Abstract
Increasing evidence suggests that land-use intensification contributes to destabilization of trophic networks of insect communities in agriculture resulting in a loss of biodiversity. However, a more detailed understanding of the causes and consequences of the widely reported insect decline is still lacking. Here, we used standardised daily long-term data on the activity of flying insects (~ 250 d/year) to describe the interactive effects of climate warming in intensively cultivated regions and changes in predatory taxa on the general long-term trend of insects and the regulation of herbivores. While the intensely managed landscapes examined in this study show a substantial decline in several taxonomic groups (95.1% total biomass loss in 24 year), the data on aphids support a general assumption that biodiversity loss is often closely associated with arising pest problems. Aphids being pests in agroecosystems develop earlier in spring in overall higher annual abundances. The data highlight that regional insect abundances have declined over recent decades in agricultural landscapes, thus indicating fundamental effects on food webs and insect herbivore performance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Key messages
-
The abundance of aerial insects in regions of intense agriculture drastically declined (35 years).
-
Taxonomic groups experienced a progressively declining density indicating community changes.
-
Aphids, however, increased in density and duration of the flight with earlier activity.
-
Low aerial insect quantities may have implications on food chains thus influencing pest management.
-
Land-use intensification and climate change contribute to the reduction of ecosystem services.
Introduction
Insect populations of terrestrial ecosystems are declining as a complex response to human-induced changes in the environment (Wagner 2020), with land-use changes or intensification particularly suspected of being involved (Sala et al. 2000). Within the last millennium, less than a quarter of the terrestrial land surface remained unaffected by humans (Winkler et al. 2021). Almost all rural areas have been shaped or altered by human activity in Western Europe and can be regarded as cultural landscape, with a large part of the European land representing landscapes of intensively managed agriculture (Van Zanten et al. 2014). In these landscapes, agricultural management is subject to further developments that may affect the performance of insect species (Beckmann et al. 2019; Klink et al. 2020). Due to their economic benefits and their importance for the environment, there is substantial concern about insect conservation while there is widespread recognition of the need to implement sustainable strategies for their protection (Firbank et al. 2008; Wagner 2020; Didham et al. 2020). Studies have already demonstrated shifts in the functional composition of both insect and plant communities, which may be strongly linked to the intensification of agricultural practices (Henle et al. 2008; Allan et al. 2015; Neff et al. 2021). In combination with recent climatic changes, they may even have a stronger impact than would be expected from the individual effects of the two factors when considered independently (Outhwaite et al. 2022). At the same time, there is evidence to suggest that susceptibility to agricultural insect pests increased in some cropping systems (Lin 2011; Gaba et al. 2014; Tilman et al. 2014; Emmerson et al. 2016; Deutsch et al. 2018).
Key threats to insects include aspects of intensification in agricultural landscapes, such as the loss of habitats, homogenisation in time and space, application of synthetic fertilizers, or herbicides and pesticides (Tilman et al. 2002, 2014; Uchida and Ushimaro 2014; Sirami et al. 2019; Powney et al. 2019). The resulting consequences for habitat quality are widely recognized as a prime threat to biodiversity because both environment and management are important in structuring communities, and they are major determinants of the abundance of individuals inhabiting a landscape (Bengtsson et al. 2005; Storch et al. 2005). Abundance in populations, in turn, can be an important aspect of biodiversity conservation, because small populations in general are more susceptible to stochastic demographic events. The same is true for reduced genetic variation as a reduction in population size has an impact on the stability of species dynamics and finally on the preservation of biological diversity (Gaston 2000; Srivastava 2002; Emmerson et al. 2016). However, how different species are affected by recent shifts in landscape structure and land-use, and the downtrend may be amplified by years with great heat and drought (Harvey et al. 2020), is not yet sufficiently known. Taking the general widespread loss of insects into account (Seibold et al. 2019; Wagner 2020; Klink et al. 2020), two scenarios may be assumed. First, a decline of all species initiated by a few common triggers, from e.g., changes in agricultural practices or local climatic conditions, and secondly, a response to environmental changes strongly determined by interactions (Huxel and McCann 1998; Mendenhall et al. 2014). Cascading trophic effects and release from predation or competition may occur, often termed ‘tipping points’, are possible scenarios that can finally lead to drastic changes in ecological communities (Snyder et al. 2006; Risch et al. 2018; Roque et al. 2018).
Considering this, reductions in insect population abundances are associated with reduced densities of antagonistic species (food webs), for instance insectivorous birds (Hallmann et al. 2014) or parasitic wasps, predatory insects (Zhao et al. 2015) and a variation in the diversity of antagonists (Neff et al. 2021). What they do have in common is that they often provide valuable biological ecosystem services, such as the biological control of herbivorous invertebrates. This interrelationship is particularly important since the entire diversity, frequency and abundance of antagonists has the potential to contribute to reduced pest dynamics in agriculture (Wilby and Thomas 2002; Meisner et al. 2014; Zhao et al. 2015). It is progressively recognized that insect-mediated biological control gains importance when sustainable farming strategies aim to reduce the applications of pesticides (Tilman et al. 2001; Lundgren and Fausti 2015; Vogel 2017).
Here, we aim to focus on long-term surveys of flying arthropods in agriculture dominated intensively managed regions to estimate the rate of change of insects from different taxonomic groups including recorded seasonal shifts caused by climate warming and to identify consequences for insect herbivore groups potentially benefitting from reduced antagonistic interaction networks (Martínez‐Núñez and Rey 2021).
Recent adjustments that took place in the agricultural sector and their impact upon the environment have to be considered (Gocht and Röder 2014) and also incorporate the proportional area of farmland in these landscapes, the crop diversity—here with a high share of cereals (mix of winter wheat and rape seed, > 60%, see Fig. 1a) and common practices such as the application of pesticides or the input of fertilizer (Goulson 2014; Brühl and Zaller 2019; Hallmann et al. 2014). In addition to monitoring in agricultural regions of low structural diversity, we considered regional climatic changes to be meaningful for the studied insect groups and discuss its influence.
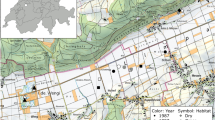
Monitoring of flying insects in landscapes of intensive farming: agriculture land-use in Germany highlighting the a share of cereal crops on arable farmland, regarding the utilized agricultural area (UAA). Trap location situated in regions of intensive farming (red dot) and a high proportion of cereal crops. The change of b permanent pasture area between 1999 and 2010 according to UAA (30% in 1999, 28.2% in 2016). Black dots display trap locations: ST I-VI. Data provided by Thuenen-Institute (Gocht and Röder 2014)
The purposes of our examination were to (i) test whether the decline of flying insects has been particularly marked in agriculture dominated landscapes by using biomass values and to (ii) detect and specify the pattern of community changes by showing annual abundances and trends in taxonomic groups. Several species and taxonomic groups contribute to the control of pest species in crop cultures. We ask whether pest species in agriculture, for which the trap systems were installed and which are extremely adaptable in many respects, showed similar trends, no changes, or even increased over the same time periods. More specifically, in section five (results), we highlight and later discuss how changes in climate, land-use and the third trophic level translate into shifts in population dynamics of aphids.
Although many studies have described insect declines in different ecosystems (Seibold et al. 2019; Klink et al. 2020) and have addressed changes in taxonomic invertebrate groups over time (Dainese et al. 2017; Wagner 2020; Outhwaite et al 2020), investigating trends in intensively managed farmland has received much less attention. This is because, estimating how strongly a high share of intensive farming areas in combination with changing practices and a changing climate affects regional insect declines can ultimately only be done with long-term monitoring studies. However, we do not claim to identify the main drivers of regional insect declines, as in long-term data the strength of the different drivers already identified, or the combined effect of different drivers might change over time (Allan et al. 2015).
Materials and methods
Insect sampling and data compilation
Data were obtained from samples of stationary suction traps (see Table 1). This trap type collects flying insects and spiders (ballooning) providing standardised daily records during the main aphids flying season between April and December (Shortall et al. 2009). It is part of a worldwide operating suction trap network (Bell et al. 2015). The volume of air intake of each trap is standardised to 3000 m3/h, and it captures at a height of 12.2 m (tube length). The traps were developed to provide an absolute population measurement for which insect abundances can be sampled more precisely in comparison to all other methods of aerial capture (Bell et al. 2015). Traps conduct sampling with a high degree of temporal resolution over many years (approximately 250 sample days per season in 8 hourly resolution) and the value of these traps to monitor insects over large areas has been demonstrated (Shortall et al. 2009).
Specifically, data of aerial arthropods from central Germany (ST1) were analysed (between 1985 and 2020 from approximately 9000 sampling days). A second trap near Rostock, established as a separate northern unit in 1997 (ST2), sampled aphids until the end of 2017 and restarted in 2019 (~ 6250 days). Furthermore, aphid data from two traps near Göttingen (ST3: 1993–1998) and near Stuttgart (ST4: 1994–1995) were included in the evaluation of regional aphid abundances during the 1990s (see Fig. 1, Table 1).
Insect biomass captures over time
To estimate the effects of land-use drivers or environmental changes on aerial arthropods, we used total biomass of trap captures at daily intervals. For each day capture, we determined the dry weight of arthropods in the exact same manner, i.e., daily arthropod-samples (kept in three 250 ml plastic bottles containing water and detergent) were directly poured into a plastic sieve (Ø = 12.5 cm) which separated arthropods from the collecting fluid. Subsequently, the samples remained on a cloth for about 10 s to dry and were then weighed. Starting the monitoring on 1st April until 5th of December, each season thus covered approximately 250 data points (days/year). In addition, daily measures were used to calculate the mean biomass weight per year (mean g/d across the season). We recorded total biomass of flying insects—including spiders—for each sample season between 2016 and 2020 (ST1) and since early spring 2019, daily biomass was routinely measured at ST2 as well. Initially, these measurements had been performed in the past during the sample periods in 1996 and 1997 (at ST1), and for the year 2004. Annual means of all available measurements of trap captures are summarized (g/d—average across the season) in Fig. 4e. Most insect samples were not stored for many years. Consequently, further biomass measures could not be conducted (1985–1995; 1998–2015).
Using the available biomass data, we initially compared measures of an earlier year of capturing (1996) against daily captures of a recent year (2018) to identify changes in both total biomass (g/year) and in the pattern of captures across the monitoring season (the comparison was also made for the year 1997 with 2019). At the biomass level, the year 1996 in comparison to 2018, or 1997 in comparison to 2019 (g/d) were considered. Pattern of daily captures is illustrated for each of the four years in Fig. 2, and annual means are added (average g/d).
a Patterns of daily captures of flying insects in an agricultural landscape (ST1). Daily measures (net weight) of flying insects, including web spiders, assessed by suction trap (12.2 m high, 3000 m3/h air intake) between 1st of April and 5th of December (250 d/year): 1996 in (blue) and 1997 (orange) in comparison to samplings in 2018 (yellow) or 2019 (grey). Dashed and solid lines indicate the averages of daily biomass (gram per day) across the season (left) or mean share of biomass (right) in 2018 (~ 0.97 g/d) and 2019 (~ 1.04 g/d) compared to the biomass in 1996 (~ 26.52 g/d, here ≙ 100%). Regional climate changes during the vegetation period (b temperature, c precipitation; annual means and sum for March-September) or winter (d temperature, e precipitation; November–February) between 1985 and 2019 (blue and red dashed lines mark the monitoring years 1996 and 2019)
Seasonal biomasses captures changing over time
To get more detailed information about the pattern of captures over the season and its variation over time, we display weekly captures for two collection periods each (ST1 mid ~ 250 d; Fig. 3). In order to facilitate comparisons with earlier years (1996 or 1997), daily, weekly (Fig. 3a, b), or annual means (Fig. 4e) are shown.
Pattern of arthropod captures across the vegetation period. Biomass of trap captures (ST1) highlighting the change in the occurrence of flying insects across the season when comparing a 1996 with 2018—or (b) 1997 with 2019—showing reduced overall biomass of captures in 2018 or 2019, and a decline of captures with ongoing vegetation period recently (the grey dotted line represents the trend, from weekly values. The grey bars indicate means and variance)
Changes in suction trap captures from taxonomic groups and total catch: a spiders, b psyllids, c thrips, d cicada (per 1000 m3 air intake). Total aerial insect biomass e assessed by suction trap for the years 1996 and 1997 compared to 2016–2019 (in addition, data of 2004 and 2020) as daily means of 1st April to 5th December (ST1: grey, ST2: blue). On the lower right, a comparison between formerly and recent samplings from f fall 2004 (10th–12th of October, 12.833 g/d ≙ 3275 insects) and for the same period in g fall 2019 (0.8533 g/d ≙ 195 insects) illustrating the change in daily suction trap catches (3 days each, see Table 2)
Therefore, daily trap captures were grouped according to the total of seven-day captures (Fig. 3a, b), thus emphasizing the relative change of insect biomass over the season by comparing data of the earlier years with recent years (1996 vs. 2018, or 1997 vs. 2019). In addition, we calculated the proportional difference for each day of the year 1996 compared to the same specific date of the year 2018, in the same way between 1997 and 2019. Illustrated is the reduction of captured biomass, in terms of percentage change, for each month of the season (as boxplots in Fig. S16).
Estimating declines in taxonomic groups
Abundances of relevant insect groups were determined according to the taxonomic level, such as aphids (superfamily), psyllids (superfamily), thrips (order), cicada (sub-order), additionally web spiders (order): hereafter called ‘taxonomic groups’. Taxonomic groups were determined in the laboratory and counted per day. Time series of taxonomic groups were arranged (here: psyllids, thrips, cicada, spiders) based on the abundance data of ST1 (see Table 1, Fig. 4a–d). However, the exact timeline varied among taxonomic groups. While the estimation of psyllid abundances started in 2006, thrips and cicada counting started in 2008, with few exceptions, e.g., thrips were excluded in 2016. We consider the abundance of taxonomic groups per air intake, because operation time of a suction trap may vary between monitoring years (according to the beginning and end of sampling) depending on the spring and fall weather conditions. Annual captures were converted to the average amount of individuals of each taxonomic group per 1000 m3 air intake per trap (hours per season at 3000 m3 air intake/h). For each taxonomic group, we computed linear regression analyses, which aimed at illustrating the trends across the different time series.
Insect composition in trap captures 2004 and 2019
More detailed determination of stored and newly sampled captures are shown for 2004 and 2019 (Table 2). Specifically, we determined a three-day sample from 2004—that had been stored in the laboratory—and compared it with a three-day capture in 2019 (each between the 10th and 12th of October) to highlight arthropods (at the family or order level) potentially contributing to the most significant changes in aerial insect composition during the past 15 years.
We classified all species into taxonomic groups (Table 2). This example may illustrate the quantity and composition of insects captured by the same trap in fall 2004 in a direct comparison with fall 2019 captures. Hence, the three-day samplings of both years are shown in Fig. 4f–g to demonstrate an average sample size during a season in 2019 and back in fall 2004, compared to daily captures of the season 1996.
Overall trends in aphid dynamics
Aphids constitute a major group of crop pests that inflict serious damages to plants and thus stationary suction traps have been used for a long time to monitor aphids in Germany (the first sampling goes back as far as 1985). Therefore, we used available data from the suction trap in central Germany (ST1) that contained records over a period of 35 years (1985–2019) and compared these with data from ST2 (1997–2017), ST3 (1993–1998) and ST4 (1994–1995) to find, e.g., similarities in the level of aphid abundances of a particular year across regions, or similarities in seasonal dynamics over a particular period of time. Annual cycles in aphids: In temperate regions, the life cycle of several aphid species involves an alternation between two species of host plants, for example between weed (or tree) species in winter and an annual crop during spring time. Consequently, winged females may develop early and late in the season, allowing the insects to colonize new plants, and thus suction traps record increasing aphid populations in spring time and a second population increase in early fall.
Total annual catch (N/year) of aphids and daily samplings, as total or mean capture over a season, generated the first categorical variable to describe changes in long-term pest dynamics, calculated as total annual counts of aphids (Fig. 5a: ST1-ST4, 1st April through 5th December) and average number of aphids per day (from ST1 totals: Fig. S10a). Basically, we used daily captures of aphids to highlight changes in annual abundances over time. We used changes in seasonal abundances of trap captures (for spring, summer, fall), phenological events (start and end of aphids’ flight period) or temporal shifts in the occurrence of peak densities (i.e. in spring and fall) during the 35-year monitoring period.
Changes in aphid captures over a period of 35 years starting in 1985. Annual counts of aphids a assessed by suction traps between 1985 and 2019, including data of ST2-4 (calculated trend: ST1; moving average: grey (ST1), yellow (ST2)). Seasonal abundances of aphids b in trap captures of ST1 for late spring (green—summarizing April/May and June), summer (orange—July/August) or fall (grey – September/October) and mean trend (dashed line)
Data handling and analyses
The data of each taxonomic group per year were summarized over a given period (1st April–5th December) and calculated as individuals per 1000 m3 air intake (Fig. 4a-d). For aphids, linear regressions were calculated to estimate the effects of weather on phenology and annual abundances (e.g., Fig. 5, Fig. S10). Here, stepwise multiple linear regression analyses were performed to identify the effect of seasonal temperature conditions (see weather data) on (i.) the number of aphids, or (ii.) the timing of peaks in a specific season (e.g., Fig. S11).
To determine the relationship between annual abundances of each taxonomic group and the respective time scale (between 1985 and 2019), linear and quadratic regressions (polynomial regression terms) were calculated. When the linear regression term in a relationship was considered significant but the quadratic term not, the relationship was categorized as monotonic negative. If both linear and quadratic effects were significant, it was then established whether it was significantly unimodal (hump-shaped or U-shaped) using a statistical test developed by Mitchell-Olds and Shaw (1987). This test determines whether the estimated maximum of annual abundances in intermediate years of the timeline is significantly higher than the annual abundances at both earlier and more recent sampling years within taxonomic groups (Chase and Leibold 2002). Statistical analyses were performed using the software SPSS 27.0.
Testing relationships between aphids and antagonists
To estimate the impact of insect antagonists in the field, we used a modelling approach by simulating aphid development under different weather conditions. We run the GETLAUS01—model, a discrete and deterministic model for simulating cereal-aphid population dynamics with a special focus on antagonist efficiency (Gosselke et al. 2001). GTLAUS01 contains submodels for winter wheat, cereal aphids (R. padi, S. avenae, M. dirhodum), and aphid predators (Coccinella septempunctata, Propylea quatuordecimpunctata, syrphids). Each model run begins with a range of starting values of density, structure and immigration type of the aphid population and their antagonists at a specific date during wheat flowering. Then, daily density values of cereal aphids and antagonists are calculated by the model. The model was previously validated and has been applied in various projects, e.g., about climate change effects in predator–prey interactions. The GTLAUS tri-trophic for simulating wheat-aphid-antagonist interaction has been revised with the aim to improve the model details to estimate antagonist effects under climate change (Klueken et al. 2009). Climate data from 1994, 1996, and 2000 (years of moderate to warm spring and winter temperatures) were used to simulate dynamics of the most abundant wheat aphids (R. padi, S. avenae, M. dirhodum) from three different regions in central Germany, i.e., Götttingen, Magdeburg, Wittenberg. We used data typical for field observations listed by Gosselke et al. (2021): wheat (584 ears/m2—without pesticide application), ladybirds (C. septempunctata: adult 0.4/m2, P. quatuordecimpunctata: eggs 8.9/m2, hoverflies (Episyrphus balteatus, and some other species): eggs 1.1/m2 and larvae 0.1/m2, lacewing larvae (Chrysoperla carnea) 0.3/m2, carabid beetles: adult 16.1/m2, staphylinids: adult 2.2/m2, spiders: adult 2.8/m2. For each successful simulation, the corresponding model run was recalculated applying reduced predator densities (10% of the field data), and the resulting differences in aphid infestations were interpreted as the predator effect (Fig. S8, see Gosselke et al. 2001).
Analyses of aphid phenology changing over time
We also tested for the relationship between population dynamics in aphids and seasonal weather to estimate the climatic effect on aphid captures. As first flight records were expected to be earlier due to global warming in spring and last longer in fall (Fig. S7a), we show changes in the first and the last occurrence of aphids each year, or season. Here listed as both the period of days with aphids and the period of days without aphid captures in summer (Fig. S10b). In addition, the counts of aphids in early spring are provided in Fig. S10c (1985–2018). Weather plays a role in both, phenology and abundance. Thus, interactions between first flight date and seasonal temperatures were calculated (Fig. S10d) using linear regression analysis (stepwise forward).
Aphid density is subject to strong seasonal variation, normally resulting in outbreak densities (peaks) in spring and summer. Generally, the date of phenological events was listed as Julian date (JD = number of days from 1st January). The relationship between timing of peaks (JD) and total aphid density (N) in spring was identified (April through June, Fig. S7b). For each year, we presented temporal occurrence of peaks (JD) during spring and fall (Fig. S7a, all with trends of linear regressions). For each year, we computed the period (Ndays) between spring peak and recovery to low densities (< 1% of seasonal means), until the end of June (Fig. 6b). This period of population decline is called retrogradation (Kindelmann and Dixon 2010) and the run of this period is strongly affected by natural antagonists (Fig. S9a; Harrington et al. 1999). Furthermore, the link between peak dates in spring (1985–2019) and seasonal temperature (definition in weather data, below) was calculated (Fig. S11a). Additionally, the interaction between peak dates in spring and seasonal temperatures was calculated for a time section of pronounced winter warming, between 1985 and 2002 (Fig. S11b).
Aphid dynamics changing over a period of 35 years. Aphid captures a between 1985 and 2019 as five-day sums from a suction trap near Quedlinburg (ST1). The dashed line indicates population growth in spring, annual peak density, and reduction in the population (also referred to as retrogradation) for the years 1985 (blue), 1996 (orange), 2009 (grey) and 2019 (yellow). b Duration of the retrogradation across sample years (1985–2019). Retrogradation: period between peak density and the decline to an inferior state of the populations, i.e., very low density (see Fig. S9)
Seasonal long-term changes in aphid dynamics for the period 1985–2019 were addressed by using trends of phenological changes in peaks and first flight date in spring or changes in seasonal aphid captures (i.e., spring, summer, late summer) over time (Figs. S12, S15). We used the (temporal) occurrence and duration of peak densities during the first half of the year, because it is a good proxy for measuring the change in aphid dynamics (from e.g., rapid emergence, or seasonal mean densities; Kindelmann and Dixon 2010), also as a combined effect of weather and antagonists (duration of increased-density period or of the retrogradation). To highlight dynamical changes over time, peak densities in relation to the occurrence in spring are shown (Fig. S12a). For each year within this time series (1985–2019), dynamical curves in three periods were added (Fig. S12b-d). Total aphid captures for three seasons (spring, early and late summer) over a 35-year period are displayed in Figure S15 to describe seasonal changes in abundances.
Weather data and climate warming
Each suction trap is operated in combination with a weather station. Air temperature at 2 m (°C) and precipitation (mm) were determined daily and used to calculate monthly averages. Seasonal mean values of temperature and precipitation (as averages or sums) were calculated to demonstrate regional weather shifts at ST1. Temperature changes between 1985 and 2019 are displayed in Fig. S14a–f from (a.) annual (Jan–Dec), (b.) growing season (March-Sept), (c.) spring (Mar-May), (d.) summer (Jun–Aug), (e.) winter I (Nov–Feb), (f.) winter II (Jan–Feb). Additionally, fall season (Sept-Oct), single months, spring (Apr–May), summer I (June-July), summer II (Aug–Sept) and sample period (Apr–Dec) temperature means (or precipitation sum) were calculated to analyse response factors in annual or seasonal aphid abundances.
Summer and winter periods for each year (1985–2019) were displayed against the climate normal period (see Fig. S13a, b: CNPwinter 1961–1990 from Magdeburg DWD weather station; CNPsummer 1985–2013 from weather station at ST1) using temperature and precipitation, thus highlighting exceptional warm and dry years for the summer (Fig. S13a) or winter (Fig. S13b) season. Datasets were provided from the German weather service (Deutscher Wetterdienst DWD) or came from weather stations associated with the respective suction trap (normally beginning with the first year of monitoring).
Landscape characteristics and agriculture
In Germany, agricultural acreage covers 16.6 million ha und thus approximately fifty per cent of the total area. In 2020, about 70.3% were farmland (11.67 million ha) of which ~ 52% were covered with cereals (6.06 million ha, see Fig. 1a), 28.5% were permanent pasture (4.73 million ha, Fig. 1b). The most common crops (in 2020) are winter wheat (23.7%), maize (19.7%) and rapeseed (8.2%), corresponding to 2.77 million ha, 2.3 million ha, or 0.96 million ha, respectively (BMELV 2020). Change in agriculture: The permanent pasture was reduced from 31.1% in 1991 (5.3 million ha) to 28.2% (4.7 million ha) in 2020, by around 10% (BMELV 2020). Spatially mapped in Fig. 1b for the period between 1999 and 2010. Since 2013, the pasture slightly increased. Cultivation of winter wheat increased from < 2.5 million ha in 1985 to 2.95 million ha by more than 20% (or 500,000 ha) in 2021. The cereal share in 2016 is shown spatially in Fig. 1a (Gocht and Röder 2014), including trap locations all located in regions with a high proportion of cereals (mostly winter wheat in the recent past). The presence of livestock and typical pasture landscape elements decreased (BMELV, various years).
The first research area, which surrounds the trap location (ST1) with a catchment area of about 30 km radius (Bell et al. 2020), is mainly covered by winter wheat (40%), rapeseed (17%), barley and maize (18%) or sugar beet (4%), grown on large fields of approximately 40.8 ha on average (farm size ~ 270 ha). The region (ST1, mid-Germany, near Quedlinburg; formerly Aschersleben) is in the transition zone between maritime and continental climate with 400–450 mm precipitation and average annual temperature of 8.8 °C. This landscape is situated in the federal state of Saxony-Anhalt, 140 m above sea level, in the slightly hilly northern Harz foreland (Fig. 1), precisely (N 51.771937, E 11.146757) on the lee side of the Harz mountains (low mountain range). Agricultural area surrounding the second trap (ST2, northern Germany, near Rostock) is located 30 m above sea level, close to the Baltic Sea (N 54.071552, E 12.324007) at around 580–620 mm precipitation and with an average annual temperature of 8.28 °C. It is mainly covered by wheat (43%), rapeseed (13%) and maize (12%), at a field size averaging about 32.5 ha (farm size ~ 275 ha). The area around ST3 (western Germany, farm size ~ 69 ha, near Göttingen) is mainly covered by winter wheat (39.5%), rapeseed (14.6%) and sugar beet (5.8%), field size 10.2 ha on average. Precipitation ranges between 624 and 664 mm at average annual temperatures of 9.2 °C. Altitude is about 150 m above sea level (N 51.54048, E 9.215428). The fourth area (ST4, near Stuttgart) is mainly covered by wheat (63%), barley (12%), maize (8.6%) and rapeseed (8.3%). This trap is in southern Germany (N 48.781776, E 9.303970, farm size ~ 35 ha, field size 4.8 ha) and 247 m above sea level; rainfall ranges between 860 and 900 mm precipitation at a mean annual temperature of 9.3 °C (according to the Climate Normal Period: CNP 1961–1990; data from a nearby climate station of the German meteorological service, DWD.de).
Results
Overall trends in insect biomass
Aerial insects from suction trap samplings declined substantially over the past two and a half decades. Comparison of the biomass-level of trap captures, calculated as means of annual day captures (annual weight divided by monitoring days), reveal that the total amount of aerial insects decreased significantly over the 24-year period, with available biomass data of the sample period in 1996 in relation to 2018 and captures in 1997 vs. 2019 (Fig. 2a). When analysing the biomass data during the vegetation period (~ 250 days/year, 1st April until 5th of December), on average, 26.52 g per day were sampled in 1996 (as a reference point in Fig. 2, ≙ 100%) or 25.47 g in 1997, as compared to 0.97 g in 2018 (4.37%), or 1.04 g per day in 2019 (4.91%). Losses of more than 90% of flying insects suggest a rapid and continuous downward trend, which is supported by data from daily biomass measures in 2004 (12.8 g/d), 2016 (1.32 g/d), 2017 (1.24 g/d) and 2020 (1.22 g/d). Presented data of ST1 for the period from 1996 till 2020 suggest a decline of 4.18% per year on average.
Over the same period, between 1985 and 2019, we observe two striking trends in weather patterns for central Germany (ST1). This is an almost linear increase in summer temperatures between 1996 and 2019, with a simultaneous decrease in precipitation over the past ten years (Fig. 2b, c). Likewise, winter temperatures increased with a tendency to greater dryness, i.e., less precipitation during winter (Fig. 2d, e).
Patterns of change of flying insects from seasonal occurrence
Complementing these findings, the second suction trap (ST2), located in northern Germany, sampled 0.664 g/d on average and thus approximately 63% biomass of flying insects of ST1 during the vegetation period in 2019 (Fig. 4e). However, the abundance of flying insects varies within the vegetation period as a response to species-specific phenologies or to changing weather conditions over days or periodically, whereas typical phenological patterns may change in the long term (with e.g., climate change or changing agricultural practices). The data from ST1 and ST2 show daily samples of insects that vary at a low level across the sample season for the years 2018 and 2019 (< 1 g/d) independently of the year of sampling and location (pattern in Fig. 2a). Besides this, during the 2018 and 2019 seasons (~ 250 d), days of markedly low captures were observed: N days < 1 g biomass (163 in 2018 / 164 in 2019), days < 0.1 g biomass (74 in 2018/68 in 2019), or days < 0.01 g biomass (34 in 2018 / 27 in 2019, Figs. 2, 3). Confirming this, a quite similar pattern of daily captures has been detected in the main flying seasons of 2016, 2017 and 2020. In contrast, the sample weight remained at a robust high level within the capture periods of 1996 and 1997 (26.52 g/d 1996, or 25.47 g/d 1997 on average) and remained on that level until the end of the collection period (Figs. 2, 3a, b). Although mean daily biomass also changed considerably across the season.
Data from ST1 furthermore highlight declining rates of insect captures between spring and fall, illustrated by a comparison of samplings (biomass over seven days sampling) between the years 1996 and 2018, or between 1997 and 2019 (Fig. 3a, b). In contrast, captures of 1996 and 1997 remained at a constant high level.
Long-term trends in taxonomic groups
Data of single taxonomic groups continuously selected, counted and stored, or selected during distinct time periods (Table 1) such as spiders (Araneae), psyllids (Sternorhyncha), or thrips (Thysanoptera) revealed a downward trend over the years (Fig. 4a-d), despite a non-significant trend of cicada (Hemiptera). Considerable declines of more than 50% suggest a general trend within two decades for spiders (R2 = 0.49, n = 10, p < 0.001, year 1999–2019), psyllids (R2 = 0.267, n = 14, p < 0.001, year 2006–2019), or thrips (R2 = 0.12, n = 8, p < 0.002, year 2008–2016) for the respective time scales, except for cicada (R2 = 0.0062, n = 11, p > 0.5, year 2008–2019). In total 28.474 spiders were sampled (1999–2019), 34.645 psyllids (2006–2019), 37.675 thrips (2008–2016) and 11.350 cicada (2008–2019).
A comparative case study of samplings in fall season
Two suction trap samples (three days each) were analysed to give more details on the change in the reported time series between former and recent trap captures. As a comparative study of two specific samples in fall 2004 and 2019 (10th–12th of October), the determination gives an insight into the shift in single insect guilds. First, daily captures in Fig. 4e illustrate the rate of change for the year 2004 (3 days in fall ≙ 38.5 g) as compared to three-day samples in 2019 (≙ 2.56 g). These three-day samples in turn (year 2004) demonstrate roughly the weight of insects that had been captured at a single day in 1996 (~ 26.52 g/d at a constant high level, see Fig. 2).
Thus, we tested whether the trend of declining insect biomasses affected all taxonomic groups or guilds equally by counting the organisms of both suction trap samples (stored in 2004 and 2019, see M&M) on the family or order level. As a result, several insect taxa that strongly contributed to total aerial biomass in fall 2004 were less frequent in 2019. For instance, the number of Diptera caught was considerably reduced in 2019 (Table 2) when compared with samplings of the year 2004 (e.g., for biting midges (Ceratopogonidae) 42.68% reduction, or for mosquitos (Culicidae) 5.12% reduction) by nearly 99%, followed by three antagonistic families, the hoverflies (Chrysopidae) and with the Ichneumonidae wasps (Ichneumonidae), or chalcid wasps (Chalcidoidae) by two parasitic wasp families. Lacewings (Chrysopidae, Neuroptera) were at a lower level in 2019, Brachycera (Diptera) made up a larger part of daily captures in 2004 (44.35% reduction in Anthomyiidae, e.g., wheat bulb fly—Della coarctata). However, rove beetles were sampled in higher abundance in 2019 (plus 75.67%).
Aphids in agricultural landscapes
The total size of aphid populations in nature is known to be affected by environmental conditions or antagonists, which is here reflected in the annual aphid abundances, and which may vary considerably over time (Fig. 5a). In total 634.155 aphids were caught over a 35-year period (at ST11985–2019), 459.603 aphids during the 24-year period in northern Germany (ST21997–2017), 48.524 near Göttingen (ST31993–1998) and 5.128 for the southern trap (ST41994-1995). Annual catches of aphids over comparable periods (~ 1st April until 5th of December) displayed here as count of aphids per year (ST1, Fig. 5a), increased considerably (R2 = 0.277, n = 34, p < 0.05, N1985-2019). This result presents a contrasting trend to the one for total biomass of insects and spiders (Fig. 4e). Despite a near cyclic dynamic of overall densities between years, the level of aphid captures proved to be significantly higher starting approximately with the 2002 season (as captures per day in Fig. S10a). Similarly, aphids in northern Germany (ST2) increased between 1997 and 2017 (R2 = 0.192, n = 20, p < 0.01, Fig. 5a). Three species, i.e., Sitobion avenae, Rhopalosiphum padi, Metopolophium dirhodum, represent the most dominant species for each of the four regions. Seasonal aphid densities in captures increased, except in the summer season in recent years (Fig. 5b).
Aphid dynamics change over time and its relation to antagonists
Currently, high aphid densities last longer than they did decades ago (R2 = 0.468, n = 34, p < 0.001, Fig. 6b). Overall, we detected a considerable increase in the duration of high-density aphid levels in recent years, as compared to seasons before 2000. The time span between peak density (i.e., the maximum density during population growth in spring and fall, see Fig. S9a) and the recovery towards a previously existing low level of aphids (the retrogradation) turned out to be considerably in three decades of monitoring (Fig. 6b). Altogether, periods of high aphids’ densities in the first half of the year are prolonged by approximately 30 days between 1985 and 2019 (R2 = 0.356, n = 34, p < 0.001).
Testing for the impact of antagonists on cereal aphids of winter wheat (S. avenae, M. dirhodum, R. padi), we used a deterministic, discrete simulation model (see M&M—GETLAUS01). All three populations increased by up to two to four times when using low antagonist densities (reduced by 90 percentage points) compared to aphid densities simulated in the presence of antagonists during warm seasons (between 1994 and 2000). The largest influence was observed during the warm dry year in 2000 for central Germany (from climate station near ST1, see Fig. S8).
Discussion
The principle aim of this study was to find indications for the rate of decline in insects within intensively managed agricultural landscapes and to investigate trends and consequences for important insect herbivores, i.e., aphids. Based upon the analysis of standardised long-term monitoring data of aerial insects (1985–2020), we demonstrated that biomasses in a cultivated landscape (ST1 > 60% agricultural area) declined at an unprecedented rate over a 24-year period (1996–2019). Monitoring data of a second high-intensity agriculture region (ST2) confirm overall low aerial insect abundances, based on a low level of daily biomass captures over recent years (still declining at a very low level until the end of time series). It provides further inside in a phenomenon that has been observed in nature reserves (Hallman et al. 2017; Klink et al. 2020) and our data suggest an even stronger reduction of insect populations in landscapes with a high share of agriculture. Here, aerial insect biomass decreased in the overall daily catches at a rate of more than 90 per cent on average. These results provide evidence in support of our expectations that the abundance of flying insects markedly decreased within agriculture-dominated landscapes during the past decades, reflected in detail by a downward trend in several taxonomic groups.
Within central Germany, the substantial decline of aerial insect biomass is not a side effect of a naturally occurring variation in insect populations because single guilds almost linearly decreased within the investigated 35-year period (Fig. 4a-d) and overall biomasses recently remained at an unprecedented low level (2016–2020—still decreasing in ST1 with similarly values for ST2, Fig. 4e). These observed patterns are in line with current observations in grasslands (Beckmann et al. 2019; Seibold et al. 2019), nature reserves (Vogel 2017; Hallmann et al. 2017; Wagner 2020), and forests surrounded by farmland (Sánchez-Bayo and Wyckhuys 2019; Powney et al. 2019; Klink et al. 2020). In general, long-term fluctuations in anthropogenically influenced temperate latitudes are a common phenomenon among insect populations and subject to various environmental influences (Shortall et al. 2009). A certain degree of variation of the naturally occurring insect biomass (annual totals) has been demonstrated by Bell et al. (2020) between 1969 and 2016 based on standardised terrestrial insect time series from England. Besides these fluctuations, their monitoring has shown that the annual count of moths in Great Britain is in significant decline and has been since about the mid-1980s, a time of considerable climatic change, whereas in their view it would seem unlikely that climate alone is responsible.
Our results indicate that regional declines in flying insects are more likely in monotonous agricultural landscapes, especially if climate change continues to accelerate. The past dry years may have amplified the land-use biodiversity effect, following unusually warm and dry conditions in 2018 and 2019. Furthermore, the effect of several extreme years in succession may have a delayed impact on populations by repeated effects on demographic traits such as adult lifespan, fecundity, and oviposition patterns (Zhang et al. 2015). Confirming this, recent studies have quantified trends in insect biodiversity in several regions and species groups (Harvey et al. 2020; Jaureguiberry et al. 2022) and they mainly declare that land-use changes and climate are main drivers of alterations in insect assemblages (Neff et al. 2022). Previous regionally scaled studies have shown that invertebrates in grassland habitats were declining at a faster rate than in woodland, and their results suggest that the effect is associated with agriculture at the landscape level (Seibold et al. 2019; Klink et al. 2020). In addition, Outhwaite et al. (2022) demonstrate that human land use and climate change have emerged as key determinants of change in the biodiversity (Harvey et al. 2020) and they show that these drivers act synergistically, particularly in areas of intensive agricultural land use when climate includes the warmest temperatures relative to seasonal and inter-annual variation. They emphasize that insect abundance declined steeply with further intensification during climate change (Outhwaite et al. 2022).
In contrast to the general trend of flying arthropods, aphids increased in density within two available long-term datasets over decades, and those represent harmful insect herbivores in agriculture. Our results suggest insect biomass and biodiversity losses—including antagonistic species—may contribute substantially to the upward trend in some agricultural pest populations. Changes in dynamics and upward trends in peak densities correlate with decreases in taxonomic groups including those that act as antagonists of aphids. In the face of land-use and climate changes over decades (Dainese et al. 2017; Wagner 2020), we were expecting fundamental shifts in the composition of insect communities. At the beginning of the observations, aerial insects were mainly represented by (i.) herbivores, i.e., pests (here: aphids, psyllids, cicada), by (ii.) antagonistic guilds (lacewings, hoverflies, parasitic wasps, spiders), or by (iii.) dipteran species (e.g., detrivores, parasites, crop pests—the year 2004 highlighted as an example of major changes compared to recent captures, see Table 2). However, a significant proportion of diurnal and nocturnal flying insects, particularly dipteran species (e.g., Brachycera) or beneficial antagonistic arthropod groups such as spiders, substantially disappeared from captures (Table 2, Fig. 4a–d).
These findings provide support for the assumption that insect populations markedly decreased during past decades and that some taxa hardly changed while others increased in abundance (Outhwaite et al. 2020). It suggests a more complex pattern of biodiversity change, but how this translates into community-level effects is not sufficiently clear. For instance, the underlying mechanisms driving different herbivore and antagonist dynamics are not specifically examined here. The most parsimonious interpretation of these results regarding aphids is that a change in agronomic practices and intercorrelated changes in the environment (Beckmann et al. 2019) are positively associated with the abundance of pests (Harrington et al. 2007), while other insect taxa decline.
A further aspect in our consideration on community structuring, i.e., the climate effect, incorporates seasonal weather events that should benefit arthropod development in general, if the conditions are below the species-specific optimum values (Pilotto et al. 2020; Filazzola et al. 2021). Based upon continuous observations, we identified seasonal temperature and precipitation variation that almost certainly affect environmental suitability for some insects, shown here at the dynamics of aphids (Fig. S7). All seasons became warmer over the last two decades, but heat and drought events occurred more often in summer (Fig. S14, Fig. 5b). For aphids, the change in phenology and survival from increasingly warm winter and spring conditions probably plays a key role in the general upward trend in annual densities (Fig. S11), although other factors most definitely contributed to this increase in abundance. Warm winter and moderate spring conditions often explain exponential growth in several species, such as R. padi, or M. dirhodum (Bell et al. 2015). Thus, climatic conditions, especially temperature, are vitally important in determining aphid dynamics, and climatic changes are likely to strongly affect their pest status (Harrington et al. 2007).
However, an increasing frequency of pest outbreaks often results from a combination of climate change and biodiversity loss (Mooney et al. 2009; Lundgren and Fausti 2015). Mass outbreaks of aphids tend to be suppressed by ladybeetles, lacewings, parasitic wasps, and spiders (Piñol et al. 2009). In this respect, we detected high average rates of decline in several insect guilds and suggest that the general increase in aphids relates to the fact that antagonists such as spiders and parasitoids became less abundant, which points to a response to reduced antagonist efficiency (Loreau and Manzancout 2013; Lundgren and Fausti 2015; Deutsch et al. 2018). Another indication, the abundance of aphids during peak densities, has recently decreased significantly slower (Fig. 6b) and densities generally remained on a higher level than three decades ago (Fig. 5a).
Supporting this, Gosselke et al. (2001) demonstrated by testing the efficiency of predatory arthropods in a tritrophic system ‘winter wheat-cereal aphids-antagonists’ in simulations (model GETLAUS01), that the aphid infestation rates rose by nearly a factor of seventeen in scenarios without predators. In our study, we repeated those simulations including changing regional weather aspects near the monitoring sites (1996 cold, 1994 medium, 2000 warm spring) and found a two to four-times higher aphid population size under reduced predator presence (Fig. S8). This illustrates that the dynamics of several insect herbivores are tightly linked to the development of parasitoids and the rate of reduction by parasitoids or further antagonists (Harrington et al. 1999), which themselves are influenced by weather and often by the exposure to pesticides (Krauss et al. 2011; Sentis et al. 2013). Exclusion of aphid predators resulted in a remarkable increase in aphid infestation, conspicuously high in warm spring conditions. Hence, low densities of sedentary predators or even low predation rates can have a disproportionate effect on the final aphid density, especially as they initially prey on small populations in early spring and thus achieve a high degree of aphid reduction (Wilby and Thomas 2002; Piñol et al. 2009).
Generally, the timing of pest occurrence relative to the growing season determines the likelihood of pest outbreaks (Meisner et al. 2014) and here peak densities tend to last longer in recent years, as aphids start earlier in the season in higher abundance (Fig. 6). This, in combination with the general losses in biodiversity, can result in significant changes in the structure and composition of species assemblages with effects on important food chains (Petchey et al. 2008; Fahrig et al. 2011). Furthermore, the impact of drought events that were observed in recent years for e.g., the observation area of ST1 (Fig. S13a, S12d), may further intensify insect declines in case these are non-tolerant to drought (Parmesan et al. 2000). In turn, losses in biodiversity contribute to less stable communities that show higher rates of decline during drought because increasing species numbers generally increases drought tolerance (Tilman et al 2014; Ma et al. 2015). Sentis et al. (2013) show that ecosystems with predators that exert a strong biotic control may be less influenced by abiotic factors and then are likely to be more resistant to extreme climatic events than impoverished ecosystems that lack predators. Thus, a low level of insect densities would also be disproportionately reduced by extreme climatic events.
Despite these overall decreasing trends in flying insects, we showed that specific time periods of the growing season, which coincide with the period when insectivorous birds raise their offspring (Poulin 2012), have experienced drastic declines in insect abundance with hardly any activity observed (Fig. 3). Besides, flying insects reach peak biomass early in the season and this at very low levels of daily captures (Fig. 3a, b). The two factors, periods of exceptional low quantities of aerial insects and peak biomass early in the season may alter ecosystem functioning, as they are representing food sources for higher trophic levels, with important implications for food chains.
These relationships demonstrate the complexity of biodiversity dynamics in cultivated landscapes. Basically, invertebrate communities have been shown to be important for maintaining ecological coupling and functioning in an increasingly defaunated world (Risch et al. 2018; dos Santos et al. 2021). This underlines once more that invertebrates are central to the functioning of ecosystems (Allan et al. 2014; Sirami et al. 2019). If insect populations become smaller, the temporal isolation after harvesting or due to crop rotation, for instance, is likely to play an increasingly important role for survival because dispersal might be less successful in otherwise unsuitable homogenous landscapes (Pilotto et al. 2020).
Another important issue is the effect from further land-use intensification and changes in land-use type in a specific region and its amplifying effect in combination with climate change including recent drought years (Meehan et al. 2011; Uchida and Ushimaro 2014; Outhwaite et al. 2020). Our dataset represents, to the best of our knowledge, an exceptional record in terms of temporal extent with a high-time resolution from standardised sampling and makes a valuable contribution to long-term biodiversity monitoring records in Europe. It demonstrates some declines in most monitored taxonomic groups across all functional groups except for some herbivores—here aphids and cicada—suggesting a more general reduction of flying insects in agricultural landscapes.
Hence, current evidence clearly shows that the results that have emerged from our insect capture analyses over the past decades are challenging and need additional research to answer the following questions: (i) to which extent in detail did changes in agriculture contribute to insect losses, (ii) is there a balance between agricultural productivity and sustainable management both within the field and across landscapes, with ecosystem functions contributing to yield improvement, (iii) how much semi-natural habitat is required to drive local maintenance of biodiversity, and how diverse must a landscape design be with respect to the spatial pattern of different habitats or changes over time.
Summarizing this, agriculture may cause many pressures on different taxa and these effects are very difficult to separate (Firbank et al. 2008). Moreover, the relative importance of different pressures might change, given the timeline of our analyses over several decades (Sax and Gaines 2003). Further studies should uncover causal factors most threatening to insects and devise strategies for counteracting their declines. Subsequently, it is necessary to understand to what extent conservation efforts can counteract biodiversity loss and which are the most important measures at the landscape scale.
Author contributions
TMZ contributed to the compilation of long-term datasets, analysed and wrote the paper with contributions and input of all authors. E.S. was responsible for the monitoring, the determination of taxonomic groups at ST1, and measured the daily biomass of trap captures during the 1990s. FO and TW developed the overall research idea and enabled the analyses of long-term data available at the Institute.
Availability of data and materials
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Allan E, Bossdorf O, Dormann CF, Prati D, Gossner MM, Tscharntke T, Blüthgen N, Bellach M, Birkhofer K, Bocha S, Böhm S, Börschig C, Chatzinotas A, Christ S, Daniel R, Diekötter T, Fischer C, Friedl T, Glaser K, Hallmann C, Hodac L, Hölzel N, Jung K, Klein AM, Klaus VH, Kleinebecker T, Krauss J, Lange M, Morris EK, Müller J, Nacken H, Pašali E, Rillig MC, Rothenwöhrer C, Schally P, Scherber C, Schulze W, Sochera SA, Steckel J, Steffan-Dewenter I, Türke M, Weiner CN, Werner M, Westphal C, Wolters V, Wubet T, Gockel S, Gorke M, Hemp A, Renner SC, Schöning I, Pfeiffer S, König-Ries B, Buscot F, Linsenmair KE, Schulze ED, Weisser WW, Fischer M (2014) Interannual variation in land-use intensity enhances grassland multidiversity. Proc Natl Acad Sci 111:308–313. https://doi.org/10.1073/pnas.1312213111
Allan E, Manning P, Alt F, Binkenstein J, Blaser S, Blüthgen N, Böhm S, Grassein F, Hölzel N, Klaus VH, Kleinebecker T, Morris EK, Oelmann Y, Prati D, Renner SC, Rillig MC, Schaefer M, Schloter M, Schmitt B, Schöning I, Schrumpf M, Solly E, Sorkau E, Steckel J, Steffen-Dewenter I, Stempfhuber B, Tschapka M, Weiner CN, Weisser WW, Werner M, Westphal C, Wilke W, Fischer M (2015) Land use intensification alters ecosystem multifunctionality via loss of biodiversity and changes to functional composition. Ecol Lett 18:834–843. https://doi.org/10.1111/ele.12469
Beckmann M, Gerstner K, Akin-Fajiye M, Ceaușu S, Kambach S, Kinlock NL, Phillips HRP, Verhagen W, Gurevitch J, Klotz S, Newbold T, Verburg PH, Winter M, Seppelt R (2019) Conventional land-use intensification reduces species richness and increases production: a global meta-analysis. Glob Change Biol 25:1941–1956. https://doi.org/10.1111/gcb.14606
Bell JR, Alderson L, Izera D, Kruger T, Parker S, Pickup J, Shortall CR, Taylor MS, Verrier P, Harrington R (2015) Long-term phenological trends, species accumulation rates, aphid traits and climate: five decades of change in migrating aphids. J Anim Ecol 84:21–34. https://doi.org/10.1111/1365-2656.12282
Bell JR, Blumgart D, Shortall CR (2020) Are insects declining and at what rate? An analysis of standardised, systematic catches of aphid and moth abundances across Great Britain. Insect Conserv Diver 13:115–126. https://doi.org/10.1111/icad.12412
Bengtsson J, Ahnström J, Weibull AC (2005) The effects of organic agriculture on biodiversity and abundance: a meta-analysis. J Appl Ecol 42:261–269. https://doi.org/10.1111/j.1365-2664.2005.01005.x
BMELV (Bundesministerium für Ernährung, Landwirtschaft und Verbraucherschutz), (2020)—various years; Statistisches Jahrbuch über Ernährung, Landwirtschaft und Forsten der Bundesrepublik Deutschland. Bonn: BMELV
Brühl CA, Zaller JG (2019) Biodiversity decline as a consequence of an inappropriate environmental risk assessment of pesticides. Front Environ Sci 177. https://doi.org/10.3389/fenvs.2019.00177
Chase JM, Leibold MA (2002) Spatial scale dictates the productivity–biodiversity relationship. Nature 416:427–429. https://doi.org/10.1038/416427a
Dainese M, Isaac NJ, Powney GD, Bommarco R, Öckinger E, Kuussaari M, Pöyry J, Benton TG, Gabriel D, Hodgson JA, Kunin WE, Lindborg R, Sait SM, Marini L (2017) Landscape simplification weakens the association between terrestrial producer and consumer diversity in Europe. Glob Change Biol 23:3040–3051. https://doi.org/10.1111/gcb.13601
Deutsch CA, Tewksbury JJ, Tigchelaar M, Battisti DS, Merrill SC, Huey RB, Naylor RL (2018) Increase in crop losses to insect pests in a warming climate. Science 361:916–919. https://doi.org/10.1126/science.aat3466
Didham RK, Basset Y, Collins CM, Leather SR, Littlewood NA, Menz MH, Müller J, Packer L, Aunders ME, Schönrogge K, Stewart AJ, Yanoviak SP, Hassal C (2020) Interpreting insect declines: seven challenges and a way forward. Insect Conserv Diver 13:103–114. https://doi.org/10.1111/icad.12408
dos Santos JS, Dodonov P, Oshima JE, Martello F, de Jesus AS, Ferreira ME, Silva-Neto CM, Ribeiro MC, Collevatti RG (2021) Landscape ecology in the Anthropocene: an overview for integrating agroecosystems and biodiversity conservation. Persp Ecol Conserv 19:21–32. https://doi.org/10.1016/j.pecon.2020.11.002
Emmerson M, Morales MB, Oñate JJ, Batáry P, Berendse F, Liira J, Aavik T, Guerrero I, Bommarco R, Eggers S, Pärt T, Tscharntke T, Weisser WW, Clement L, Bengtsson J (2016) How agricultural intensification affects biodiversity and ecosystem services. Adv Ecol Res 55:43–97. https://doi.org/10.1016/bs.aecr.2016.08.005
Fahrig L, Baudry J, Brotons L, Burel FG, Crist TO, Fuller RJ, Sirami C, Siriwardena GM, Marti JL (2011) Functional landscape heterogeneity and animal biodiversity in agricultural landscapes. Ecol Lett 14:101–112. https://doi.org/10.1111/j.1461-0248.2010.01559.x
Filazzola A, Matter SF, MacIvor JS (2021) The direct and indirect effects of extreme climate events on insects. Sci Total Environ 769:145161. https://doi.org/10.1016/j.scitotenv.2021.145161
Firbank LG, Petit S, Smart S, Blain A, Fuller RJ (2008) Assessing the impacts of agricultural intensification on biodiversity: a British perspective. Philos Trans R Soc B 363:777–787. https://doi.org/10.1098/rstb.2007.2183
Gaba S, Fried G, Kazakou E, Chauvel B, Navas ML (2014) Agroecological weed control using a functional approach: a review of cropping systems diversity. Agron Sustain Dev 34:103–119. https://doi.org/10.1007/s13593-013-0166-5
Gaston KJ (2000) Global patterns in biodiversity. Nature 405:220–227. https://doi.org/10.1038/35012228
Gocht A, Röder N (2014) Using a Bayesian estimator to combine information from a cluster analysis and remote sensing data to estimate high-resolution data for agricultural production in Germany. Int J Geogr Inf Sci 28:1744–1764. https://doi.org/10.1080/13658816.2014.897348
Gosselke U, Triltsch H, Rossberg D, Freier B (2001) GETLAUS01: the latest version of a model for simulating aphid population dynamics in dependence on antagonists in wheat. Ecol Model 145:143–157. https://doi.org/10.1016/S0304-3800(01)00386-6
Goulson D (2014) Pesticides linked to bird declines. Nature 511:295–296. https://doi.org/10.1038/nature13642
Hallmann CA, Foppen RP, van Turnhout CA, de Kroon H, Jongejans E (2014) Declines in insectivorous birds are associated with high neonicotinoid concentrations. Nature 511:341–343. https://doi.org/10.1038/nature13531
Hallmann CA, Sorg M, Jongejans E, Siepel H, Hofland N, Schwan H, Stenmans W, Müller A, Sumser H, Hörren T, Goulson D, de Kroon H (2017) More than 75 percent decline over 27 years in total flying insect biomass in protected areas. PLoS ONE 12:e0185809. https://doi.org/10.1371/journal.pone.0185809
Harrington R, Woiwod I, Sparks T (1999) Climate change and trophic interactions. Trends Ecol Evol 14:146–150. https://doi.org/10.1016/S0169-5347(99)01604-3
Harrington R, Clark SJ, Welham SJ, Verrier PJ, Denholm CH, Hulle M, Maurice D, Rounsevell MD, Cocu N (2007) Environmental change and the phenology of European aphids. Glob Change Biol 13:1550–1564. https://doi.org/10.1111/j.1365-2486.2007.01394.x
Harvey JA, Heinen R, Gols R, Thakur MP (2020) Climate change-mediated temperature extremes and insects: from outbreaks to breakdowns. Glob Change Biol 26:6685–6701. https://doi.org/10.1111/gcb.15377
Henle K, Alard D, Clitherow J, Cobb PC, Firbank L, Kull T, McCracken D, Moritz RF, Niemelä J, Rebane M, Wascher D, Watt A, Young J (2008) Identifying and managing the conflicts between agriculture and biodiversity conservation in Europe: a review. Agric Ecosyst Environ 124:60–71. https://doi.org/10.1016/j.agee.2007.09.005
Huxel GR, McCann K (1998) Food web stability: the influence of trophic flows across habitats. Am Nat 152:460–469. https://doi.org/10.1086/286182
Jaureguiberry P, Titeux N, Wiemers M, Bowler DE, Coscieme L, Golden AS, Guerra CA, Jacob U, Takahashi Y, Settele J, Diaz S, Molnár Z, Purvis A (2022) The direct drivers of recent global anthropogenic biodiversity loss. Sci Adv 8:eabm9982. https://doi.org/10.1126/sciadv.abm9982
Klueken AM, Hau B, Freier B, Friesland H, Kleinhenz B, Poehling HM (2009) Comparison and validation of population models for cereal aphids. ‘Vergleich und Validierung von Populationsmodellen für Getreideblattläuse.’ J Plant Dis Protect. https://doi.org/10.1007/BF03356299
Krauss J, Gallenberger I, Steffan-Dewenter I (2011) Decreased functional diversity and biological pest control in conventional compared to organic crop fields. PLoS ONE 6:e19502. https://doi.org/10.1371/journal.pone.0019502
Lin BB (2011) Resilience in agriculture through crop diversification: adaptive management for environmental change. Bioscience 61:183–193. https://doi.org/10.1525/bio.2011.61.3.4
Loreau M, de Mazancourt C (2013) Biodiversity and ecosystem stability: a synthesis of underlying mechanisms. Ecol Lett 16:106–115. https://doi.org/10.1111/ele.12073
Lundgren JG, Fausti SW (2015) Trading biodiversity for pest problems. Sci Adv 1:e1500558. https://doi.org/10.1126/sciadv.150055
Ma G, Rudolf VH, Ma CS (2015) Extreme temperature events alter demographic rates, relative fitness, and community structure. Glob Change Biol 21:1794–1808. https://doi.org/10.1111/gcb.12654
Martínez-Núñez C, Rey PJ (2021) Hybrid networks reveal contrasting effects of agricultural intensification on antagonistic and mutualistic motifs. Funct Ecol 35:1341–1352. https://doi.org/10.1111/1365-2435.13800
Meehan TD, Werling BP, Landis DA, Gratton C (2011) Agricultural landscape simplification and insecticide use in the Midwestern United States. Proc Natl Acad Sci 108:11500–11505. https://doi.org/10.1073/pnas.1100751108
Meisner MH, Harmon JP, Ives AR (2014) Temperature effects on long-term population dynamics in a parasitoid-host system. Ecol Monogr 84:457–476. https://doi.org/10.1890/13-1933.1
Mendenhall CD, Karp DS, Meyer CF, Hadly EA, Daily GC (2014) Predicting biodiversity change and averting collapse in agricultural landscapes. Nature 509:213–217. https://doi.org/10.1038/nature13139
Mitchell-Olds T, Shaw RG (1987) Regression analysis of natural selection: statistical inference and biological interpretation. Evolution 41:1149–1161. https://doi.org/10.1111/j.1558-5646.1987.tb02457.x
Mooney H, Larigauderie A, Cesario M, Elmquist T, Hoegh-Guldberg O, Lavorel S, Mace GM, Palmer M, Scholes R, Yahara T (2009) Biodiversity, climate change, and ecosystem services. Curr Opin Envir Sust 1:46–54. https://doi.org/10.1016/j.cosust.2009.07.006
Neff F, Brändle M, Ambarli D, Ammer C, Bauhus J, Boch S, Hölzel N, Klaus VH, Kleinebecker T, Prati D, Schall P, Schäfer D, Schulze ED, Seibold S, Simons NK, Weisser WW, Pellissier L, Gossner MM (2021) Changes in plant-herbivore network structure and robustness along land-use intensity gradients in grasslands and forests. Sci Adv 7:eabf3985. https://doi.org/10.1126/sciadv.abf398
Neff F, Korner-Nievergelt F, Rey E, Albrecht M, Bollmann K, Cahenzli F, Chittaro Y, Gossner MM, Martinez-Nunez C, Meier ES, Monnerat C, Moretti M, Roth T, Herzog F, Knop E (2022) Different roles of concurring climate and regional land-use changes in past 40 years’ insect trends. Nat Commun 13:7611. https://doi.org/10.1038/s41467-022-35223-3
Outhwaite CL, Gregory RD, Chandler RE, Collen B, Isaac NJ (2020) Complex long-term biodiversity change among invertebrates, bryophytes, and lichens. Ecol Evol 4:384–392. https://doi.org/10.1038/s41559-020-1111-z
Outhwaite CL, McCann P, Newbold T (2022) Agriculture and climate change are reshaping insect biodiversity worldwide. Nature 605:97–102. https://doi.org/10.1038/s41586-022-04644-x
Parmesan C, Root TL, Willig MR (2000) Impacts of extreme weather and climate on terrestrial biota. Bull Am Meteorol Soc 81:443–450. https://doi.org/10.1175/1520-0477(2000)081%3c0443:IOEWAC%3e2.3.CO;2
Petchey OL, Beckerman AP, Riede JO, Warren PH (2008) Size, foraging, and food web structure. Proc Natl Acad Sci 105:4191–4196. https://doi.org/10.1073/pnas.0710672105
Pilotto F, Kühn I, Adrian R, Alber R, Alignier A, Andrews C, Bäck J, Barbaro L, Beaumont D, Beenaerts N, Benham S, Boukal DS, Bretagnolle V, Camatti E, Canullo R, Cardoso PG, Ens BJ, Everaert G, Evtimova V, Feuchtmayr H, García-González R, Gómez García D, Grandin U, Gutowski JM, Hadar L, Halada L, Halassy M, Hummel H, Huttunen KL, Jaroszewicz B, Jensen TC, Kalivoda H, Schmidt IK, Kröncke I, Leinonen R, Martinho F, Meesenburg H, Meyer J, Minerbi S, Monteith D, Nikolov BP, Oro D, Ozoliņš D, Padedda BM, Pallett D, Pansera M, Ângelo Pardal M, Petriccione B, Pipan T, Pöyry J, Schäfer SM, Schaub M, Schneider SC, Skuja A, Soetaert K, Spriņģe G, Stanchev R, Stockan JA, Stoll S, Sundqvist L, Thimonier A, van Hoey G, van Ryckegem G, Visser ME, Vorhauser S, Haase P (2020) Meta-analysis of multidecadal biodiversity trends in Europe. Nat Commun 11:3486. https://doi.org/10.1038/s41467-020-17171-y
Piñol J, Espadaler X, Pérez N, Beven K (2009) Testing a new model of aphid abundance with sedentary and non-sedentary predators. Ecol Model 220:2469–2480. https://doi.org/10.1016/j.ecolmodel.2009.06.031
Poulin B (2012) Indirect effects of bioinsecticides on the nontarget fauna: the Camargue experiment calls for future research. Acta Oecol 44:28–32. https://doi.org/10.1016/j.actao.2011.11.005
Powney GD, Carvell C, Edwards M, Morris RK, Roy HE, Woodcock BA, Isaac NJ (2019) Widespread losses of pollinating insects in Britain. Nat Commun 10:1–6. https://doi.org/10.1038/s41467-019-08974-9
Risch AC, Ochoa-Hueso R, van der Putten WH, Bump JK, Busse MD, Frey B, Gwiazdowicz DJ, Page-Dumroese DS, Vandegehuchte ML, Zimmermann S, Schütz M (2018) Size-dependent loss of aboveground animals differentially affects grassland ecosystem coupling and functions. Nat Commun 9:1–11. https://doi.org/10.1038/s41467-018-06105-4
Roque F, Menezes JF, Northfield T, Ochoa-Quintero JM, Campbell MJ, Laurance WF (2018) Warning signals of biodiversity collapse across gradients of tropical forest loss. Sci Rep 8:1–7. https://doi.org/10.1038/s41598-018-19985-9
Sala OE, Chapin FS, Armesto JJ, Berlow E, Bloomfield J, Dirzo R, Huber-Sanwald E, Huenneke LF, Jackson RB, Kinzig A, Leemans R, Lodge DM, Mooney HA, Oesterheld M, Poff NL, Sykes MT, Walker BH, Walker M, Wall DH (2000) Global biodiversity scenarios for the year 2100. Science 287:1770–1774. https://doi.org/10.1126/science.287.5459.1770
Sánchez-Bayo F, Wyckhuys KA (2019) Worldwide decline of the entomofauna: a review of its drivers. Biol Conserv 232:8–27. https://doi.org/10.1016/j.biocon.2019.01.020
Sax DF, Gaines SD (2003) Species diversity: from global decreases to local increases. Trends Ecol Evol 18:561–566. https://doi.org/10.1016/S0169-5347(03)00224-6
Seibold S, Gossner MM, Simons NK, Blüthgen N, Müller J, Ambarlı D, Ammer C, Bauhus J, Fischer M, Habel JC, Linsenmair KE, Nauss T, Penone C, Prati D, Schall P, Schulze ED, Vogt J, Wöllauer S, Weisser WW (2019) Arthropod decline in grasslands and forests is associated with landscape-level drivers. Nature 574:671–674. https://doi.org/10.1038/s41586-019-1684-3
Sentis A, Hemptinne JL, Brodeur J (2013) Effects of simulated heat waves on an experimental plant-herbivore-predator food chain. Glob Change Biol 19:833–842. https://doi.org/10.1111/gcb.12094
Shortall CR, Moore A, Smith E, Hall MJ, Woiwod IP, Harrington R (2009) Long-term changes in the abundance of flying insects. Insect Conserv Diver 2:251–260. https://doi.org/10.1111/j.1752-4598.2009.00062.x
Sirami C, Gross N, Baillod AB, Bertrandi C, Carrié R, Hass A, Henckel L, Miguet P, Vuillot C, Alignier A, Girard J, Batáry P, Clough Y, Violle C, Giralt D, Bota G, Badenhausser I, Lefebvre G, Gauffree B, Vialatte A, Calatayud F, Gil-Tena A, Tischendorf L, Mitchell S, Lindsay K, Georges R, Hilaire S, Recasens J, Solé-Senany XO, Robleñoy I, Bosch J, Barrientos JA, Ricarte A, Marcos-Garcia MÁ, Miñano J, Mathevet R, Gibon A, Baudry J, Balent G, Poulin B, Burel F, Tscharntke T, Bretagnolle V, Siriwardena G, Ouin A, Brotons L, Martin JL, Fahrig L (2019) Increasing crop heterogeneity enhances multitrophic diversity across agricultural regions. Proc Natl Acad Sci 116:16442–16447. https://doi.org/10.1073/pnas.1906419116
Snyder WE, Snyder GB, Finke DL, Straub CS (2006) Predator biodiversity strengthens herbivore suppression. Ecol Lett 9:789–796. https://doi.org/10.1111/j.1461-0248.2006.00922.x
Srivastava DS (2002) The role of conservation in expanding biodiversity research. Oikos 98:351–360. https://doi.org/10.1034/j.1600-0706.2002.980216.x
Storch D, Evans KL, Gaston KJ (2005) The species-area-energy relationship. Ecol Lett 8:487–492. https://doi.org/10.1111/j.1461-0248.2005.00740.x
Tilman D, Fargione J, Wolff B, D’Antonio C, Dobson A, Howarth R, Schindler D, Schlesinger WH, Simberloff D, Swackhamer D (2001) Forecasting agriculturally driven global environmental change. Science 292:281–284. https://doi.org/10.1126/science.1057544
Tilman D, Cassman KG, Matson PA, Naylor R, Polasky S (2002) Agricultural sustainability and intensive production practices. Nature 418:671–677. https://doi.org/10.1038/nature01014
Tilman D, Isbell F, Cowles JM (2014) Biodiversity and ecosystem functioning. Annu Rev Ecol Syst 45:471–493. https://doi.org/10.1146/annurev-ecolsys-120213-091917
Uchida K, Ushimaru A (2014) Biodiversity declines due to abandonment and intensification of agricultural lands: patterns and mechanisms. Ecol Monogr 84:637–658. https://doi.org/10.1890/13-2170.1
Vogel G (2017) Where have all the insects gone? Science 356:576–579. https://doi.org/10.1126/science.356.6338.57
Wagner DL (2020) Insect declines in the Anthropocene. Annu Rev Entomol 65:457–480. https://doi.org/10.1146/annurev-ento-011019-025151
Wilby A, Thomas MB (2002) Natural enemy diversity and pest control: patterns of pest emergence with agricultural intensification. Ecol Lett 5:353–360. https://doi.org/10.1046/j.1461-0248.2002.00331.x
Winkler K, Fuchs R, Rounsevell M, Herold M (2021) Global land use changes are four times greater than previously estimated. Nat Commun 12:2501. https://doi.org/10.1038/s41467-021-22702-2
Van Klink R, Bowler DE, Gongalsky KB, Swengel AB, Gentile A, Chase JM (2020) Meta-analysis reveals declines in terrestrial but increases in freshwater insect abundances. Science 368:417–420. https://doi.org/10.1126/science.aax9931
van Zanten BT, Verburg PH, Espinosa M, Gomez-y-Paloma S, Galimberti G, Kantelhardt J, Kapfer M, Lefebvre M, Manrique R, Piorr A, Raggi M, Schaller L, Targetti S, Zasada I, Viaggi D (2014) European agricultural landscapes, common agricultural policy and ecosystem services: a review. Agron Sustain Dev 34:309–325. https://doi.org/10.1007/s13593-013-0183-4
Zhang W, Rudolf VH, Ma CS (2015) Stage-specific heat effects: timing and duration of heat waves alter demographic rates of a global insect pest. Oecologia 179:947–957. https://doi.org/10.1007/s00442-015-3409-0
Zhao ZH, Hui C, He DH, Li BL (2015) Effects of agricultural intensification on ability of natural enemies to control aphids. Sci Rep 5:1–7. https://doi.org/10.1038/srep08024
Acknowledgements
Thanks to Diana Bowler and Martin Gossner for critical reading and helpful comments on an earlier draft of the manuscript and two anonymous referees for the helpful comments. We are also very grateful to all the technicians involved—specifically K. Welzel, H. Dobrowolski, R. Schmidt, E. Betke, K. Stein, or volunteers/students—for sample and data processing over many years. The authors thank the Plant Protection Department in Rostock (LALLF) for providing us a dataset of aphids (ST2, visualized in Fig. 5a). In addition, we gratefully acknowledge the datasets from Göttingen and Stuttgart, kindly provided by Bernd Ulber (Uni Göttingen). And for delivery of climate data from the German Meteorological Service (DWD).
Funding
Open Access funding enabled and organized by Projekt DEAL. The authors declare that no funds, grants, or other support were received during the preparation of this manuscript. No external funding was received for this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethics approval and consent to participate
This study was performed in line with the principles of the Federal Agency for Nature Conservation. The investigation was conducted in compliance with current laws of Germany and the federal state of Saxony-Anhalt. No informed consent was necessary.
Consent for publication
No consent to participate was necessary for this study. Not applicable, no human research participants.
Additional information
Communicated by Blas Lavandero.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ziesche, T.M., Ordon, F., Schliephake, E. et al. Long-term data in agricultural landscapes indicate that insect decline promotes pests well adapted to environmental changes. J Pest Sci (2023). https://doi.org/10.1007/s10340-023-01698-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10340-023-01698-2