 × interquartile range (IQR) and smallest values within 25% quantiles—1.5 × IQR. Numbers of replicate plants are indicated at the top of the panels by n
Plant performance
While the main effect of amendment of brassica soil with black soldier fly frass on B. oleracea seed germination was significant (GLM: χ2 = 12.32, df = 3, P = 0.006), there were no significant differences in germination between the treatments (EMM: P > 0.05; Fig. 2). Amendment had no significant effect on shoot dry biomass (Kruskal–Wallis test: H = 4.2442, df = 3, P = 0.24) or root dry biomass (LM: F = 0.7681, df = 3, P = 0.516) of B. oleracea plants.
Seed germination of Brassica oleracea in brassica soil amended with black soldier fly (BSF) frass at different ratios. Germination did not differ significantly among treatments (EMM, P > 0.05). Error bars represent standard errors. Numbers of replicate pots are indicated at the top of the panels by n
Experiment 2: effects of soil amendment with black soldier fly frass on D. radicum performance in different soils
D. radicum performance
Survival of D. radicum was significantly affected by soil type (GLM: χ2 = 77.051, df = 2, P < 0.001) and soil amendment with black soldier fly frass (GLM: χ2 = 5.182, df = 1, P = 0.023), while the interaction of the two factors was marginally insignificant (GLM: χ2 = 5.442, df = 2, P = 0.066). Survival was significantly lower in brassica field soil than in either crop rotation field soil (EMM: P = 0.006; Fig. 3a) or blocks of potting soil that had been transplanted to brassica field soil 3 weeks after sowing (EMM: P < 0.001; Fig. 3a). Soil amendment significantly reduced survival only in brassica field soil (EMM: P = 0.027; Fig. 3a), with survival being significantly lower than when either of the other soils was amended (EMM: P < 0.001; Fig. 3a). Pupal fresh biomass was significantly affected by soil type (SRH test: H = 8.5535, df = 2, P = 0.014), soil amendment (SRH test: H = 6.9195, df = 1, P = 0.009) and the interaction of both factors (SRH test: H = 7.4527, df = 2, P = 0.024). Amendment significantly reduced pupal biomass only in crop rotation field soil (Dunn’s test: P = 0.007; Fig. 3b), with pupal biomass being significantly lower than when potting soil was amended (Dunn’s test: P = 0.020; Fig. 3b).
Survival (a) and pupal fresh biomass (b) of Delia radicum after feeding on Brassica oleracea plants growing in different soils amended with black soldier fly (BSF) frass (5 g/kg) or control soil (unamended). Treatments denoted with the same letter are not significantly different (EMM/Dunn's test, P > 0.05). Error bars (a) represent standard errors. Box plot whiskers (b) represent largest values within 75% quantiles + 1.5 × interquartile range (IQR) and smallest values within 25% quantiles—1.5 × IQR. Numbers of replicate plants are indicated at the top of the panels by n
Plant performance
Germination of B. oleracea seeds was significantly affected by soil type (SRH test: H = 71.862, df = 2, P < 0.001) and soil amendment with black soldier fly frass (SRH test: H = 8.374, df = 1, P = 0.004) but not by the interaction of the two factors (SRH test: H = 5.544, df = 2, P = 0.063). Amendment of crop rotation field soil resulted in a significantly lower seed germination rate than in amended or unamended potting soil (Dunn’s test: P < 0.001; Fig. 4a). Seed germination rates in brassica field soil were significantly lower than in potting soil, irrespective of soil amendment (Dunn’s test: P < 0.001; Fig. 4a). Root dry biomass of B. oleracea plants was significantly affected by soil type (SRH test: H = 15.8027, df = 2, P < 0.001), soil amendment (SRH test: H = 6.1019, df = 1, P = 0.014) and the interaction of both factors (SRH test: H = 6.0678, df = 2, P = 0.048). Plants grown in unamended brassica field soil had a significantly higher root biomass than plants grown in blocks of potting soil that were transplanted to brassica field soil 3 weeks after sowing (Dunn’s test: P < 0.001; Fig. 4b). Soil amendment significantly reduced root biomass only in brassica field soil (Dunn’s test: P = 0.013; Fig. 4b).
Seed germination (a) and root dry biomass (b) of Brassica oleracea 8 weeks after planting and 3 weeks after infestation with Delia radicum larvae in different soils amended with black soldier fly (BSF) frass (5 g/kg) or control soil (unamended). Treatments denoted with the same letter are not significantly different (Dunn’s test, P > 0.05). Error bars (a) represent standard errors. Box plot whiskers (b) represent largest values within 75% quantiles + 1.5 × interquartile range (IQR) and smallest values within 25% quantiles—1.5 × IQR. Numbers of replicate pots (a) or plants (b) are indicated at the top of the panels by n
Discussion
In both experiments reported here, the negative effect of soil amendment with black soldier fly frass on D. radicum performance observed in a previous study was confirmed (Wantulla et al. 2023b). Similar to the previously tested amendment ratio of 10 g/kg, amending brassica field soil with frass at a ratio of 5 g/kg reduced D. radicum survival by 32% in Experiment 1 and by 50% in Experiment 2. In addition, amendment of brassica field soil reduced pupal biomass by 19% in Experiment 1 while amendment with crop rotation field soil reduced pupal biomass by 18% in Experiment 2. At a ratio of 1 or 2 g/kg, however, D. radicum performance was not significantly affected by soil amendment with black soldier fly frass. This suggests that a minimum amendment ratio of 5 g/kg might be necessary to reduce D. radicum survival and growth, though ratios between 2 and 5 g/kg were not tested and may also prove to be sufficient.
Brassica field soil also proved to be suppressive to D. radicum without soil amendment. Larval survival in brassica field soil was lower than in potting soil or in crop rotation field soil and was reduced further by amendment with black soldier fly frass. Although the addition of frass did not significantly affect larval survival in crop rotation field soil, it did appear to have sublethal effects on D. radicum as pupal biomass was reduced. Soil amendment with black soldier fly frass was previously found to have no effect on the biomass of adult D. radicum, suggesting that pupae with reduced biomass are less likely to develop into flies (Wantulla et al. 2023b).
Although the tested soils differed vastly in their physical texture, experience with D. radicum infestation in the field suggests that this is not likely to be the cause of the observed differences in larval survival. Delia radicum is known to perform better in lighter, sandy soils than in heavier, loamy soils (Beirne 1971). However, D. radicum-suppressive brassica field soil was 81% sand, whereas non-suppressive crop rotation field soil had a sand content of 47%. Moreover, suppressiveness due to soil texture would not explain the observed enhancement of suppression by soil amendment. The inherent suppressiveness of brassica field soil to D. radicum may more likely be caused by native microbes whose suppressive activity is enhanced by the addition of black soldier fly frass. Soil amendment with frass possibly promoted the growth of responsible microorganisms by providing nutrients to these microbes (Barragán-Fonseca et al. 2022; Mazzola and Freilich 2017). While some of these microbes might also be present in crop rotation field soil, they are possibly less abundant since amendment of this soil had only a minor effect on D. radicum performance. Such differences in the native soil microbiome might be due to the different cropping histories of the two agricultural fields. Whereas brassicaceous plants had been grown in the field in Wageningen for years, various crops had been rotated on the field in Lelystad, none of which belonged to the Brassicaceae. Furthermore, D. radicum infestations had been a common occurrence in the brassica field in Wageningen. Nonetheless, the potential role of cropping history in natural D. radicum suppression requires further investigation and different soils that have been under long-term monoculture of host crops of D. radicum need to be tested for suppressiveness. The prolonged presence of brassicaceous plants might lead to the development of a D. radicum-suppressive soil microbiome, e.g., via plant–soil or plant–soil–insect feedback (Pineda et al. 2017). Alternatively, the presence of D. radicum might stimulate an increased abundance of antagonistic soil microbes independently of its host plants. Root-feeding by D. radicum has been reported to induce specific changes in the rhizosphere microbial community of cabbage plants and reduce D. radicum performance on plants subsequently grown in the same soil (Friman et al. 2021a). Interestingly, D. radicum feeding was found to increase the abundance of bacteria from the genus Pseudomonas, a taxon that also responds positively to soil amendment with black soldier fly exuviae (Wantulla et al. 2023a).
Several Pseudomonas species have been identified in D. radicum larvae and may be beneficial to them, whereas others exhibit varying degrees of pathogenicity to D. radicum (Flury et al. 2019; van den Bosch and Welte 2020). Pseudomonads are well known for their plant-protective properties and different root-colonizing species can influence insect pest numbers by inducing systemic plant resistance or via insecticidal activity (Kupferschmied et al. 2013). Induced systemic resistance (ISR), however, works primarily against generalist herbivores and soil inoculation with an ISR-eliciting Pseudomonas strain was found to positively affect D. radicum performance (Friman et al. 2021b; Pineda et al. 2013). On the other hand, plant–soil–insect feedback effects associated with D. radicum infestation were shown to reduce D. radicum performance but did not seem to enhance plant defense (Friman et al. 2021a). Rather than mediating ISR, bacteria that are enriched in the cabbage rhizosphere by D. radicum feeding or by soil amendment with black soldier fly residues might thus be pathogenic to D. radicum. A root-colonizing Pseudomonas strain with insecticidal activity was demonstrated to persist in D. radicum after ingestion by root-feeding larvae and to be transmitted to other plants by adult flies. Although the bacterium only had minor effects on D. radicum, persistence throughout different life stages was observed in highly susceptible insect species, too (Flury et al. 2019). This suggests that more virulent strains could also be dispersed by D. radicum, e.g., if individuals that have been exposed to lower doses survive an infection. The introduction of such D. radicum pathogens to soil would be increasingly likely as D. radicum infestations repeatedly occur in a field. Consequently, uncontrolled D. radicum populations could eventually result in natural D. radicum suppression.
Despite the suppression of D. radicum, brassica field soil and amendment with black soldier fly frass also had negative effects on B. oleracea. Seed germination in brassica field soil was lower than in potting soil. Similarly, the main effect of frass addition was a reduction in B. oleracea seed germination. In brassica field soil in Experiment 2 soil amendment with frass reduced root biomass. This is in line with a previous study which showed a negative effect of soil amendment with black soldier fly frass on B. oleracea shoot biomass (Wantulla et al. 2023b). While amendment with frass did not affect plant biomass in Experiment 1, it also had a less pronounced effect on D. radicum than in Experiment 2. Whereas amendment of brassica field soil halved D. radicum survival in Experiment 2 and did not affect pupal biomass, it only reduced survival by a third in Experiment 1 but reduced pupal biomass, too. Furthermore, control survival in brassica field soil was much higher in Experiment 1 than in Experiment 2. It should be noted that the field soil used in Experiment 1 had been stored for 10 months, whereas in Experiment 2 soil was used only 1 month after collection in the field. Longer storage might have caused changes in the soil microbiome, possibly resulting in the depletion of D. radicum-suppressive or plant growth-inhibiting microbes.
Overall, the present study corroborates that soil amendment with black soldier fly frass can negatively affect both D. radicum and B. oleracea performance. Intriguingly, the extent of these effects depends on the soil type and might be influenced by factors such as cropping history, the native microbiome and inherent suppressiveness of the soil. While this context-dependence may pose a challenge to the application of black soldier fly frass for D. radicum control, it might also provide new opportunities for its use in crop production. Since it is likely that the negative effects of soil amendment with frass on D. radicum and B. oleracea are caused by different microorganisms, it might be possible to disentangle these effects. In unamended brassica field soil, D. radicum survival was lower than in other soils while B. oleracea biomass was higher. This suggests that D. radicum suppression is not necessarily associated with inhibited plant growth.
If it is possible to isolate insect pathogens from D. radicum-suppressive soil, coapplying them with black soldier fly frass might be an effective method to control D. radicum in other soils. Ideally, such an approach would circumvent microbes that are detrimental to crops and that could also be stimulated by the soil amendment. Therefore, future research should focus on isolating entomopathogenic microbes from D. radicum-suppressive environments, which may be fields that have frequently been cultivated with brassicaceous plants. Amendment with black soldier fly frass might result in enrichment of D. radicum pathogens in the soil and potentially facilitate their isolation. Addressing these topics may lead to novel approaches in the control of this important pest of brassicaceous crops.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Barragán-Fonseca KY, Nurfikari A, van de Zande EM, Wantulla M, van Loon JJA, de Boer W, Dicke M (2022) Insect frass and exuviae to promote plant growth and health. Trends Plant Sci 27:646–654. https://doi.org/10.1016/j.tplants.2022.01.007
Beirne BP (1971) Pest insects of annual crop plants in Canada: part I, Lepidoptera; II, Diptera; III, Coleoptera. Mem Entomol Soc Can 103:1–124. https://doi.org/10.4039/entm10378fv
Collier R, Mazzi D, Folkedal Schjoll A, Schorpp Q, Thoming G, Johansen TJ, Meadow R, Meyling NV, Cortesero AM, Vogler U, Gaffney MT, Hommes M (2020) The potential for decision support tools to improve the management of root-feeding fly pests of vegetables in Western Europe. InSects 11:369. https://doi.org/10.3390/insects11060369
Cordova-Kreylos AL, Fernandez LE, Koivunen M, Yang A, Flor-Weiler L, Marrone PG (2013) Isolation and characterization of Burkholderia rinojensis sp. nov., a non-Burkholderia cepacia complex soil bacterium with insecticidal and miticidal activities. Appl Environ Microbiol 79:7669–7678. https://doi.org/10.1128/AEM.02365-13
Dinno A (2017) dunn.test: Dunn's test of multiple comparisons using rank sums. R package version 1.3.5.
Edgington S, Thompson E, Moore D, Hughes KA, Bridge P (2014) Investigating the insecticidal potential of Geomyces (Myxotrichaceae: Helotiales) and Mortierella (Mortierellacea: Mortierellales) isolated from Antarctica. Springerplus 3:289. https://doi.org/10.1186/2193-1801-3-289
European Union (2021) Commission Regulation (EU) 2021/1925 of 5 November 2021. Off J Eur Union 64:4–8. https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=OJ:L:2021:393:FULL&from=EN
Ferry A, Le Tron S, Dugravot S, Cortesero AM (2009) Field evaluation of the combined deterrent and attractive effects of dimethyl disulfide on Delia radicum and its natural enemies. Biol Control 49:219–226. https://doi.org/10.1016/j.biocontrol.2009.01.013
Flury P, Vesga P, Dominguez-Ferreras A, Tinguely C, Ullrich CI, Kleespies RG, Keel C, Maurhofer M (2019) Persistence of root-colonizing Pseudomonas protegens in herbivorous insects throughout different developmental stages and dispersal to new host plants. ISME J 13:860–872. https://doi.org/10.1038/s41396-018-0317-4
Fox J, Weisberg S (2019) An R Companion to Applied Regression, 3rd edn. Sage, Thousand Oaks CA
Friman J, Karssemeijer PN, Haller J, Kreek K, van Loon JJA, Dicke M (2021a) Shoot and root insect herbivory change the plant rhizosphere microbiome and affects cabbage–insect interactions through plant–soil feedback. New Phytol 232:2475–2490. https://doi.org/10.1111/nph.17746
Friman J, Pineda A, Gershenzon J, Dicke M, van Loon JJA (2021b) Differential effects of the rhizobacterium Pseudomonas simiae on above- and belowground chewing insect herbivores. J Appl Entomol 145:250–260. https://doi.org/10.1111/jen.12842
Fuhrmann A, Wilde B, Conz RF, Kantengwa S, Konlambigue M, Masengesho B, Kintche K, Kassa K, Musazura W, Spath L, Gold M, Mathys A, Six J, Hartmann M (2022) Residues from black soldier fly (Hermetia illucens) larvae rearing influence the plant-associated soil microbiome in the short term. Front Microbiol 13:994091. https://doi.org/10.3389/fmicb.2022.994091
Kupferschmied P, Maurhofer M, Keel C (2013) Promise for plant pest control: Root-associated pseudomonads with insecticidal activities. Front Plant Sci 4:287. https://doi.org/10.3389/fpls.2013.00287
Lenth RV (2021) emmeans: Estimated Marginal Means, aka Least-Squares Means. R package version 1.5.5–1.
Mangiafico S (2022) rcompanion: Functions to support extension education program evaluation. R Package Version 2(4):18
Mazzola M, Freilich S (2017) Prospects for biological soilborne disease control: application of indigenous versus synthetic microbiomes. Phytopathology 107:256–263. https://doi.org/10.1094/PHYTO-09-16-0330-RVW
Pineda A, Dicke M, Pieterse CMJ, Pozo MJ, Biere A (2013) Beneficial microbes in a changing environment: are they always helping plants to deal with insects? Funct Ecol 27:574–586. https://doi.org/10.1111/1365-2435.12050
Pineda A, Kaplan I, Bezemer TM (2017) Steering soil microbiomes to suppress aboveground insect pests. Trends Plant Sci 22:770–778. https://doi.org/10.1016/j.tplants.2017.07.002
Poveda J (2021) Insect frass in the development of sustainable agriculture. A Rev Agron Sustain Dev 41:5. https://doi.org/10.1007/s13593-020-00656-x
R Core Team (2021) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria.
Santolamazza-Carbone S, Velasco P, Cartea ME (2017) Resistance to the cabbage root fly, Delia radicum (Diptera, Anthomyiidae), of turnip varieties (Brassica rapa subsp. rapa). Euphytica 213:1–13. https://doi.org/10.1007/s10681-017-2069-z
Schmitt E, de Vries W (2020) Potential benefits of using Hermetia illucens frass as a soil amendment on food production and for environmental impact reduction. Curr Opin Green Sustain Chem 25:100335. https://doi.org/10.1016/j.cogsc.2020.03.005
Shuhang W, Voorrips RE, Steenhuis-Broers G, Vosman B, van Loon JJA (2016) Antibiosis resistance against larval cabbage root fly, Delia radicum, in wild Brassica-species. Euphytica 211:139–155. https://doi.org/10.1007/s10681-016-1724-0
Torgerson KL, Meijering JV, Sok J, Dicke M, Oude Lansink AGJM (2021) Towards circular agriculture – exploring insect waste streams as a crop and soil health promoter. J Insects as Food Feed 7:357–368. https://doi.org/10.3920/jiff2020.0095
Van den Bosch TJM, Welte CU (2020) The microbial diversity of cabbage pest Delia radicum across multiple life stages. Front Microbiol 11:315. https://doi.org/10.3389/fmicb.2020.00315
Van Huis A (2021) Prospects of insects as food and feed. Org Agric 11:301–308. https://doi.org/10.1007/s13165-020-00290-7
Wantulla M, van Loon JJA, Dicke M (2023a) Soil amendment with insect exuviae causes species-specific changes in the rhizosphere bacterial community of cabbage plants. Appl Soil Ecol 188:104854. https://doi.org/10.1016/j.apsoil.2023.104854
Wantulla M, van Zadelhoff K, van Loon JJA, Dicke M (2023b) The potential of soil amendment with insect exuviae and frass to control the cabbage root fly. J Appl Entomol 147:181–191. https://doi.org/10.1111/jen.13097
Acknowledgements
This work was supported by the Dutch Research Council, NWO (grant number ALWGK.2016.010). We thank Esther Kangha for her help in conducting one of the experiments, Klaas van Rozen for providing field soil from Lelystad, Andre Ramaker and Melissa Spoor for helping to maintain plants and Sarah Kalisvaart and Els van de Zande for their assistance in collecting D. radicum larvae and pupae.
Funding
This work was supported by the Dutch Research Council, NWO (grant number ALWGK.2016.010).
Author information
Authors and Affiliations
Contributions
MW, MD and JJAvL conceived and designed the research. MW conducted experiments and analyzed data. MW wrote the manuscript with input from MD and JJAvL. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Communicated by Don Weber.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wantulla, M., Dicke, M. & van Loon, J.J.A. Effects of amending soil with black soldier fly frass on survival and growth of the cabbage root fly (Delia radicum) depend on soil type. J Pest Sci (2023). https://doi.org/10.1007/s10340-023-01710-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10340-023-01710-9