Abstract
Companion dogs are at risk of tick infestations. This paper describes cases of transfer of Dermacentor reticulatus ticks by dogs to apartments in eastern Poland, tick development in household conditions, and potential consequences for the residents. For the first time, the preoviposition and oviposition of D. reticulatus females removed from dogs or spontaneously detached from these hosts were studied in household conditions. Similar analyses were performed simultaneously in laboratory settings (25 °C and 75% RH). In the household characterized by a temperature range of 18.5–21.3 °C and 46.9–56% humidity, the preoviposition and oviposition periods with the development of D. reticulatus larvae lasted 20.8 ± 3.1 days and 29.9 ± 1.4 days, respectively. Greater numbers of eggs (2415.8 ± 983.1) were laid by females in the household than laboratory conditions. There were no statistically significant differences in the hatching success between both experiments. The study also provides the first description of infestation of a human by a partially engorged D. reticulatus female that had detached from dog’s skin. Ticks transferred by dogs can develop successfully in human homes. After feeding on dogs, females achieve high reproductive performance. This suggests that dogs may play an important role in the biology of this tick species in urbanized areas.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The growth in the range of tick occurrence and the increase in their number recorded in recent years have enhanced the threat to the health of humans and companion animals posed by pathogens transmitted by these arthropods. Ticks are transferred to new habitats mainly by migratory birds (Lommano et al. 2014; Buczek et al. 2020) and wild mammals (Qviller et al. 2013; Mysterud et al. 2016; Ciebiera et al. 2021).
The spread of alien and autochthonous tick species may also be attributed to companion animals traveling with their owners to different geographic regions (Hansford et al. 2018; Buczek and Buczek 2020). The increase in the importance of dogs and cats in the transmission of ticks and pathogens is associated with the increase in the number of these animals in many European countries, including Poland (Animals in Polish homes 2017).
In Europe, dogs are parasitized by various tick species, depending on the region (Nowak-Chmura 2013; Estrada-Peña et al. 2017). Some tick species can also attack humans. In the western, central, and northern part of the continent, the sheep tick Ixodes ricinus is the most frequent parasite of these two hosts (Bartosik et al. 2011a; Namina et al. 2019; Lernout et al. 2019; Pawełczyk et al. 2021). In recent years, there have been reports on human infestations by tick species that were previously found exclusively on animals, e.g. the hedgehog tick Ix. hexagonus (Lernout et al. 2019; Springer et al. 2020) and the meadow tick Dermacentor reticulatus (Bartosik et al. 2011a; Földvári et al. 2013; Gałęziowska et al. 2018; Lernout et al. 2019; Springer et al. 2020; Pawełczyk et al. 2021).
Dermacentor reticulatus, i.e. one of the tick species parasitizing companion animals and humans, is characterized by particularly high dynamics of territorial migration (Földvári et al. 2016). The tick mainly occurs in meadow and forest ecosystems; however, its presence in wooded areas, glades, and wastelands located in cities or near urban areas has been confirmed in recent years (Biernat et al. 2014; Földvári et al. 2016; Pavlović et al. 2016; Rogovskyy et al. 2017; Kohn et al. 2019).
The host-seeking activity of adult D. reticulatus ticks infesting humans, dogs, and other domestic animals usually begins in early spring and lasts until late autumn, with a brief decline in summer. Due to the global warming and weather changes, this tick species can be active even in winter months (Buczek et al. 2014; Kiewra et al. 2016). As reported by Bartosik et al. (2012), the questing behavior of adult D. reticulatus ticks depends on the part of the day and season. During spring and autumn, these ticks were found to be active throughout the day, with a tendency to intensify their activity from the morning to early afternoon and a peak at 2:00 p.m. (1400 h military time). The high activity persisted until late afternoon (6:00 p.m.; 1800 h) in spring and declined before nightfall at 6:00 p.m. (1800 h) in the autumn period.
The wide distribution and the long duration of seasonal and diurnal activity of D. reticulatus ticks pose a high risk of contact of companion animals and their owners with this species in its habitat. In some habitats and seasons, D. reticulatus is the most common tick species collected from dogs (Mierzejewska et al. 2015).
Damage to the host’s skin caused by the mouthparts of this tick and the components of its saliva secreted during blood ingestion stimulate inflammatory reactions at the attachment site (Buczek et al. 2002). Additionally, various pathogens can be introduced into the host with the saliva of infected D. reticulatus ticks, such as tick-borne encephalitis virus, Omsk hemorrhagic fever virus, spotted fever group (SFG) rickettsia, e.g., Rickettsia raoultii and Rickettsia slovaca, Babesia canis, Babesia caballi, and Theileria equi, which cause serious diseases and economic loss in domesticated animals (Nosek 1972; Földvári et al. 2016; Rubel et al. 2016; Sprong et al. 2019; Ličková et al. 2020). As reported by many authors (Król et al. 2016; Skotarczak 2018; Banović et al. 2021), companion animals (dogs and cats) play an important role in the environmental circulation of tick-borne pathogens (TBPs) causing animal and human diseases.
The spread of D. reticulatus into urbanized areas prompts the need for more comprehensive knowledge of its biology in an environment influenced by strong anthropopressure-related factors. The current paper describes for the first time the development of D. reticulatus in household conditions. We also report a case of human infestation within the apartment due to interrupted tick feeding.
Materials and methods
Collection and identification of ticks infesting dogs
The research material comprised D. reticulatus ticks transferred by dogs to their owners’ apartments in Lublin (Lubelskie Province, eastern Poland) in September 2022, i.e., during the autumn peak activity of this species in eastern Poland (Bartosik et al. 2011b). The dogs were walked in green areas with a total surface of about 150 ha located within the Lublin agglomeration (approximately 3 km from the city center). The area was covered mainly by a mosaic of meadow communities and xerothermic plant communities, and to a lesser extent scrub and synanthropic plant communities. In the process of collecting ticks, we were supported by 43 dog owners who were also dog keepers taking care of 45 dogs. According to the declarations of their owners/keepers, the animals involved in the study did not suffer from chronic diseases, including skin diseases requiring medication. The ticks used in the study came from 20 animals that had not been treated against ectoparasites for the previous 6 months. Some ticks spontaneously detached from the dogs’ skin, while others were removed by the keepers. The species, the developmental stage, and the sex of all collected ticks were identified with the use of guide to species identification by Nowak-Chmura (2013). Dermacentor reticulatus females selected in the laboratory on the basis of their body mass (≥ 0.063 g) were included in the experiment on the day of removal from the host. To minimize the influence of the host's individual characteristics on the development of ticks, only one D. reticulatus female removed from single animal was included in the study.
Research procedure
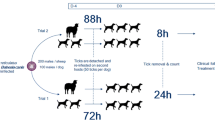
Twenty D. reticulatus females transferred to apartments by dogs were weighed using the RADWAG XA 110 analytical balance with the accuracy of 0.0001 g (RADWAG, Radom, Poland). After weighing, the females were placed in 50-ml sterile polypropylene containers. A single female was placed in each chamber. Two experiments were performed to compare the development of D. reticulatus in household conditions with that in optimal laboratory conditions. In the first experiment, 10 females were moved to an apartment inhabited by the investigator only (group I). Throughout the observation period, the temperature and humidity in the apartment were monitored using a thermo-hygrometer with the accuracy of 0.1 °C and 0.1% RH (R6030, Reed Instruments, Wilmington, NC, USA). The tick chambers were protected with a single layer of gauze to provide adequate air and vapor flow in the household conditions. When the observation of the eggs indicated the end of embryonic development and the approaching hatching, the gauze was replaced with a perforated cover to prevent the escape of the larvae also throughout the experiment in the laboratory conditions.
In the second experiment, 10 females were left in the laboratory and kept at a constant temperature of 25 °C and 75% humidity until oviposition and larval hatching (group II). As shown by our previous observations, these conditions are favorable for the development of this tick species.
In the laboratory and in the household conditions, the course of the experiments was checked at the same time every day. This facilitated determination of the length of the preoviposition period, i.e., the period of egg maturation in D. reticulatus females (the period between the detachment of the engorged female from the dog’s skin and oviposition of the first egg), the length of oviposition and larval development (the period from oviposition of the first egg to the hatching of the first larvae), and the egg laying frequency (the percentage of the study females that laid eggs). After 40 days from the hatch of the first larvae, the developmental stages of the ticks were preserved in 70% ethanol. The larvae that hatched from eggs deposited by each female as well as undeveloped eggs were counted separately under the binocular microscope mentioned above. We determined the hatching success as the number of larvae that hatched in a single egg batch expressed as a percentage. The number of eggs laid by a particular female was obtained by adding up the number of larvae and the number of undeveloped eggs derived from that female.
A case of female Dermacentor reticulatus tick attachment to human skin
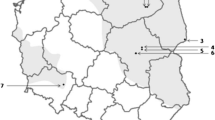
A female of D. reticulatus tick was found attached to the forearm skin of a woman living with her dog in a three-story building in the Lublin city center (Fig. 1). The woman had not left her apartment for 13 days, and her dog was taken for walks by neighbors. The feeding tick was removed from the forearm with tweezers, and the skin at the tick feeding site was disinfected. The morphological analysis performed using a stereomicroscope (Stemi DV4/DR, Carl Zeiss Light Microscopy, Göttingen, Germany) confirmed that it was a partially engorged D. reticulatus female, and the morphological features (e.g., central fat body that undergoes considerable changes during feeding) suggested that the feeding period of this specimen was much longer than 2 h.
The interview indicated that the tick attached to the woman’s forearm while she was resting with the dog on the couch and fed on the host for a maximum 2 h. After finding the tick in the human host’ skin, the dog was examined carefully, and two other partially engorged specimens of this species were removed. As declared by the owner, the dog had previously been repeatedly infested by ticks in the surroundings. Probably, the ticks attacked the dog in an undeveloped green area, where it was taken for walks every day. The dog was not taken for walks outside the urban area. No repellents or acaricides had ever been used to protect the dog against tick attacks.
Statistical analysis
The measurable variables were described using basic parameters: arithmetic mean, standard deviation (SD), and minimum and maximum values (Min and Max). The categorical variables were described using counts and frequencies.
Due to the non-normal distribution of the measurable variables and the very low counts, nonparametric tests were used: Mann–Whitney U test to check the significance of differences within the two groups and Spearman’s rank correlation coefficient test to examine correlations between the measurable variables. Fisher’s exact test was used to analyze the relationships between the conditions and larval hatching. The value of p < 0.05 was considered statistically significant. Statistical calculations were performed using the STATISTICA 10 PL package.
Results
The body mass of the D. reticulatus females that detached from the skin of the dogs in the apartments or were removed by the dog keepers ranged from 0.0650 to 0.2623 g (on average 0.1807 g).
Although the egg development in the female ticks after infestations of the dogs lasted longer (on average 20.8 days) in the household conditions with temperature fluctuations from 18.5 to 21.3 °C and relative humidity ranging from 46.9 to 56% (group I) than in the stable laboratory conditions at 25 °C and 75% humidity (17.8 days; group II), there were no statistically significant differences in the preoviposition course between both experimental groups (Table 1).
In turn, the Mann–Whitney U test revealed a significant difference in the length of the oviposition period and the development of D. reticulatus larvae between the variable household conditions and the stable laboratory conditions. The oviposition and larval hatching lasted on average 29.9 days in the household conditions and 13.9 days at the higher constant temperature of 25 °C and 75% RH in the laboratory (Table 1).
Spearman’s rank correlation coefficient (Rs) test showed no significant correlations between the body mass of the tick females and the length of the preoviposition period in group I kept in the variable conditions (Rs = 0.036; p = 0.9323) and in group II (Rs = 0.133; p = 0.7544) or jointly in both groups (Rs = 0.378; p = 0.1488). Similarly, this test did not reveal a statistically significant correlation between the body mass of the females and the length of oviposition and larval development in group I (Rs = 0.31; p = 0.4597), group II (Rs = − 0.66; p = 0.0778), and both groups (Rs = 0.48; p = 0.0590).
After feeding on the dogs, the D. reticulatus females laid a higher number of eggs (2415.8 ± 983.1) at the variable temperatures and humidity prevailing in the household conditions than in the stable laboratory conditions (1817.4 ± 693.2) (Fig. 2). The statistically significant difference in the number of eggs between the two groups was confirmed by the Mann–Whitney U test (U = 12.0; p = 0.0406).
Spearman’s rank correlation coefficient test showed significant strong positive correlations (Rs > 0.8) between the female body mass and the number of eggs laid in the stable conditions (Rs = 0.905; p = 0.0020), in the variable conditions (Rs = 0.810; p = 0.0149), and jointly in both experimental groups (Rs = 0.947; p < 0.0001). The number of eggs increased with the increase in the body mass of the female ticks (Fig. 2). In both studied groups, the number of females that laid eggs (Fig. 3A) was the same (8 in each group, the egg laying frequency 80%).
We noted larvae hatching in a case of 100% of D. reticulatus egg batches in each studied group (Fig. 3B, Video S1).
The hatching success in groups I and II was estimated at 95.48% and 93.18%, respectively. The Mann–Whitney U test showed no significant differences in this parameter between the groups from the variable and stable conditions (Table 1).
No significant correlation was found between the body mass of female ticks and the hatching success in the variable (Rs = − 0.595; p = 0.1195) and stable (Rs = − 0.524; p = 0.1827) conditions.
Discussion
The transfer of ixodid ticks by companion animals to human homes is commonly reported. However, only a few studies were focused on the development of engorged ticks removed from dogs and cats or detaching from these animals in the homes of their owners (Szymański 1979; Gilot et al. 1992; Uspensky and Ioffe-Uspensky 2002).
The present study indicates for the first time the possibility of indoor development of D. reticulatus eggs and hatching of larvae, which are the most sensitive to external factors of all tick development stages. The oviposition was observed in 80% of D. reticulatus females both in the stable laboratory conditions and in the household conditions characterized by temperature and humidity fluctuations from 18.5 to 21.3 °C and from 46.9 to 56% RH, respectively. Only four females (two in the laboratory and two in the apartment) removed from the dogs before the end of feeding did not lay eggs. They were probably not fertilized by males. D. reticulatus and other Metastriata ticks mate only on the host (Balashov 1972). No males were found on the dogs infested by the tick females that failed to lay eggs. At least 80% oviposition in D. reticulatus females was reported by Zahler and Gothe (1995a) in their study carried out at constant temperature values of 5 °C, 20 °C, and 27 °C and at 15%, 50%, and 100% relative humidity. In our previous laboratory studies conducted at a constant temperature of 25 °C and 75% relative humidity, the percentage of ovipositing females after rabbit blood ingestion in the presence of males was 100% (Buczek et al. 2013a).
The course of feeding and the reproductive capacity of female D. reticulatus ticks depend on various factors, primarily on the infested host species (Šimo et al. 2004), amounts of ingested blood (Balashov 1972; Buczek et al. 2013a), and host immunity (Bartosik et al. 2017). In the present study conducted in the different experimental conditions, we confirmed a high correlation between the body mass of females parasitizing dogs and the number of produced eggs.
The lower number of eggs oviposited by D. reticulatus females after feeding on dogs than on guinea pigs, mice, and rabbits (Šimo 2004; our unpublished data) is probably related to the fact that most of the specimens used in the present study were removed from the hosts before reaching full engorgement and maximum body mass. The dog owners reported that their pets had previously been frequently attacked by ticks in the urban area. Probably, the immunity acquired by the dogs against ticks reduced the foraging efficiency of D. reticulatus females in reinvasions, and consequently, reduced their reproductive performance. Further research is required to determine the impact of the immune status of dogs on the course of feeding, preoviposition, and oviposition in this tick species.
The magnitude of blood ingestion by ticks is also influenced by the intensity of host infestation. D. reticulatus females parasitizing the host simultaneously with a large number of other specimens on the same host were reported to reach lower body mass than in the case of lower infestation levels. In the presence of males in groups of 10 and 20 females, the average body mass of engorged females was 0.3554 ± 0.0380 g and 0.3559 ± 0.0654 g, respectively. In turn, when the intensity of infestation was increasing (40 female specimens), the body weight of females after completion of feeding was lower, i.e., on average 0.2511 ± 0.1135 g (Bartosik and Buczek 2012).
The larval hatching and the high hatching success observed in the D. reticulatus kept in the apartment in Lublin in the variable temperature and humidity range (18.5–21.3 °C and 46.9–56.3% RH) confirm the high tolerance of environmental conditions in this species, which is consistent with the results of our multiyear research on the activity of D. reticulatus in habitats in eastern Poland (Bartosik et al. 2011b; Buczek et al. 2013b). In laboratory conditions, D. reticulatus larvae were shown to hatch at a temperature ranging from 20 to 34 °C and 90% RH, but they achieved higher hatching success only at 20 °C and 27 °C at 100% RH (Zahler and Gothe 1995b). Over 98% hatching success of larvae from eggs oviposited by D. reticulatus females in the eastern Polish population was observed after infestation of rabbits (Buczek et al. 2013a). The higher reproductive capacity of D. reticulatus females kept in the apartment suggests that fluctuations of abiotic factors, i.e., temperature and humidity (within the ecological tolerance of this species), have a more favorable effect on the course of preoviposition and oviposition than their constant levels in laboratory conditions. The range of temperature and humidity, similar to that in the examined dwellings, was observed in the habitats of these ticks in June and July, i.e., during the laying of eggs by some D. reticulatus females in eastern Poland.
In the present study, the large number of eggs laid by the D. reticulatus females after feeding on dogs and the larval hatching indicate that dogs are competent hosts for adult stages of this tick and may ensure the reproductive success and spread of the parasite in the environment. The large dog populations in some countries and regions prompt the need to elucidate the role of these animals in D. reticulatus development and the maintenance of large populations of these ticks in urban areas.
To date, mass reproduction of ixodid ticks in an apartment (in Warsaw) has been described only once in Poland (Szymański 1979). These were Rhipicephalus sanguineus ticks transferred by a dog of a Polish family spending holidays in the Mazury region together with an Italian family that had a dog. In the apartment, engorged Rh. sanguineus female ticks detached from the dog’s skin and laid eggs; later, the eggs developed into larvae. However, Szymański did not monitor the parameters of tick development in his study. Cases of indoor occurrence of Rh. sanguineus ticks have been reported from other European countries as well, e.g., Germany (Dongus et al. 1996), England (Best et al. 1969; Fox and Sykes 1985; Hansford et al. 2015), and France (Gilot et al. 1992; Renvoisé et al. 2012), and in other areas of the geographic distribution of this tick, e.g., in Israel (Uspensky and Ioffe-Uspensky 2002), the USA (Demma et al. 2005), and Brazil (Dantas-Torres et al. 2006). This species is linked with the human environment, in which the entire development cycle can be completed. Kahl et al. (2022) reported an interesting case of the presence of over 30 specimens of fed I. hexagonus females in the bedroom of a cat owner’s house in Jena (central Germany).
Unfed (female and male) ticks transferred to apartments and detached from the skin of a dog or a cat can attack other hosts, including humans. There are literature reports of migration of male ticks, which, after detaching from one dog infested another one that was present in the same room. In a study conducted by Little et al. (2007), the percentage of Rh. sanguineus male ticks migrating from one dog to another ranged from 0 to 46% (mean 31.1%). Similarly, Amblyomma hebraeum males were observed to migrate onto other animals present nearby (Andrew and Norval 1989). The migration of male Metastriata ticks on one host or between different hosts is a natural phenomenon. Male ticks attach to the skin of the host for a short time and mate with many feeding females (Balashov 1972). In search of other females, they may migrate onto another host.
In this study, we highlight the possibility that, after interrupted feeding on companion animals, D. reticulatus ticks may infest humans. This is the first recorded case of migration of a partially engorged D. reticulatus female from an animal host to a human in dwelling. The morphometric traits of the specimen removed from the woman’s skin indicate that the partially engorged female of this species detached from the dog in the first/early feeding phase and then infested the human host.
The causes of the detachment of the female tick from the dog before completion of feeding are unknown. Typically, after attachment to host’s skin, ticks ingest blood until they are engorged and then detach and hide in the immediate vicinity. Unengorged ticks may detach from a host that was immunized by previous infestations (Gebbia et al. 1995; Narasimhan et al. 2021). Ticks may also be removed by the animal during grooming. In our unpublished studies, we observed disruption of tick feeding on animals that had earlier been treated with acaricides or repellents.
In the infestation case described in this study, the partially engorged D. reticulatus female started feeding shortly after reaching the new host’s skin. This is an interesting observation, as humans are not common hosts for this tick species. As shown by our laboratory studies, in the presence of male ticks, the first unfed D. reticulatus females attached to the skin within 0.5–48 h after the transfer onto a rabbit host. Depending on the number of accompanying males, the average attachment time ranged from 10.20 ± 19.92 to 14.15 ± 17.55 h (Bartosik and Buczek 2012). Males of this species placed on the host in homogeneous sex groups comprising 15 and 30 male specimens and in a mixed group composed of 15 males and 15 females at 18 ± 2 °C and 50 ± 2% RH attached after 2.43 ± 2.46, 7.75 ± 11.85, and 9.07 ± 10.97 days, respectively (Bartosik et al. 2019). We did not find any literature studies on the course of attachment of D. reticulatus ticks to the skin of dogs.
The high aggressiveness of the partially fed female in our study was probably related to the need to complete the blood meal, which is necessary for egg development and production of offspring. For ethical reasons, we did not carry out observations of the course of the D. reticulatus female feeding on the human host. Nevertheless, noteworthy is the appearance of an inflammatory reaction visible on the woman’s skin at the tick attachment site shortly after the infestation.
Discontinuation of tick feeding on one host and continuation on another may promote the transmission of tick-borne pathogens in the environment. The scale of this phenomenon in nature is unknown. The case described in this study prompts the need to investigate the epidemiological significance of the zoonotic transmission of pathogens to humans through the change of hosts by unengorged tick specimens.
In Poland, including its eastern part, adult D. reticulatus ticks infesting humans have been reported several times (Bartosik et al. 2011a; Gałęziowska et al. 2018; Pawełczyk et al. 2021). However, the direct effects of their parasitism have not been described. Due to the possibility of D. reticulatus infestations of humans, infested patients living in the occurrence range of this species may develop symptoms of various tick-borne diseases. In the eastern part of Poland, D. reticulatus ticks collected from vegetation and/or dogs were infected by TBEV, Rickettsia raoultii, Borrelia burgdorferi s.l., Babesia species, Toxoplasma gondii, and Anaplasma phagocytophilum (Zając et al. 2017; Wójcik-Fatla et al. 2012; Pańczuk et al. 2022). These pathogens were also identified in this tick species in other regions of Poland (Biernat et al. 2014; Król et al. 2016; Michalski et al. 2020; Grochowska et al. 2021) and Europe (Geurden et al. 2018; Ben and Lozynskyi 2019; Răileanu et al. 2022).
Infections of humans with TBPs transmitted by ticks transferred by companion animals to households have been described in the literature. In France, symptoms of spotted fever group (SFG) rickettsiosis were observed in family members living in a house where Rh. sanguineus ticks were found on the floor behind furniture. The ticks were infected by Rickettsia conorii subsp. caspia and Rickettsia massiliae (Renvoisé et al. 2012). In turn, rickettsiosis caused by Rickettsia sibrica mongolitimonae was diagnosed in patients who had companion animals attacked by infected ticks. R. massiliae DNA was identified in Rh. sanguineus ticks collected from the dogs, whereas the presence of Ri. sibrica mongolitimonae DNA was detected in ticks feeding on the cats (Edouard et al. 2013).
In Serbia, Haemaphysalis punctata, Rhipicephalus sanguineus, and Ixodes ricinus ticks were found on the outside walls of a farmhouse and on the family’s dogs (Banović et al. 2021). Five pathogens (Rickettsia helvetica, Rickettsia monacensis, Rickettsia felis, A. phagocytophilum, and Hepatozoon canis) were detected in these ticks or in the blood of their hosts (the family’s dogs and alpine goats). Additionally, IgG against B. burgdorferi sensu stricto was detected in a sample of human serum of one of the family members with a history of Lyme disease.
The wide tick occurrence range and the frequent infestations of companion animals by these arthropods suggest the necessity of dissemination of anti-tick prophylaxis methods among animal owners. Dogs and cats should be carefully inspected after return from tick habitats to remove any unattached ticks from their fur or to remove already feeding specimens. The longer ticks feed, the greater the risk of infection of the host with different species of pathogens (Eisen 2018). Ticks should be removed using tweezers or with other methods that eliminate the possibility of damaging the feeding specimen. There are known cases of human infection with Ri. conorii during removal of ticks from dogs’ skin (Oztoprak et al. 2008). An effective method for protection of companion animals and their owners against tick attacks and tick-borne diseases is the regular use of repellent or acaricidal chemicals (Buczek et al. 2013b; Duscher et al. 2013; Pfister and Armstrong 2016).
Conclusions
The present study has demonstrated that D. reticulatus eggs and larvae can successfully develop in household conditions. The high reproductive performance of D. reticulatus females after feeding on dogs and the larval hatching success suggest that this host species may play an important role not only in the circulation of TBPs, as reported by other authors, but also in the biology of D. reticulatus ticks in urbanized areas.
This first case report of a partially fed female D. reticulatus infesting the human host shows that, once detached or removed from a dog, such ticks may continue feeding on other hosts present in the surroundings, including humans. The transfer of ticks to homes by dogs can therefore pose a threat to the health of their residents. For this reason, it is necessary to take measures to reduce the tick population size in urban areas and disseminate knowledge of the methods for anti-tick prophylaxis among companion animal owners. In the event of appearance of disease symptoms in dog owners, the diagnostics should consider potential infection with TBPs as a result of tick infestations both outside and inside the household.
Author contributions
WB and KB did conceptualization; AB and KB performed methodology; AB and KB analyzed validation and formal analysis; WB and KB carried out investigation and data curation; WB provides writing original draft; KB and AB did writing, review and editing; WB and KB contributed to visualization; AB performed supervision. All authors read and approved the final manuscript.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Andrew HR, Norval RA (1989) The role of males of the bont tick (Amblyomma hebraeum) in the transmission of Cowdria ruminantium (heartwater). Vet Parasitol 34:15–23. https://doi.org/10.1016/0304-4017(89)90159-3
Animals in Polish homes (2017) Kantar Public. Available online: http://www.tnsglobal.pl/archiwumraportow/files/2017/05/K.021_Zwierzeta_domowe_O04a-17.pdf. Accessed 12 January 2023
Balashov YS (1972) Blood sucking ticks (Ixodidae)- vectors of diseases of man and animals. Misc Publ Entomol Soc Am 8:161–376
Banović P, Díaz-Sánchez AA, Galon C, Foucault-Simonin A, Simin V, Mijatović D, Papić L, Wu-Chuang A, Obregón D, Moutailler S, Cabezas-Cruz A (2021) A One Health approach to study the circulation of tick-borne pathogens: a preliminary study. One Health 13:100270. https://doi.org/10.1016/j.onehlt.2021.100270
Bartosik K, Buczek A (2012) The impact of intensity of invasion of Ixodes ricinus and Dermacentor reticulatus on the course of the parasitic phase. Ann Agric Environ Med 19:651–655
Bartosik K, Sitarz M, Szymańska J, Buczek A (2011a) Tick bites on humans in the agricultural and recreational areas in south-eastern Poland. Ann Agric Environ Med 18:151–157
Bartosik K, Wiśniowski L, Buczek A (2011b) Abundance and seasonal activity of adult Dermacentor reticulatus (Acari: Amblyommidae) in eastern Poland in relation to meteorological conditions and the photoperiod. Ann Agric Environ Med 18:340–344
Bartosik K, Wiśniowski Ł, Buczek A (2012) Questing behavior of Dermacentor reticulatus adults (Acari: Amblyommidae) during diurnal activity periods in eastern Poland. J Med Entomol 49:859–864. https://doi.org/10.1603/ME11121
Bartosik K, Buczek A, Borzęcki A, Kulina D (2017) Study of the non-parasitic stage in Ixodes ricinus after co-feeding with Dermacentor reticulatus in three infestations. Ann Agric Environ Med 24:90–95. https://doi.org/10.5604/12321966.1234005
Bartosik K, Buczek A, Buczek W, Buczek AM, Kulina D, Koman-Iżko A (2019) Host feeding behaviour of Dermacentor reticulatus males in relation to the transmission of pathogens. Ann Agric Environ Med 26:227–230. https://doi.org/10.26444/aaem/105402
Ben I, Lozynskyi I (2019) Prevalence of Anaplasma phagocytophilum in Ixodes ricinus and Dermacentor reticulatus and Coinfection with Borrelia burgdorferi and tick-borne encephalitis virus in Western Ukraine. Vector Borne Zoonotic Dis 19:793–801. https://doi.org/10.1089/vbz.2019.2450
Best JM, Butt KM, Rohrbach JA (1969) Occurrence of Rhipicephalus sanguineus in London. Vet Rec 85:633. https://doi.org/10.1136/vr.85.22.633
Biernat B, Karbowiak G, Werszko J, Stańczak J (2014) Prevalence of tick-borne encephalitis virus (TBEV) RNA in Dermacentor reticulatus ticks from natural and urban environment, Poland. Exp Appl Acarol 64:543–551. https://doi.org/10.1007/s10493-014-9836-5
Buczek A, Buczek W (2020) Importation of ticks on companion animals and the risk of spread of tick-borne diseases to non-endemic regions in Europe. Animals 11:6. https://doi.org/10.3390/ani11010006
Buczek A, Kuśmierz A, Olszewski K, Buczek L, Czerny K, Lańcut M (2002) Comparison of rabbit skin changes after feeding of Ixodes ricinus (L.) and Dermacentor reticulatus (Fabr.). In: Bernini F, Nannelli R, Nuzzaci G, de Lillo E (eds) Acarid phylogeny and evolution, adaptation in mites and ticks. Kluwer Academic Publishers, Dordrecht, pp 419–424
Buczek A, Bartosik K, Kuczyński P (2013a) Evaluation of the effect of various concentrations of selected pyrethroids on the devel-opment of Dermacentor reticulatus eggs and larvae. Ann Agric Environ Med 20:447–451
Buczek A, Bartosik K, Wiśniowski Ł, Tomasiewicz K (2013b) Changes in population abundance of adult Dermacentor reticulatus (Acari: Amblyommidae) in long-term investigations in eastern Poland. Ann Agric Environ Med 20:269–272
Buczek A, Bartosik K, Zając Z (2014) Changes in the activity of adult stages of Dermacentor reticulatus (Ixodida: Amblyommidae) induced by weather factors in eastern Poland. Parasit Vectors 7:245. https://doi.org/10.1186/1756-3305-7-245
Buczek AM, Buczek W, Buczek A, Bartosik K (2020) The potential role of migratory birds in the rapid spread of ticks and tick-borne pathogens in the changing climatic and environmental conditions in Europe. Int J Environ Res Public Health 17:2117. https://doi.org/10.3390/ijerph17062117
Ciebiera O, Łopińska A, Gabryś G (2021) Ticks on game animals in the fragmented agricultural landscape of western Poland. Parasitol Res 120:1781–1788. https://doi.org/10.1007/s00436-021-07132-9
Dantas-Torres F, Figueredo LA, Brandão-Filho SP (2006) Rhipicephalus sanguineus (Acari: Ixodidae), the brown dog tick, parasitizing humans in Brazil. Rev Soc Bras Med Trop 39:64–67. https://doi.org/10.1590/S0037-86822006000100012
Demma LJ, Traeger MS, Nicholson WL, Paddock CD, Blau DM, Eremeeva ME, Dasch GA, Levin ML, Singleton J Jr, Zaki SR, Cheek JE, Swerdlow DL, McQuiston JH (2005) Rocky Mountain spotted fever from an unexpected tick vector in Arizona. N Engl J Med 353:587–594. https://doi.org/10.1056/NEJMoa050043
Dongus H, Zahler M, Gothe R (1996) The brown dog tick, Rhipicephalus sanguineus (Ixodidae), in Germany: an epidemiologic study and control measures. Berl Münch Tierärztl Wochenschr 109:245–248
Duscher GG, Feiler A, Leschnik M, Joachim A (2013) Seasonal and spatial distribution of ixodid tick species feeding on naturally infested dogs from Eastern Austria and the influence of acaricides/repellents on these parameters. Parasit Vectors 6:76. https://doi.org/10.1186/1756-3305-6-76
Edouard S, Parola P, Socolovschi C, Davoust B, La Scola B, Raoult D (2013) Clustered cases of Rickettsia sibirica mongolitimonae infection, France. Emerg Infect Dis 19:337–338. https://doi.org/10.3201/eid1902.120863
Eisen L (2018) Pathogen transmission in relation to duration of attachment by Ixodes scapularis ticks. Ticks Tick Borne Dis 9:535–542. https://doi.org/10.1016/j.ttbdis.2018.01.002
Estrada-Peña A, Roura X, Sainz A, Miró G, Solano-Gallego L (2017) Species of ticks and carried pathogens in owned dogs in Spain: results of a one-year national survey. Ticks Tick Borne Dis 8:443–452. https://doi.org/10.1016/j.ttbdis.2017.02.001
Földvári G, Rigó K, Lakos A (2013) Transmission of Rickettsia slovaca and Rickettsia raoultii by male Dermacentor marginatus and Dermacentor reticulatus ticks to humans. Diagn Microbiol Infect Dis 76:387–389. https://doi.org/10.1016/j.diagmicrobio.2013.03.005
Földvári G, Široký P, Szekeres S, Majoros G, Sprong H (2016) Dermacentor reticulatus: a vector on the rise. Parasit Vectors 9:314. https://doi.org/10.1186/s13071-016-1599-x
Fox MT, Sykes TJ (1985) Establishment of the tropical dog tick, Rhipicephalus sanguineus, in a house in London. Vet Rec 116:661–662. https://doi.org/10.1136/vr.116.25.661
Gałęziowska E, Rzymowska J, Najda N, Kołodziej P, Domżał-Drzewicka R, Rząca M, Muraczyńska B, Charzyńska-Gula M, Szadowska-Szlachetka Z, Ślusarska B, Guty E (2018) Prevalence of Borrelia burgdorferi in ticks removed from skin of people and circumstances of being bitten—research from the area of Poland, 2012–2014. Ann Agric Environ Med 25:31–35. https://doi.org/10.5604/12321966.1233906
Gebbia JA, Bosler EM, Evans RD, Schneider EM (1995) Acquired resistance in dogs to repeated infestation with Ixodes scapularis (Acari: Ixodidae) reduces tick viability and reproductive success. Exp Appl Acarol 19:593–605. https://doi.org/10.1007/BF00048814
Geurden T, Becskei C, Six RH, Maeder S, Latrofa MS, Otranto D, Farkas R (2018) Detection of tick-borne pathogens in ticks from dogs and cats in different European countries. Ticks Tick Borne Dis 9:1431–1436. https://doi.org/10.1016/j.ttbdis.2018.06.013
Gilot B, Diop S, Laforge ML (1992) Spatio-temporal dynamics of Rhipicephalus sanguineus (Latreille, 1806) populations (Acari, Ixodoidea) in a social housing complex at Marseilles. Acarologia 33:127–140
Grochowska A, Dunaj J, Pancewicz S, Czupryna P, Majewski P, Wondim M, Tryniszewska E, Moniuszko-Malinowska A (2021) Detection of Borrelia burgdorferi s.l., Anaplasma phagocytophilum and Babesia spp. in Dermacentor reticulatus ticks found within the city of Białystok. Poland-First Data Exp Appl Acarol 85:63–73. https://doi.org/10.1007/s10493-021-00655-x
Hansford KM, Pietzsch M, Cull B, Medlock JM (2015) Brown dog tick infestation of a home in England. Vet Rec 176:129–130. https://doi.org/10.1136/vr.h496
Hansford KM, Pietzsch ME, Cull B, Gillingham EL, Medlock JM (2018) Potential risk posed by the importation of ticks into the UK on animals: records from the Tick Surveillance Scheme. Vet Rec 182:107. https://doi.org/10.1136/vr.104263
Kahl O, Bulling I, Chitimia-Dobler L (2022) Some new findings on the endophilic vector tick Ixodes hexagonus in Germany. Ticks Tick Borne Dis 13:101954. https://doi.org/10.1016/j.ttbdis.2022.101954
Kiewra D, Czułowska A, Lonc E (2016) Winter activity of Dermacentor reticulatus (Fabricius, 1794) in the newly emerging population of Lower Silesia, south-west Poland. Ticks Tick Borne Dis 7:1124–1127. https://doi.org/10.1016/j.ttbdis.2016.08.012
Kohn M, Krücken J, McKay-Demeler J, Pachnicke S, Krieger K, von Samson-Himmelstjerna G (2019) Dermacentor reticulatus in Berlin/Brandenburg (Germany): activity patterns and associated pathogens. Ticks Tick Borne Dis 10:191–206. https://doi.org/10.1016/j.ttbdis.2018.10.003
Król N, Obiegala A, Pfeffer M, Lonc E, Kiewra D (2016) Detection of selected pathogens in ticks collected from cats and dogs in the Wrocław Agglomeration. South-West Poland Parasit Vectors 9:351. https://doi.org/10.1186/s13071-016-1632-0
Lernout T, De Regge N, Tersago K, Fonville M, Suin V, Sprong H (2019) Prevalence of pathogens in ticks collected from humans through citizen science in Belgium. Parasit Vectors 12:550. https://doi.org/10.1186/s13071-019-3806-z
Ličková M, Fumačová Havlíková S, Sláviková M, Slovák M, Drexler JF, Klempa B (2020) Dermacentor reticulatus is a vector of tick-borne encephalitis virus. Ticks Tick Borne Dis 11:101414. https://doi.org/10.1016/j.ttbdis.2020.101414
Little SE, Hostetler J, Kocan KM (2007) Movement of Rhipicephalus sanguineus adults between co-housed dogs during active feeding. Vet Parasitol 150:139–145. https://doi.org/10.1016/j.vetpar.2007.08.029
Lommano E, Dvořák C, Vallotton L, Jenni L, Gern L (2014) Tick-borne pathogens in ticks collected from breeding and migratory birds in Switzerland. Ticks Tick Borne Dis 5:871–882. https://doi.org/10.1016/j.ttbdis.2014.07.001
Michalski MM, Kubiak K, Szczotko M, Chajęcka M, Dmitryjuk M (2020) Molecular detection of Borrelia burgdorferi sensu lato and Anaplasma phagocytophilum in ticks collected from dogs in urban areas of North-Eastern Poland. Pathogens 9:455. https://doi.org/10.3390/pathogens9060455
Mierzejewska EJ, Welc-Faleciak R, Karbowiak G, Kowalec M, Behnke JM, Bajer A (2015) Dominance of Dermacentor reticulatus over Ixodes ricinus (Ixodidae) on livestock, companion animals and wild ruminants in eastern and central Poland. Exp Appl Acarol 66:83–101. https://doi.org/10.1007/s10493-015-9889-0
Mysterud A, Qviller L, Meisingset EL, Viljugrein H (2016) Parasite load and seasonal migration in red deer. Oecologia 180:401–407. https://doi.org/10.1007/s00442-015-3465-5
Namina A, Capligina V, Seleznova M, Krumins R, Aleinikova D, Kivrane A, Akopjana S, Lazovska M, Berzina I, Ranka R (2019) Tick-borne pathogens in ticks collected from dogs, Latvia, 2011–2016. BMC Vet Res 15:398. https://doi.org/10.1186/s12917-019-2149-5
Narasimhan S, Kurokawa C, DeBlasio M, Matias J, Sajid A, Pal U, Lynn G, Fikrig E (2021) Acquired tick resistance: the trail is hot. Parasite Immunol 43:e12808. https://doi.org/10.1111/pim.12808
Nosek J (1972) The ecology and public health importance of Dermacentor marginatus and D. reticulatus ticks in Central Europe. Folia Parasitol (praha) 19:93–102
Nowak-Chmura M (2013) Fauna of ticks of Central Europe. Scientific Publishing House of the Pedagogical University, Cracow
Oztoprak N, Celebi G, Aydemir H, Pişkin N, Bektaş S, Koca R, Kuloğlu F (2008) Mediterranean spotted fever due to contact with dog-tick. Mikrobiyol Bul 42:701–706
Pańczuk A, Tokarska-Rodak M, Teodorowicz P, Pawłowicz-Sosnowska E (2022) Tick-borne pathogens in Dermacentor reticulatus collected from dogs in eastern Poland. Exp Appl Acarol 86:419–429. https://doi.org/10.1007/s10493-022-00700-3
Pavlović I, Jovčevski S, Rogožarski D, Csordás F, Mitrović N, Mijatović I, Marčić D, Ilić Ž, Ćirković D, Šekler M, Jovčevski S, Ristić M (2016) Biodiversity of ticks and fleas of dogs in thewestern Balkans—preliminary examinations. Bull UASVM Vet Med Cluj-Napoca 73:195–197. https://doi.org/10.15835/buasvmcn-vm:11344
Pawełczyk A, Bednarska M, Hamera A, Religa E, Poryszewska M, Mierzejewska EJ, Welc-Falęciak R (2021) Long-term study of Borrelia and Babesia prevalence and co-infection in Ixodes ricinus and Dermacentor recticulatus ticks removed from humans in Poland, 2016–2019. Parasit Vectors 14:348. https://doi.org/10.1186/s13071-021-04849-5
Pfister K, Armstrong R (2016) Systemically and cutaneously distributed ectoparasiticides: a review of the efficacy against ticks and fleas on dogs. Parasit Vectors 9:436. https://doi.org/10.1186/s13071-016-1719-7
Qviller L, Risnes-Olsen N, Bærum KM, Meisingset EL, Loe LE, Ytrehus B, Viljugrein H, Mysterud A (2013) Landscape level variation in tick abundance relative to seasonal migration in red deer. PLoS ONE 8:e71299. https://doi.org/10.1371/journal.pone.0071299
Răileanu C, Tauchmann O, Silaghi C (2022) Sympatric occurrence of Ixodes ricinus with Dermacentor reticulatus and Haemaphysalis concinna and the associated tick-borne pathogens near the German Baltic coast. Parasit Vectors 15(1):65. https://doi.org/10.1186/s13071-022-05173-2
Renvoisé A, Delaunay P, Blanchouin E, Cannavo I, Cua E, Socolovschi C, Parola P, Raoult D (2012) Urban family cluster of spotted fever rickettsiosis linked to Rhipicephalus sanguineus infected with Rickettsia conorii subsp. caspia and Rickettsia massiliae. Ticks Tick Borne Dis 3:389–392. https://doi.org/10.1016/j.ttbdis.2012.10.008
Rogovskyy AS, Nebogatkin IV, Scoles GA (2017) Ixodid ticks in the megapolis of Kyiv, Ukraine. Ticks Tick Borne Dis 8:99–102. https://doi.org/10.1016/j.ttbdis.2016.10.004
Rubel F, Brugger K, Pfeffer M, Chitimia-Dobler L, Didyk YM, Leverenz S, Dautel H, Kahl O (2016) Geographical distribution of Dermacentor marginatus and Dermacentor reticulatus in Europe. Ticks Tick Borne Dis 7:224–233. https://doi.org/10.1016/j.ttbdis.2015.10.015
Šimo L, Kocáková P, Sláviková M, Kubeš M, Hajnická V, Vančová I, Slovak M (2004) Dermacentor reticulatus (Acari, Ixodidae) female feeding in laboratory. Biologia 59:655–660
Skotarczak B (2018) The role of companion animals in the environmental circulation of tick-borne bacterial pathogens. Ann Agric Environ Med 25:473–480. https://doi.org/10.26444/aaem/93381
Springer A, Raulf MK, Fingerle V, Strube C (2020) Borrelia prevalence and species distribution in ticks removed from humans in Germany, 2013–2017. Ticks Tick Borne Dis 11:101363. https://doi.org/10.1016/j.ttbdis.2019.101363
Sprong H, Fonville M, Docters van Leeuwen A, Devillers E, Ibañez-Justicia A, Stroo A, Hansford K, Cull B, Medlock J, Heyman P, Cochez C, Weis L, Silaghi C, Moutailler S (2019) Detection of pathogens in Dermacentor reticulatus in northwestern Europe: evaluation of a high-throughput array. Heliyon 5:e01270. https://doi.org/10.1016/j.heliyon.2019.e01270
Szymański S (1979) Case of a mass development of tick Rhipicephalus sanguineus (Latreille, 1806) in a Warsaw residence. Wiad Parazytol 25:453–459
Uspensky I, Ioffe-Uspensky I (2002) The dog factor in brown dog tick Rhipicephalus sanguineus (Acari: Ixodidae) infestations in and near human dwellings. Int J Med Microbiol 291:156–163. https://doi.org/10.1016/S1438-4221(02)80030-3
Wójcik-Fatla A, Bartosik K, Buczek A, Dutkiewicz J (2012) Babesia microti in adult Dermacentor reticulatus ticks from eastern Poland. Vector Borne Zoonotic Dis 12:841–843. https://doi.org/10.1089/vbz.2011.0904
Zahler M, Gothe R (1995a) Effect of temperature and humidity on longevity of unfed adults and on oviposition of engorged females of Dermacentor reticulatus (Ixodidae). Appl Parasitol 36:200–211
Zahler M, Gothe R (1995b) Effect of temperature and humidity on egg hatch, moulting and longevity of larvae and nymphs of Dermacentor reticulatus (Ixodidae). Appl Parasitol 36:53–65
Zając V, Wójcik-Fatla A, Sawczyn A, Cisak E, Sroka J, Kloc A, Zając Z, Buczek A, Dutkiewicz J, Bartosik K (2017) Prevalence of infections and co-infections with 6 pathogens in Dermacentor reticulatus ticks collected in eastern Poland. Ann Agric Environ Med 24:26–32. https://doi.org/10.5604/12321966.1233893
Acknowledgements
We would like to thank the dog keepers for providing ticks for this research, which could not have been carried out without their help and involvement.
Funding
Publication financed by the Polish Ministry of Education and Science, under the internal Grant of the Medical University of Lublin no. DS508.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Ethical approval
Our study does not include experiments on humans or vertebrate animals. The ticks used for the study were collected from companion animals by their keepers as part of hygienic procedures. The author that surveyed the development of ticks in the household had many years of experience in research on the development of ticks. In addition, the construction of rearing chambers guaranteed no threat to the health of the person monitoring the experiment. Therefore, the description of interrupted tick feeding included in this study is not a medical experiment and, based on Polish law and Good Clinical Practice, does not need Ethical Approval.
Informed consent
The authors affirm that human research participants provided informed consent for publication of the images in Fig. 1A, B.
Additional information
Communicated by Antonio Biondi.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 (MOV 24739 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Buczek, W., Bartosik, K. & Buczek, A. Development of Dermacentor reticulatus ticks in human household conditions. J Pest Sci 97, 1069–1079 (2024). https://doi.org/10.1007/s10340-023-01695-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10340-023-01695-5